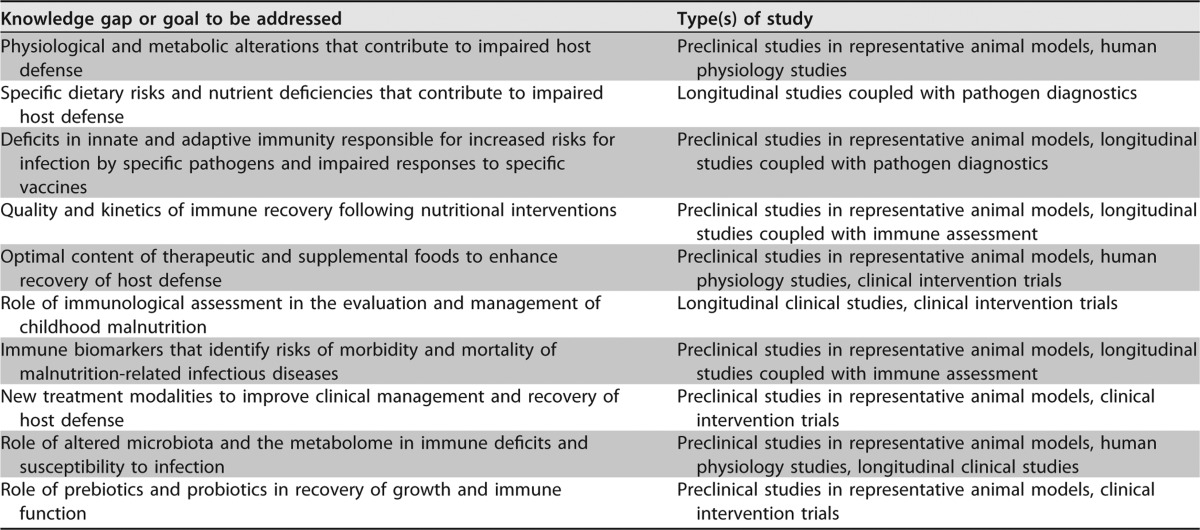
SUMMARY
The global impact of childhood malnutrition is staggering. The synergism between malnutrition and infection contributes substantially to childhood morbidity and mortality. Anthropometric indicators of malnutrition are associated with the increased risk and severity of infections caused by many pathogens, including viruses, bacteria, protozoa, and helminths. Since childhood malnutrition commonly involves the inadequate intake of protein and calories, with superimposed micronutrient deficiencies, the causal factors involved in impaired host defense are usually not defined. This review focuses on literature related to impaired host defense and the risk of infection in primary childhood malnutrition. Particular attention is given to longitudinal and prospective cohort human studies and studies of experimental animal models that address causal, mechanistic relationships between malnutrition and host defense. Protein and micronutrient deficiencies impact the hematopoietic and lymphoid organs and compromise both innate and adaptive immune functions. Malnutrition-related changes in intestinal microbiota contribute to growth faltering and dysregulated inflammation and immune function. Although substantial progress has been made in understanding the malnutrition-infection synergism, critical gaps in our understanding remain. We highlight the need for mechanistic studies that can lead to targeted interventions to improve host defense and reduce the morbidity and mortality of infectious diseases in this vulnerable population.
KEYWORDS: Mycobacterium tuberculosis, host defense, immunology, infectious disease, malaria, malnutrition, micronutrients, pneumonia, sepsis
INTRODUCTION
The synergistic association between malnutrition and infection has been recognized for more than 50 years. Our understanding of this association largely comes from retrospective and prospective cross-sectional studies of children in resource-poor settings. Few longitudinal studies clearly define malnutrition as a risk factor for the increased incidence and/or severity of infection. Even fewer studies address causal mechanisms that lead to the increased risk of infection in the malnourished host. Recent studies have shed some light on the mechanistic underpinnings of the malnutrition-infection relationship, but much work remains to address the large gaps in both knowledge and practice. A number of important aspects about the impact of malnutrition on host defense have not been well studied, and very few studies have investigated the impact of nutritional interventions on ameliorating malnutrition-infection synergism.
In this review, we summarize what is known about the influence of the most common nutrient deficiencies on host defense and the risk of infectious diseases. Areas of future research needed to address the knowledge gaps are highlighted. This review focuses on literature related to primary childhood malnutrition (as a result of the inadequate quantity or quality of food and associated macro- and micronutrients) available through PubMed over the past 15 years, with selected references to previous seminal work. In some instances, findings related to adult malnutrition and host defense that are relevant to childhood malnutrition are also discussed. Particular attention is given to longitudinal and prospective cohort human studies and studies of experimental animal models that address causal, mechanistic relationships between malnutrition and host defense. Some experimental animal studies have given little regard to age, and older animals may not accurately represent the period of early childhood development. As such, their direct applicability to early childhood malnutrition is uncertain. It is becoming increasingly clear that maternal and prenatal nutrition plays an important role in the shaping of immune function during postnatal life and even into adulthood. For this topic, the reader is referred to several recent excellent reviews (1, 2).
DEFINITIONS OF MALNUTRITION
The World Health Organization (WHO) defines malnutrition as the imbalance between the intake of nutrients and energy and the body's requirement to ensure homeostasis, specific functions, and, in the case of children, growth. A number of terms have been used to classify childhood malnutrition (Table 1). Protein-energy malnutrition (PEM) in children is a term broadly used to describe malnutrition resulting from dietary deficiencies (inadequate intake) in protein and energy (calories) (reviewed in reference 3). It is often accompanied by various deficiencies in micronutrients, especially iron and zinc. It may be acute, chronic, or acute superimposed on chronic. Acute malnutrition is defined as insufficient weight relative to height, while stunting, or chronic malnutrition, is defined by poor linear growth (length or height) for age. WHO reference growth standards for age and sex enable the grading of malnutrition into severe, moderate, or mild categories (WHO classification; see http://www.who.int/childgrowth/standards/chart_catalogue/en/index.html).
TABLE 1.
Definitions and clinical features of malnutritiona
| Classification | Description | Criterion and/or grading |
|---|---|---|
| PEM | General term describing acute malnutrition resulting from inadequate dietary intake of protein and energy (calories); it probably has a spectrum of clinical manifestations but is typically classified as marasmus or kwashiorkor (severe acute malnutrition [see below]) | Not well defined except for clinical marasmus and kwashiorkor (see below) |
| Acute malnutrition | Malnutrition resulting from inadequate food intake leading to acute loss of body mass with respect to length/ht for age; it can be classified as MAM or SAM; it is reversible with adequate nutritional rehabilitation | WHO (WFH z scores below median); mild, z score between −1 and −2; moderate, z score between −2 and −3 or MUAC between 125 mm and 115 mm; severe, z score of <−3 or MUAC of <115 mm |
| SAM (kwashiorkor) | Severe from of malnutrition resulting from poor-quality diet and probably other environmental factors; children with kwashiorkor have pitting edema in both feet and lower extremities and in severe cases may have total body edema (anasarca); liver steatosis is common; sores develop on the skin and at the corner of the mouth; skin is pale and peels (“flaky-paint” dermatosis); these children are apathetic and have little appetite | Diagnosis of kwashiorkor does not rely upon anthropometric measures but only on the presence of bilateral pitting edema |
| SAM (wasting [marasmus]) | Acute malnutrition leading to overt loss of subcutaneous adipose tissue and muscle mass; the wasted child is thin for his/her ht but not necessarily short; children with marasmus have a thin face with wrinkled skin, sunken cheeks, and large eyes; the loss of normal subcutaneous adipose tissue gives the face an old appearance; the abdomen may be swollen; they have sagging skin on legs and buttocks; they are irritable and have increased appetite | WHO (WFH z scores below median); severe, z score of <−3 or MUAC of <115 mm |
| Chronic malnutrition (stunting) | Malnutrition resulting from chronic or recurrent inadequate food intake and, possibly, chronic systemic inflammation; it leads to chronic growth faltering, typically evident by short stature for age, neurocognitive impairment, and metabolic changes associated with chronic adult diseases like diabetes mellitus or hypertension; the effects of chronic malnutrition are largely irreversible after 24 mo of age | WHO (HFA z scores below median); mild, z score between −1 and −2; moderate, z score between −2 and −3; severe, z score of <−3 |
| Underweight | Faltering of linear growth (low ht for age), wt gain (low wt for age), or a combination of both (acute on chronic malnutrition) | Median WFAb; mild (grade 1), 75%–90% WFA; moderate (grade 2), 60%–74% WFA; severe (grade 3), <60% WFA |
| Micronutrient deficiency | Deficit of essential vitamins and minerals required for normal physiological function, growth, and development; micronutrient deficiencies may have no overt clinical signs or symptoms unless they are chronic or severec | Based on biochemical measurements with comparison to reference values derived from normal populations |
Severe acute malnutrition (SAM) is commonly categorized into two major syndromes, marasmus and kwashiorkor. Marasmus is defined by a weight-for-height (WFH) value more than 3 standard deviations (SDs) below the mean for age and sex (or a weight-for-height z score [WHZ] of less than −3), whereas kwashiorkor is characterized by the presence of bilateral pitting pedal edema, independent of anthropometric values (3). Patients may also present with marasmic kwashiorkor, with edema superimposed on severe wasting. Similarly, severe stunting is defined as a height for age more than 3 SDs below the expected value for age or a height-for-age z score [HAZ] of <−3. Moderate malnutrition is defined by anthropometric values between −3 and −2 SDs from expected values. Mild or “at-risk” malnutrition is considered if any of the above-described indexes fall below 1 standard deviation below the median value for the reference population (z value <−1 SD). The mid-upper-arm circumference (MUAC) is a measure of lean body mass, strongly correlates with WHZ, is a strong predictor of mortality (4), and can be assessed quickly, even by staff with very little training. Thus, MUAC is now widely used for nutritional assessment for children between 6 and 59 months of age: a MUAC of <115 mm defines SAM, and a MUAC of ≥115 but less than 125 mm defines moderate acute malnutrition (MAM). Few studies so far have looked at the accuracy of MUAC in the diagnosis of stunting, but the available data suggest a significant correlation between MUAC and HAZ (4). Specific nutrient assessment is rarely performed in the classification of childhood malnutrition, but children with anthropometric evidence of malnutrition almost certainly have, or are at risk for, multiple nutrient deficiencies. Better characterization of the comorbidity of multiple nutrient deficiencies is needed.
GLOBAL BURDEN AND IMPACT OF CHILDHOOD MALNUTRITION
Malnutrition is a serious public health problem affecting millions of people worldwide. It is observed most frequently in developing countries among children less than 5 years of age. It was estimated in 2010 that more than 925 million people in the world were undernourished and that more than one-third of the global disease burden would be eliminated by adequate nutrition (5). Stunting affected 159 million and wasting affected at least 50 million children younger than 5 years of age in 2014 (6). While many parts of the world have made progress in reducing the prevalence of stunting, the high burdens in south Asia and sub-Saharan Africa remain, where, in 2014, 25.1% and 32.0% of children under 5 years of age were stunted, and there were an estimated 34.3 million and 13.9 million children affected by wasting, respectively (6). Undernutrition has been estimated to contribute to more than 45% of all deaths among children younger than 5 years of age (7). The highest mortality rate is found among children with SAM, who have 12 times the risk of dying compared with same-age, well-nourished children (4). However, children with less severe forms of malnutrition still have substantially increased mortality. Most of the deaths occurring among malnourished children are attributable to infections.
MALNUTRITION AND HOST DEFENSE
The increased predisposition of the nutrient-deficient host to infection is presumed to be largely due to impaired immune function. Most of what is reported relating to the impact of malnutrition on host defense involves children or animal models that are broadly described as suffering from protein-energy malnutrition, but this is often poorly defined. Studies of children are limited mostly to the descriptive quantitation of specific cells or factors, often without an assessment of function or consequence. Little is known about the impact of malnutrition on mucosal and skin defense, leukocyte trafficking, leukocyte effector function, and inflammatory mediator activity in an in vivo context. Animal studies have shed some mechanistic light on the effect of malnutrition on host defense, but these models are not always representative of human conditions and have frequently utilized adult animals rather than animals of ages representative of young children with a developing immune system. Furthermore, the multifactorial nature of childhood malnutrition is difficult to represent in an animal model. Despite these caveats, a large body of information is available regarding the effects of malnutrition on multiple components of the host defense.
Malnutrition and Mucosal and Skin Barrier Function
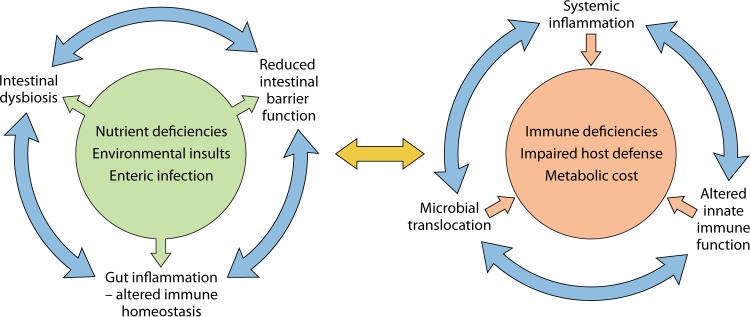
The integrity of the gastrointestinal mucosa is commonly impaired in malnutrition and, together with reduced gastric acid secretion, leads to an increased susceptibility to some pathogens (8, 9). The high rates of cell proliferation and DNA replication in the intestinal epithelium make this tissue particularly vulnerable to the effects of a diet deficient in protein, zinc, vitamin A, or folate. Moreover, many children living in areas with poor sanitation are affected by so-called environmental enteric dysfunction (EED) or environmental enteropathy (EE), a small intestinal disease characterized by villous atrophy, moderate to severe crypt hyperplasia, chronic inflammatory cell infiltration, and increased permeability (10). The mechanisms that drive EED are unclear, but exposure to high loads of intestinal pathogens and disruption of the normal gut microbiota (dysbiosis) have important roles. Central to these is a common factor of poor sanitation (11, 12). Dietary deficiencies in zinc, vitamin A, vitamin D, and protein may also play a role by altering intestinal epithelial barrier function and inflammation (13, 14). Several studies have found a strong association between markers of EED and childhood malnutrition (15–17). A pig model of severe stunting (pigs fed solely maize flour) showed that malnutrition led to atrophy of the small intestinal mucosa (18). Rats subjected to a low-protein diet suffered from impaired gastric epithelial cell proliferation (19). Disruption of the intestinal epithelial barrier is associated with a loss of lymphoid tissue and altered intestinal microbiota (see below), both of which influence the risk of enteric infection. Disruption of the epithelial gut barrier with increased levels of markers of intestinal inflammation (e.g., fecal calprotectin, neopterin, and myeloperoxidase) and microbial translocation (serum soluble CD14 and antiendotoxin antibody) is associated with EED (16, 20–22). Similarly, chronic malnutrition (stunting) is at least partially mediated by the chronic translocation of bacteria or bacterial products, which leads to chronic inflammation and the suppression of the growth hormone–insulin-like growth factor 1 (IGF-1) axis (20, 23, 24). Presumably, there is also a metabolic cost of the chronic inflammation associated with bacterial translocation, but this has not been investigated. Chronic inflammation in malnourished hosts may also contribute to the high frequency of anemia, not all of which is explained by iron deficiency. Recently, intestinal and systemic inflammation was associated with mortality in children with complicated severe acute malnutrition (25). In a model of recently weaned mice, undernutrition (low levels of dietary protein and fat) coupled with repeated exposure to specific enteric bacteria (a cocktail of several commensal Bacteroidales species and Escherichia coli) resulted in bacterial overgrowth, inflammation, villous blunting, and increased permeability in the small intestine, all of which are characteristic of EED (26). These mice also showed an increased susceptibility to an enteric pathogen. A proposed mechanistic understanding of the interplay of malnutrition with EED is shown in a schematic in Fig. 1.
FIG 1.
Interplay of malnutrition with environmental enteric dysfunction and systemic inflammation. Exposure to intestinal pathogens and intestinal dysbiosis, as a consequence of poor sanitation and possibly specific nutrient deficiencies (e.g., zinc, vitamin A, and protein), lead to intestinal inflammation and disruption of intestinal barrier function. Impaired barrier function allows the translocation of bacteria and bacterial products from the intestine, which activate innate immune cells in the mesenteric lymph nodes, liver, and systemic circulation to generate proinflammatory cytokines. The increased systemic inflammation carries a metabolic cost and leads to impaired host defense. Collectively, these vicious cycles lead to growth faltering and increased mortality.
Nutrient deficiencies lead to diverse dermatological manifestations (reviewed in reference 27). Surprisingly, there are no studies that have evaluated the risk of cutaneous infection in malnourished children. One can presume, however, that malnutrition-related skin changes, most notably the edema, desquamation, and severe “flaky-paint” dermatosis of kwashiorkor (3), would predispose one to pathogen entry and infection. Experimental animal studies identified the effect of malnutrition on the physical barrier of the skin. Thinning of the dermis and reduced collagen levels were evident in rats fed inadequate or poor-quality protein (28). Mice fed insufficient food (marasmus model) had a thinner epidermis with decreased stratum corneum hydration and reduced epidermal cell proliferation (29). Malnutrition also has a deleterious influence on wound healing (30). Rats receiving dietary protein restriction showed delayed wound healing that included impaired wound contraction, increased numbers of inflammatory cells, poor collagen deposition, an edematous extracellular matrix, and altered neovascularization (31).
Malnutrition and Hematopoietic and Lymphoid Organs
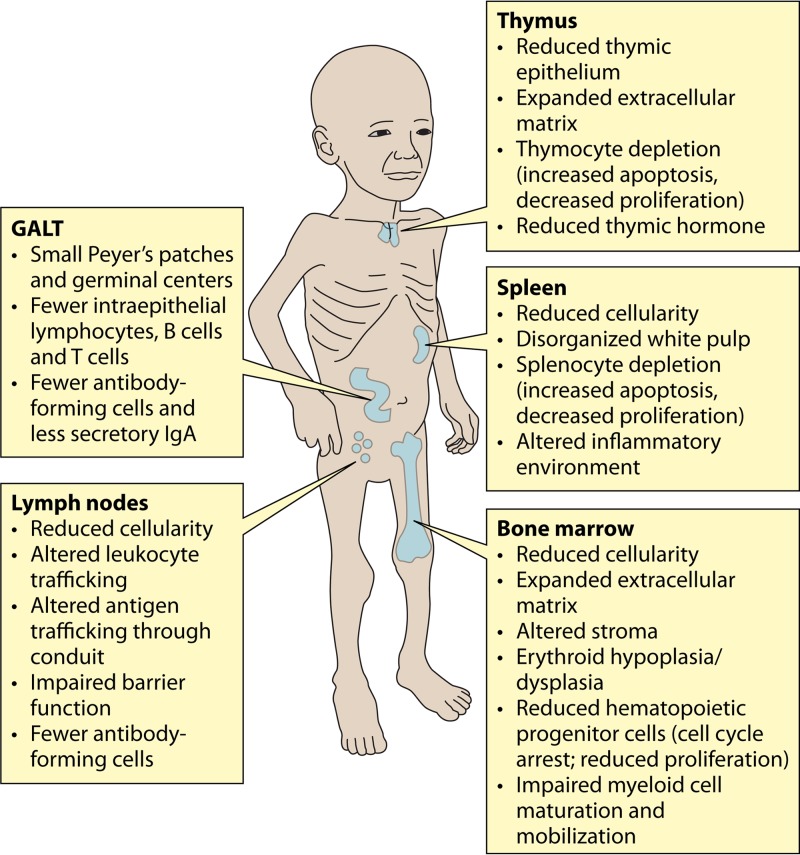
Malnutrition has multiple effects on the hematopoietic and lymphoid organs. These are summarized in Fig. 2.
FIG 2.
Effects of acute malnutrition on lymphoid and hematopoietic organs. The effects of acute malnutrition on the thymus, lymph nodes, spleen, and bone marrow are shown. Note that observations for the spleen and lymph node are based largely on data from animal studies. The effect of malnutrition on the immune and hematopoietic functions of the liver has not been investigated.
Thymus.
The thymus is the primary lymphoid organ where bone marrow-derived lymphocytes undergo differentiation prior to migration to peripheral lymphoid tissues. Autopsy studies of malnourished children describe profound thymic atrophy, thymocyte depletion, and an alteration of the extracellular matrix (32). However, many of these children died from severe infection, itself a cause of acute thymic atrophy (33). Malnutrition- and infection-related thymocyte depletion is caused by the increased apoptosis of CD4- and CD8-double-positive (immature), -double-negative, and -single-positive thymocyte populations (34). Reduced thymocyte proliferation also contributes to thymic hypocellularity (35). Deficiencies in both dietary protein and zinc lead to thymocyte apoptosis (36, 37). Thymocyte apoptosis during malnutrition is driven by elevated levels of circulating glucocorticoids (38) and reduced leptin levels (37). Treatment of protein-deprived rats with leptin abrogated malnutrition-related thymocyte apoptosis (39). In a model of mild maternal protein deprivation during lactation, thymocytes in the offspring were protected from apoptosis by enhanced leptin activity (37). Alteration of the thymic microenvironment, including a reduced volume of the thymic epithelium, expansion of the extracellular matrix, and reduced thymic hormone production, is associated with thymocyte depletion (reviewed in reference 40).
Bone marrow.
The high rates of cell proliferation and self-renewal make bone marrow particularly vulnerable to the effects of nutrient deficiencies, especially protein-energy malnutrition and iron deficiency. Megaloblastic and dysplastic changes with erythroid-series hypoplasia were found in the bone marrow of children (n = 34) with marasmus (28.5%), kwashiorkor (50%), and marasmic kwashiorkor (30%) (41). In mice fed a protein-deficient diet, bone marrow atrophy with gelatinous degeneration, expansion of the extracellular matrix, and a loss of markers of cell proliferation was observed (42). Protein malnutrition suppressed the cell cycle progression of hematopoietic progenitor cells, with arrest in the G0/G1 phase (43, 44). This was associated with reduced levels of cell cycle-inducing proteins and increased levels of inhibitory proteins (44). The arrest of progenitor cells led to a reduction in myeloid and erythroid lineages (42). Altered erythropoiesis in protein-deficient mice occurred independently of iron or erythropoietin deficiency (45). Bone marrow granulocytic cells showed losses at all developmental stages, blunted maturation, an impaired blastic response to granulocyte colony-stimulating factor (G-CSF) (46), and reduced mobilization in response to lipopolysaccharide (LPS) (47). Lymphoid populations, which are relatively rare in bone marrow, were also reduced in malnourished mice (48).
Nonhematopoietic stromal cells play a role in the growth and maintenance of hematopoietic progenitor cells. The stroma of malnourished mice did not sustain CD34+ hematopoietic stem cell growth (42). Bone marrow mesenchymal stem cells in protein-deficient mice were found to differentiate into adipose cells, leading to an altered cytokine microenvironment and compromised hematopoiesis (49).
Blood.
Malnourished children with bacterial infection showed no difference in total blood leukocyte counts or numbers of lymphocytes, granulocytes, or monocytes compared to well-nourished children with bacterial infection (50). Children with severe acute malnutrition had normal numbers of total mononuclear cells but reduced numbers of dendritic cells (DCs) in peripheral blood (51). Protein-malnourished mice were anemic and leukopenic, with reduced numbers of neutrophils, lymphocytes, and monocytes (49, 52).
Spleen and lymph nodes.
The effect of malnutrition on secondary lymphoid tissues (spleen and lymph nodes) in children is unknown, but animal models suggest significant pathological changes. Mice fed a protein-deficient diet had a small, hypocellular spleen with a thickened capsule. There were reduced numbers of total splenocytes and splenic mononuclear cells (52, 53). Spleen cells showed reduced proliferation and were increasingly observed in the G0/G1 cell cycle phase (52). Similarly, malnutrition in weanling rats led to reduced proportions of cells in the S and G2/M phases, with abnormal lengths of both the G1 and S phases (54). Lactating malnourished mice showed increased splenocyte apoptosis (34). The splenic inflammatory milieu was altered in protein-malnourished mice. The production of interferon gamma (IFN-γ) and interleukin (IL-5) was unchanged, but IL-2 production was reduced and IL-10 production was increased in activated splenocytes from protein-malnourished mice (52). Activated STAT3 expression (involved in IL-10 production) was increased, but STAT1 expression (involved in IFN-γ responses) was reduced (52). There was a disorganization of the splenic white pulp in protein-malnourished mice, which was accentuated when malnourished mice were chronically infected with Leishmania infantum (55).
Lymph node cellularity is similarly affected by malnutrition. In a mouse model of moderate multinutrient deficiency (reduced zinc, iron, protein, and energy levels), the lymph node had fewer DCs, fibroblastic reticular cells, and macrophages. The reduction in myeloid cell populations (macrophages, DCs, and neutrophils) was amplified following challenge with the protozoan parasite Leishmania donovani (3 days postinfection), and the lymph node had an impaired capacity to act as a barrier to pathogen dissemination (56, 57). Trafficking of soluble antigens through the lymph node conduit system was also altered in this model (57).
Gut-associated lymphoid tissue.
Children with malnutrition had reduced numbers of cells positive for IgA in the jejunal mucosa, but other immunoglobulin subtypes were not affected (58). Reduced levels of secretory IgA were also found in the intestinal fluid (59). Extrapolating from the malnutrition-related hypocellularity of other lymphoid organs, one would expect the sizes of Peyer's patches to be reduced, but this and other analyses of gut-associated lymphoid tissue (GALT) in malnourished children have not been reported. Malnutrition-related low secretory IgA levels in protein-deprived mice were restored following supplementation with dietary protein (60). In the above-mentioned mouse model of chronic malnutrition (26), the typical histological and functional features of environmental enteropathy were reproduced by serial exposure to a diet poor in proteins and fat and a bacterial gavage of Bacteroidales species and E. coli. The deprived diet alone did not induce structural changes in the small intestinal mucosa but was associated with an increased number of intraepithelial lymphocytes, predominantly γδ CD8+ T cells, compared to those in mice fed a normal diet. Sequential exposure to the bacterial cocktail induced the flattening of mucosal villi and an influx of natural killer (NK) cells. Intraepithelial lymphocytes obtained from the duodenum of these mice secreted significantly higher levels of tumor necrosis factor alpha (TNF-α) and IFN-γ (26). Increased numbers of langerin-positive DCs were found in the gut lamina propria and mesenteric lymph nodes of vitamin A-deficient mice (61). Malnutrition of rat neonates during suckling reduced the numbers and delayed the maturation of B cells and T cells (including recent thymic emigrants) in Peyer's patches (62, 63). Following mucosal immunization with cholera toxin, specific IgG, IgA, and IgM antibody-forming cells were diminished in Peyer's patches and mesenteric lymph nodes of malnourished rats (63).
Alterations of the gastrointestinal mucosal barrier and GALT function suggest that the efficacy of oral vaccines would be reduced in malnourished children. Indeed, childhood malnutrition and environmental enteropathy are considered to be contributors to the so-called “tropical barrier,” referring to the phenomenon of a reduced efficacy of live oral vaccines in developing countries (10, 64, 65). The oral poliovirus, rotavirus, and cholera vaccines have shown reduced immunogenicity and efficacy in children in a number of developing countries (65–67). However, recent studies in children indicated that there was no effect of mild underweight (weight for age, ≤10th percentile) on vaccine responses (68). Thus, failures of oral vaccine-induced immunity may be limited to children with more severe malnutrition and are likely to have multiple contributing factors. In mice, PEM impaired the mucosal IgA response to rotavirus vaccine but not protective efficacy (69).
Malnutrition and Innate Immune Function
Studies of the association of polymorphisms in Toll-like receptors (TLRs) with disease susceptibility suggest that even subtle changes in innate immune signaling can profoundly influence susceptibility to infectious diseases (70, 71). A number of human and experimental animal studies have identified malnutrition-related deficits in innate immune function. However, few studies have connected specific functional nutritional deficits to susceptibility to infection. The impact of PEM on the function of complement and innate immune cells, including monocytes/macrophages, neutrophils, NK cells, and DCs, is discussed below.
Blood inflammatory mediators, complement, and acute-phase proteins.
The acute-phase response is a systemic response to infection or other causes of inflammation. It leads to appetite suppression and a negative energy balance. Energy expenditure is increased by 7 to 11% for each unit (degrees Celsius) increase in fever (72, 73). The acute-phase response is accompanied by proinflammatory cytokine production, which drives the catabolism of muscle protein and increased hepatic protein synthesis. Insulin resistance and hepatic glycogenolysis and gluconeogenesis contribute to increased plasma glucose levels during the acute-phase response. Owing at least in part to insulin resistance, there is also increased peripheral lipolysis and hepatic triglyceride and very-low-density lipoprotein (VLDL) synthesis but decreased cholesterol synthesis. All of these metabolic changes amplify growth faltering in children with insufficient nutrient intake. Children with severe malnutrition often have a blunted febrile response to infection. Consistent with this clinical observation, some studies have reported the reduced production of acute-phase proteins and proinflammatory cytokines (IL-1, IL-6, and TNF) in children with kwashiorkor and marasmus (74–77). Furthermore, the acute-phase proteins C-reactive protein (CRP) and procalcitonin were not reliable predictors of invasive bacterial infection in severely malnourished children (78). However, other studies demonstrated high levels of circulating TNF and increased cellular responsiveness to bacterial lipopolysaccharide in uninfected malnourished children (79, 80). This discordance may be due to differences in intestinal barrier function, bacterial translocation, and endotoxemia, which are common in severely malnourished children (17, 51, 81). Endotoxin tolerance may have a role in blunting the acute-phase response and the production of inflammatory mediators in severely malnourished children. Children with protein and energy deficits showed reduced levels and impaired activities of components of the complement system (82, 83).
There is a long-recognized need for noninvasive biomarkers to identify children at risk for growth faltering. Most studies have focused on markers of systemic inflammation, such as the cytokines and acute-phase proteins described above. More recently, markers of intestinal barrier disruption, bacterial translocation, and intestinal inflammation have been evaluated (84). Recent work from the MAL-ED Network demonstrated the interaction of inflammation, linear growth, and the growth hormone axis, suggesting that serum growth hormone, IGF-1, and IGF binding protein 3 (IGFBP-3) could be useful biomarkers of growth faltering (85). Fecal markers of inflammation have also been evaluated (21, 86, 87). To our knowledge, there has been no study of biomarkers that might identify impaired host defense and an increased risk of infection in malnourished children.
Monocytes/macrophages.
A number of clinical and experimental animal studies demonstrated reduced numbers of monocytes and macrophages in malnourished hosts. Infants with PEM had elevated expression levels of the apoptotic marker CD95 (Fas) in peripheral blood neutrophils, lymphocytes, and monocytes, which were decreased after nutritional rehabilitation (88). This suggests that the life span of monocytes is reduced in malnourished children. Protein-deficient mice had reduced numbers of circulating blood monocytes (49, 52). Acutely starved mice had decreased numbers of peritoneal macrophages, which were restored by refeeding (89). Polynutrient (protein, energy, zinc, and iron)-deficient mice had reduced numbers of resident (subcortical) and subcapsular sinus macrophages in their lymph nodes compared to those in nourished controls (57). Rats exposed to dietary protein restriction during lactation had fewer alveolar macrophages (90).
Macrophage effector function is also decreased in the malnourished host. Peritoneal macrophages from mice suffering from PEM showed impaired phagocytosis (91, 92) and diminished production of reactive oxygen and nitrogen intermediates (93). Peritoneal macrophages from protein-deficient mice exhibited dysregulated NF-κB activation, decreased TRAF-6 expression, dysregulated proinflammatory cytokine expression with low-level TNF-α production, and lower expression levels of the CD14 and TLR4/MD-2 receptors upon exposure to lipopolysaccharide (94–96). TNF-α-stimulated macrophages from protein-deficient mice showed lower expression levels of TNF-RI and reduced NF-κB phosphorylation together with the reduced production of IL-1β and IL-12 (97). NF-κB dysregulation was also found in a model of moderate polynutrient (protein, iron, and zinc) deficiency (93).
Neutrophils.
Surprisingly little is known about neutrophil function in childhood malnutrition. Neutrophil chemotaxis and microbicidal activity were impaired in children with PEM (98–100). Impaired synthesis of lysosomal enzymes and reduced glycolytic activity in neutrophils from malnourished children were reported (98, 99). Retinoic acid plays a critical role in neutrophil maturation. Neutrophils from vitamin A-deficient rats displayed impaired chemotaxis, phagocytosis, and generation of reactive oxygen species (101). A single dose of vitamin A supplementation enhanced the phagocytic capacity of neutrophils in 68 preschool children evaluated at a Venezuelan nutrition clinic (25% were vitamin A deficient) (102). The numbers of neutrophils in the skin-draining lymph nodes of mice deficient in protein, energy, iron, and zinc were reduced (57). Folate-deficient rats also had lower numbers of neutrophils and eosinophils (103). Conversely, zinc-deficient rats were shown to have increased circulating neutrophil counts, which were probably the result of increased corticosterone levels and enhanced release from the bone marrow (104, 105). Circulating granulocyte counts (and elevated corticosterone levels) returned to normal after 2 weeks of feeding a zinc-sufficient diet (105). Neutrophils from vitamin C (ascorbate)-deficient animals failed to undergo spontaneous apoptosis, resulting in reduced clearance (106).
Natural killer cells.
Children 8 to 36 months of age with moderate or severe malnutrition showed no decrease in the number of circulating natural killer (NK) cells (107), but NK cell activity was depressed (108) and recovered with therapeutic nutritional intervention (109). NK cell numbers and cytotoxic activity were reduced in the lungs and spleen of energy-restricted mice in response to influenza virus infection (110). The number and activity of splenic NK cells were also reduced in vitamin A-deficient rats and returned to normal after vitamin A repletion (111). Total numbers of NK cells (103) and their cytotoxicity (112) were reduced in rats fed a folate-deficient diet.
Dendritic cells.
Dendritic cells (DCs) bridge innate and adaptive immunity through the production of cytokines and the initiation of antigen presentation. Severely malnourished children from Zambia had reduced numbers of DCs that recovered after standard nutritional treatment (51). In a subpopulation of these children who had evidence of endotoxemia, DCs showed impaired maturation (failure to upregulate HLA-DR) and a reduced capacity to stimulate T cell proliferation (51). In a murine model of multinutrient deficiencies (protein, zinc, and iron deficiencies), a reduced number of lymph node-resident DCs was associated with the dysregulation of DC chemoattractants under inflammatory conditions (56, 57). The adoptive transfer of immortalized syngeneic DCs (but not CD3+ T cells) to protein-energy-deficient mice partially restored the impaired delayed-type hypersensitivity response (113). The effect of PEM on the DC antigen-presenting capacity and the induction of T cell activation was found to be nil (114) or impaired (115, 116). Differences in model systems, including the age of the mice and purity of DCs, probably account for these discrepancies. Studies of highly purified, defined DC subsets under inflammatory and noninflammatory conditions are needed to resolve this important issue. The critical role of vitamin A in DC differentiation (117) is discussed in the section on vitamin A, below.
Malnutrition and Adaptive Immune Function
The impact of malnutrition on adaptive immunity has significant implications for both the control of a pathogen and the response to vaccination. Several descriptive studies identify defects in adaptive immune function in malnourished children, but a mechanistic understanding of these deficits is incomplete. A number of studies examined the impact of malnutrition on the response to childhood vaccines, and readers are referred to several reviews on the topic (65, 118, 119).
T cells.
Malnourished children hospitalized with bacterial infection showed no difference in numbers of peripheral CD8+ and CD4+ T cells (50) but had reduced numbers of CD4+ CD45RO+ memory T cells (120) and reduced numbers of effector T cell (CD4+ CD62L− and CD8+ CD28−) subsets (121). Anemic children with vitamin A deficiency showed remarkable increases in the total numbers of CD4+ and CD8+ T cells after vitamin A supplementation (122). As noted above, malnutrition may impair antigen-presenting cell function, so altered adaptive T cell responses may not be due to an intrinsic change in T cell function. Peripheral blood mononuclear cells from malnourished children with bacterial infection had reduced levels of key cytokines required for both Th1 differentiation (IL-7, IL-12, IL-18, and IL-21) and function (IFN-γ and IL-2) (123, 124) and overexpression of the Th2 cytokines IL-4 and IL-10 (125). Increased apoptosis of CD3+ T cells, which was associated with decreased IL-7/IL-7 receptor alpha (IL-7Rα) and increased Fas (CD95) and PD-1 expression levels, was reported for children with severe acute malnutrition and respiratory and/or gastrointestinal infection (123). In mice, dietary protein restriction led to splenic atrophy but variable T cell numbers in the spleen (55, 126). Fasting for as few as 2 days decreased the numbers of T cells in the spleen (127, 128). PEM and zinc deficiency in rats caused a decreased level of production of immature CD4+ CD8+ cells due to enhanced thymocyte apoptosis and reduced lymphocyte proliferation (34).
The ability of T cells to respond to inflammatory stimuli is also negatively affected by malnutrition. In response to DNA vaccination (ovalbumin expression plasmid), protein-deficient mice exhibited an impaired antigen-specific T cell response (decreased numbers of ova-specific CD8+ T cells and lower-level IL-2 production by CD4+ T cells) but an unaltered antigen-specific antibody response (129). Similar to what was described for malnourished children, malnourished mice showed enhanced Th2 cytokine polarization and skewing of the Th1-Th2 balance (130). Mice fed a very-low-protein diet and infected with lymphocytic choriomeningitis virus (LCMV) showed fewer activated (CD44hi) virus-specific CD8+ T cells in the spleen and reduced virus clearance (131). Virus-specific CD8+ T cells from protein-deficient mice showed effective T cell activation when transferred into normally nourished LCMV-infected mice. This suggests that protein deficiency does not lead to intrinsic defects in T cells, but rather, the malnourished environment does not effectively support T cell activation (131). Acute malnutrition inhibits glucose metabolism-dependent T cell activation (proliferation and cytokine production) (128, 132, 133). The in vitro activation of T cells from mice fasted for 48 h showed an impaired production of the Th1 cytokines IL-2 and IFN-γ that was rescued by exogenous leptin (128).
B cells and antibody responses.
Malnourished children with respiratory or gastrointestinal bacterial infection had reduced numbers of B cells compared to those of infected well-nourished controls (50). B lymphocyte function generally appears to be maintained in PEM, although specific antibody-mediated immune responses may be affected. Levels of Th2-type immunoglobulins (IgG1 and IgE) are increased, whereas levels of Th1-type immunoglobulins (IgG2a and IgG3) are unaltered (134). Numbers of IgA-secreting cells and secretory IgA concentrations are reduced (58, 59). However, oral administration of the probiotic Lactobacillus pentosus to protein-deficient mice restored the levels of intestinal IgA and numbers of splenic B and Th2 cells to the levels of normal controls (135). This suggests that malnutrition mediates its effect on mucosal immunity by affecting the intestinal microbiota (see below). Folate-deficient rats had lower numbers of B and T cells than those in the controls (103). Vitamin A-deficient mice produced a poor IgG response that was restored with vitamin A repletion (136). Vitamin A-deficient rats had reduced numbers of IgA+ plasma cells and CD4+ cells and increased numbers of CD8+ cells in their Peyer's patches (137). Zinc deficiency depleted immature and mature cells of the B cell lineage in bone marrow (138).
Dietary Lipids in Immune Function and Host Defense
Dietary lipids have immunomodulatory properties, but their role in host defense in malnourished children has received little attention. Dietary lipids are important components of therapeutic interventions for malnourished children because of their high energy density and importance in brain development (139). n-6 (omega-6) and n-3 (omega-3) polyunsaturated fatty acids (PUFAs) are of particular interest because metabolites of n-6 PUFAs are mostly proinflammatory, whereas n-3 PUFAs are largely anti-inflammatory (140). The long-chain n-6 PUFA arachidonic acid (AA) is the source of prostaglandin E2 (PGE2) synthesis, but the long-chain n-3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) inhibit the synthesis of cyclooxygenase 2 (COX2) and PGE2 (140). The incorporation of n-3 fatty acids into T cell membranes affects T cell receptor signaling via changes in membrane fluidity and lipid raft formation (141, 142). Infants who received a dietary supplement of n-3 fatty acids showed an increased production of IFN by LPS-stimulated whole-blood leukocytes (143). Breastfeeding infants showed altered cytokine production when their mothers received a fish oil supplement (144). Currently used formulations for nutritional interventions for malnourished children are being questioned because the high ratio of n-6 to n-3 fatty acids (145) may be suboptimal for neural growth and development (146). The high ratio of n-6 to n-3 fatty acids may also favor heightened inflammatory responses, which could be particularly detrimental to intestinal barrier function, which is commonly impaired in malnourished children (17, 81). Recent clinical trials indicate that plasma long-chain n-3 PUFA levels decline in children with SAM during rehabilitation with standard ready-to-use therapeutic food (RUTF) formulations (147, 148). Treatment with RUTF modified by increasing the amount of preformed n-3 PUFA (fish oil) or decreasing the amount n-6 PUFA (by replacement of the n-6 PUFA source with high-oleic-acid peanuts) resulted in increased plasma n-3/n-6 ratios (147, 148). The n-3 PUFA α-linolenic acid (ALA), found in plant oils, can act as a precursor for the synthesis of longer-chain n-3 PUFAs (EPA and DHA), but this is a very inefficient process. Therefore, alteration of the n-3/n-6 PUFA ratio will be best achieved by the dietary intake of marine sources of preformed long-chain n-3 PUFAs. However, a cautionary note should be considered: the anti-inflammatory effects of n-3 PUFAs could also impair protective cellular immune responses against intracellular pathogens. Macrophages infected in vitro with Mycobacterium tuberculosis and exposed to high levels of n-3 PUFAs had diminished IFN-γ-induced signaling and bactericidal activity (149). Further research to define the optimal sources, types, and amounts of dietary lipids for the prevention and therapy of malnutrition is needed.
Micronutrients in Immune Function and Host Defense
Micronutrient deficiencies are commonly unnoticed but can cause a number of clinical manifestations when the deficiency is chronic and/or severe (Table 2). Deficiencies of micronutrients can also have profound effects on immune function and host defense (reviewed in reference 150). Their common association with PEM is likely to lead to an additive or synergistic impairment of host defenses, but this has not been thoroughly studied. The roles of iron, zinc, selenium, vitamin A, vitamin C, and vitamin D in immune function are discussed below. The immunological effects of deficiencies in other micronutrients have not been investigated in children, but studies in other subjects and experimental models suggest a possible influence on immune function. These are summarized in Table 2.
TABLE 2.
Clinical manifestations and potential immunological effects of micronutrient deficienciesa
| Micronutrient | Deficiency symptom(s) | Potential effect(s) on immune function | Reference(s) |
|---|---|---|---|
| Iron | Hypochromic anemia, cognitive deficits, behavioral abnormalities | See the text | |
| Zinc | Anorexia, reduced growth, skin lesions, impaired wound healing, frequent infections | See the text | |
| Iodine | Goiter, impaired cognitive development | Granulocyte function, myeloperoxidase activity | 539 |
| Copper | Sideroblastic anemia, reduced growth, osteoporosis | No. of phagocytes, phagocyte activation, T cell activation | 540 |
| Selenium | Cardiomyopathy | See the text | |
| Vitamin A | Xerophthalmia (night blindness, xerosis), keratomalacia (blindness); increased susceptibility to and severity of infections | See the text | |
| Vitamin C | Scurvy (diarrhea, gingivitis, arthropathy), skin changes (petechiae, perifollicular hemorrhage, and bruising) | See the text | |
| Vitamin D | Rickets, osteomalacia | See the text | |
| Vitamin E | Neuropathy, ataxia, retinal degeneration, hemolytic anemia (almost never observed from simple dietary deficiency) | Epithelial barrier, T cell activation, NK cell activity | 541, 542 |
| Vitamin K | Bleeding diathesis (almost never observed from simple dietary deficiency) | T cell proliferation, regulation of inflammation (NF-κB) | 543 |
| Thiamine (vitamin B1) | Beriberi (peripheral neuropathy, cardiomyopathy, seizures; in infants, laryngeal paralysis with aphonic cry), Wernicke-Korsakoff syndrome | Unknown | |
| Riboflavin (vitamin B2) | Angular stomatitis, glossitis, cheilitis, seborrheic dermatitis | Phagocyte activation | 544 |
| Niacin (vitamin B3) | Pellagra (diarrhea, photosensitive dermatitis, dementia) | Unknown | |
| Pantothenic acid (vitamin B5) | Paresthesias and dysesthesias (“burning-feet syndrome”) | Unknown | |
| Pyridoxine (vitamin B6) | Dermatitis, angular stomatitis, glossitis, neuropathy | Antibody production, T cell activity and phenotype, DTH response, NK cell activity | 541 |
| Biotin (vitamin B7) | Hypotonia, exfoliative dermatitis | Regulation of inflammation, DC function, and NK cell and CTL activity | 545, 546 |
| Folate (vitamin B9) | Megaloblastic anemia, neural tube defects, cleft lip | No. of lymphoid and myeloid cells, T cell activation, NK cell activity | 103, 112 |
| Cobalamin (vitamin B12) | Megaloblastic anemia, ataxia, muscle weakness, spasticity, incontinence, dementia | Antibody production, no. and activity of T cells, NK cell activity | 541, 547, 548 |
DTH, delayed-type hypersensitivity; CTL, cytotoxic T lymphocyte.
Iron.
Dietary iron exists as heme iron and nonheme iron, with the former being found exclusively in animal foods and the latter being found in both animal and plant foods. The efficiency of intestinal heme iron absorption is much higher than the efficiency of absorption of nonheme iron. Iron deficiency is the world's most widespread micronutrient disorder. Anemia affects over 1.6 billion people worldwide, one-quarter of the world's population (151), and half of these anemia cases are associated with iron deficiency (152). Worldwide, nearly 47% of preschool children, 42% of pregnant women, 30% of nonpregnant women, and 12.7% of men are anemic (151). The prevalence of iron deficiency in the poorest populations is attributed to cereal-based diets that lack heme iron and contain low levels of nonheme iron and high levels of inhibitors of iron absorption (153). Severe anemia in children is associated with fatigue and may result in developmental delays and behavioral problems. Iron is critically important for both innate and adaptive immunity (154, 155). Intracellular iron has been shown to activate NF-κB via promoting the release of reactive oxygen species (156, 157). Hypoxia-inducible factor-1 alpha (HIF-1α), an iron-dependent transcription factor, promotes the production of antimicrobial peptides by macrophages (158). Peripheral blood mononuclear cells from iron-deficient patients showed increased TNF-α, IL-6, and IL-10 mRNA expression levels after the administration of iron (159). Mitogen-activated spleen cells from iron-deficient mice showed reduced IFN-γ production (160). Transferrin receptor 1 (TfR1)-deficient mice, which have reduced cellular iron uptake, exhibited impaired T cell development and fewer mature B cells than wild-type mice (161). The proliferation of human B and T lymphocytes was also reduced by TfR1-blocking antibodies (155). Mice with a conditional deletion of ferritin H in their bone marrow had fewer mature B and T cell populations in lymphoid tissues (162). On the other hand, too much iron is detrimental to host defense. Macrophages from Hfe−/− mice, which have enhanced iron absorption that leads to iron overload, produced low levels of inflammatory cytokines (IL-6 and TNF-α) in response to Salmonella infection (163). Similarly, children with low levels of the cellular iron transporter ferroportin, which leads to reduced iron efflux and increased accumulation of intracellular iron, had low levels of circulating TNF-α (164). Collectively, these findings indicate that an alteration of iron homeostasis, whether resulting from too much or too little iron uptake, impairs innate and adaptive responses.
The impact of iron deficiency on susceptibility to infection is difficult to dissect because free iron is essential for the growth of many pathogens (reviewed in reference 165). Some human and animal studies demonstrated that iron deficiency increased the risk of infection (155), but other studies observed that iron supplementation increased susceptibility to malaria and tuberculosis (TB) (166, 167). Host cells may harness pathways involved in iron homeostasis as an antimicrobial defense system. Upon infection, reticuloendothelial cells sequester iron from the blood and phagocytes by the release of lactoferrin. Lactoferrin binds iron more avidly (specifically at low pH) (168) than do bacterial siderophores, with a consequent deprivation of iron required for the replication of the pathogen (165). Therefore, iron deficiency results in the impaired killing of bacteria by phagocytes but may also lead to impaired pathogen replication. Clearly, iron deficiency leading to anemia is a major public health problem, but further research is needed to determine optimal iron levels and the impacts of iron repletion on maximizing host defense and minimizing pathogen replication and virulence.
Zinc.
Zinc deficiency affects one-fifth of the world's population and is responsible for the deaths of nearly 450,000 children under the age of 5 years annually (5, 169). Zinc deficiency often accompanies childhood PEM (170–172), and a protein-deficient diet led to zinc deficiency in experimental animals (173). Foods of animal origin (e.g., meat, shellfish, and organs such as liver) are the richest sources of zinc, and the bioavailability of this mineral from animal sources is higher than that of zinc found in plant sources. Animal-derived foods rich in both protein and zinc are severely limited in the diets of children whose families have inadequate resources. Zinc is a cofactor for more than 200 enzymatic reactions and thus has profound effects on cellular function and is critical to proper childhood growth and sexual maturation. It plays critical roles in the structure and functioning of biomembranes and in stabilizing DNA, RNA, and ribosomal structures (174). Zinc also regulates a wide range of immune functions (reviewed in references 153 and 175). It is important for the activity of thymic hormone (176–178), which regulates T cell maturation. Zinc promotes Th1 cell differentiation and Th1 cell responses by increasing IL-2, IFN-γ, and IL-12Rbβ2 expression levels (179, 180). Additionally, zinc regulates the release of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α by innate immune cells (181–183). It regulates neutrophil function by modulating the oxidative burst (184, 185). As a result, zinc deficiency leads to thymic atrophy, lymphopenia, a reduced CD4/CD8 ratio, and a reduced synthesis of Th1 cytokines. It is also associated with impaired NK cell function and impaired phagocytosis by macrophages (174, 175, 186, 187). Zinc deficiency may impair mucosal immune function through altered epithelial homeostasis.
Dietary zinc supplementation has been widely studied for its effects on childhood growth and mortality (reviewed in reference 188). Its effects on immune function and the risk of infection are somewhat controversial, but the general consensus is that zinc supplementation reduces the risk of diarrheal disease and pneumonia. Gender-related differences in response to zinc supplementation may contribute to some of the conflicting results of clinical trials (189). A double-blind, randomized, placebo-controlled study of daily zinc supplementation in a cohort of children aged 6 to 30 months in a New Delhi, India, slum demonstrated reduced frequency, duration, and severity of diarrheal disease in the zinc-supplemented group (190). In the same cohort, zinc supplementation had no effect on the rate of acute lower respiratory tract infection (LRTI) but was associated with a significant decline in the incidence of pneumonia (190). This large-cohort trial confirmed the results of previous smaller studies that demonstrated a benefit of dietary zinc for diarrheal disease and respiratory infections (191–193). A trial of zinc supplementation showed no effect on the incidence and morbidity of malaria but showed a reduction in the prevalence of diarrhea (194). Zinc reduced biofilm formation, adherence to epithelial cells, and virulence factor expression of enteroaggregative Escherichia coli (EAEC) (195). Zinc supplementation in deficient mice reduced EAEC stool shedding and abrogated infection-related growth stunting (195).
Selenium.
Selenium deficiency usually accompanies PEM in geographic regions with soil deficient in selenium (174). It is more pronounced in kwashiorkor than marasmus (196). Selenium plays a pivotal role in major metabolic pathways (197, 198) and contributes to antioxidant activity via selenoproteins (199). It has major anti-inflammatory effects through the mitogen-activated protein kinase (MAPK)-, NF-κB-, and peroxisome proliferator-activated receptor γ (PPARγ)-dependent regulation of proinflammatory mediators (200, 201). The genetic deletion of the whole family of selenoproteins by a knockout of the selenocysteine tRNA gene in mice resulted in fewer functional T cells, impaired T cell-dependent antibody responses, and an impaired migration of macrophages (202, 203). The genetic deletion of selenoprotein K did not alter the numbers of immune cells but resulted in impaired T cell responses, neutrophil migration, and phagocyte oxidative burst through the alteration of cellular calcium flux (204). Dietary selenium deficiency leads to several immune deficits (205), including reduced CD4+ T cell proliferation and function (reduced NFAT [nuclear factor of activated T cells] activation, IL-2 production, and IL-2 receptor expression and impaired calcium mobilization) (202, 205, 206). Supplementation with selenium along with vitamin A, the vitamin B complex, vitamin C, and vitamin E increased CD3+ and CD4+ T cell counts but did not augment the antituberculous T cell response in patients with active tuberculosis (207, 208).
Vitamin A.
Vitamin A, or retinol, is acquired exclusively through the diet, absorbed by enterocytes, and stored in the liver. Vitamin A deficiency is a global health problem that affects 100 million to 140 million children, with 4.4 million having xerophthalmia (209). Indeed, vitamin A deficiency is the leading cause of childhood blindness worldwide. PEM compounds vitamin A deficiency due to inadequate amino acid availability in the liver, which is required for the synthesis of vitamin A transport proteins such as retinol binding protein. Vitamin A, through its primary active metabolite retinoic acid, plays key roles in the proper differentiation of epithelial cells in skin; the cornea of the eye; and mucosal surfaces of the gastrointestinal, respiratory, and urogenital tracts. The lack of adequate epithelial barrier function makes pathogenic bacterial and viral invasion more easily accomplished. Retinoic acid is also involved in the regulation of a number of innate and adaptive immune functions (reviewed in references 210 and 211) (Fig. 3). Retinoic acid production is highly enriched in the intestinal tract, where it modulates intestinal immune homeostasis and defense. Its effects are highly cell specific and influenced by whether the tissue microenvironment is homeostatic or inflammatory. Maternal vitamin A intake plays a critical role in secondary lymphoid development in utero through the regulation of prenatal innate lymphoid cells, which determine the size of lymphoid organs in adult life (212). Retinoic acid has an essential role in mucosal immunity (213) through the regulation of mucin gene expression (214), the production of IgA (215, 216), the regulation of innate lymphoid cell development (217), and the regulation of DC and T cell differentiation in the lamina propria and gut-associated lymphoid tissue (117, 218). Retinoic acid acts on, and is secreted by, mucosal DCs and macrophages. It regulates specific DC subpopulations, most notably CD11b+ CD103+ DCs in the intestine (219). It enhances DC migration to draining lymph nodes (220) and, by doing so, regulates T cell differentiation and activation. It also promotes the activation of IFN-γ signaling through STAT1 and interferon regulatory factor 1 (IRF1) activation in lung epithelial cells (221). The effects of retinoic acid on T cell differentiation and function appear to be context dependent. Under homeostatic conditions, retinoic acid promotes (with the help of transforming growth factor β [TGF-β]) the conversion of naive CD4+ T cells into regulatory T cells and inhibits the development of Th17 cells. Both processes promote immune tolerance against commensal bacteria (222, 223). Retinoic acid regulates small intestine inflammation via the generation of regulatory and gut-homing IL-10-producing T cells (218, 224, 225). DC-induced T cell recruitment is mediated by the retinoic acid-induced expression of the gut-homing molecules α4β7 and CCR9 on CD4+ T cells (226, 227). Under inflammatory conditions, retinoic acid promotes CD4+ and CD8+ effector T cell responses (228–231) and in particular favors the development of Th2 over Th1 responses (231–233). Retinoic acid treatment of M. tuberculosis-infected rats led to reduced bacterial burdens in the lung and spleen, which were associated with the increased accumulation of CD4+ and CD8+ T cells, NK cells, and CD163+ macrophages at the site of lung infection (234). It also enhanced the proinflammatory response to and killing of tubercle bacilli by alveolar macrophages. Retinoic acid secreted by DCs and alveolar macrophages enhances the differentiation of T cells to regulatory T cells (222). Previous studies demonstrated that retinoic acid enhances the ability of regulatory T cells and gut-homing T cells to suppress acute small intestinal inflammation after adoptive transfer in mice (218, 225).
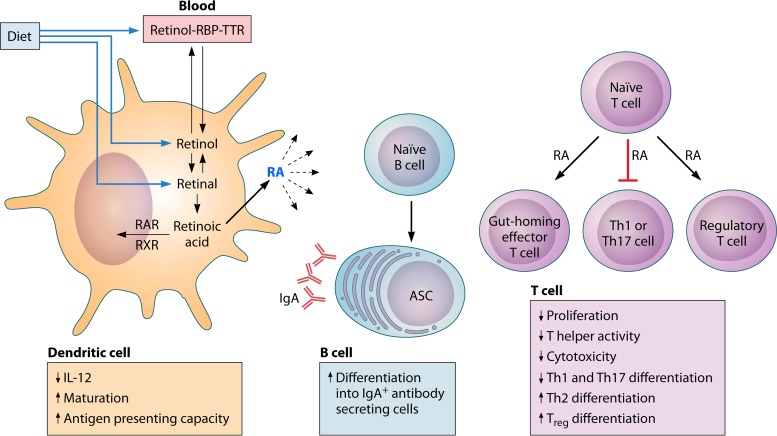
FIG 3.
Vitamin A metabolism and effect on immune cells in mucosa- and gut-associated lymphoid tissues. The fat-soluble vitamin A is acquired in the diet in the form of all-trans-retinol, retinyl esters, or β-carotene. These forms are solubilized in products of fat digestion and absorbed in micelles through the enterocyte membrane. Retinol circulates in the blood, complexed with retinol binding protein (RBP) and transthyretin (TTR). Retinol is oxidized to all-trans-retinal, which is then oxidized to all-trans-retinoic acid (RA) by retinal dehydrogenases, which are found in intestinal epithelial cells and gut-associated dendritic cells. Retinoic acid is exported from the cell and exerts autocrine and paracrine effects on immune cells by binding to nuclear receptors of the retinoic acid receptor (RAR) family, which heterodimerize with receptors of the retinoic X receptor (RXR) family. Together, these forms bind to retinoic acid response elements within promoters of retinoic acid response genes. In the presence of inflammatory stimuli, RA enhances dendritic cell maturation and antigen-presenting capacity. Dendritic cells also store and release RA to act on other immune cells. RA acts on naive T cells to upregulate the expression of gut-homing receptors. It reduces Th1 differentiation by blocking the expression of IL-12 by dendritic cells and T cell expression of the transcription factor Tbet and Th1 cytokines. It also blocks the induction of the transcription factor retinoic acid receptor-related orphan receptor γt (RORγt) and the differentiation of Th17 cells. In contrast, RA induces GATA3 and IL-4 expression, leading to enhanced Th2 differentiation, and promotes the differentiation of naive T cells to FoxP3+ regulatory T cells in intestinal tissue. B cells in mucosa- and gut-associated lymphoid tissues activated in the presence of RA differentiate into IgA+ antibody-secreting cells (ASC) (211).
In light of the above-mentioned role of retinoic acid, it is not surprising that vitamin A-deficient mice possess altered innate and adaptive immunity. Vitamin A deficiency leads to a marked reduction in the number of type 3 innate lymphoid cells (ILC3s), leading to reduced IL-17 and IL-22 levels and increased susceptibility to acute enteric bacterial infection (217). At the same time, vitamin A-deficient mice exhibited an expansion of the IL-13-producing ILC2 population with consequent increases in the amount of intestinal mucus, goblet cell hyperplasia, and resistance to intestinal helminthes (217). This effect was dependent on signaling through the retinoic acid receptor (RARα). Thus, dietary vitamin A regulates intestinal barrier immunity by regulating the balance between these two subsets of ILCs. This enhances one arm of innate immunity to defend against nutrient-depleting worms at the expense of increased susceptibility to enteric bacterial pathogens. The numbers and functions of natural killer T (NKT) cells and NK cells are also modulated by the availability of retinoic acid (235, 236).
Regarding adaptive immunity, vitamin A deficiency altered homeostatic DC maintenance and differentiation in the gut-associated lymphoid tissue (61, 237). Gestational vitamin A deficiency in rats also decreased the numbers of CD11c+ DCs in Peyer's patches of offspring (238). CD4+ (Th1, Th2, and Th17) and CD8+ T cell numbers in the intestinal lamina propria were also altered (217, 226, 228, 239). Vitamin A deficiency promotes the differentiation of T cells toward Th2 cells and increases the ratio of Th2 to Th1 cytokines by suppressing the Th1 immune response (218, 240). This explains, at least in part, why vitamin A deficiency is associated with reduced effector T cell responses, suboptimal immune responses to some vaccines (241), and an increased risk for certain infections. A large number of clinical trials of vitamin A supplementation have been conducted, collectively involving several hundred thousand participants. Most of these trials have shown a reduction in all-cause mortality (20 to 30%) and reductions in the incidences and severities of diarrheal disease and measles but not lower respiratory tract infections (reviewed in references 242–245).
Vitamin C.
Vitamin C is an essential water-soluble vitamin important for metabolic function and antioxidant activity (246), and it increases the absorption of nonheme iron when coingested in the same meal. Vitamin C deficiency affects approximately 10% of adults in the industrialized world (247, 248). It occurs more frequently in impoverished populations, but there is little information on its prevalence in children in the developing world. Its potential role in leukocyte function is suggested by the ascorbic acid (reduced form of vitamin C) content in leukocytes being severalfold higher than that in plasma (249). Vitamin C blunts the inflammatory cytokine response to LPS in peripheral blood mononuclear cells from adult human subjects (250) but paradoxically enhances inflammatory cytokine responses in neonatal cord blood leukocytes (251). Vitamin C regulates apoptosis in monocytes/macrophages, neutrophils, and B cells (106, 252–254). DCs cultured in the presence of vitamin C showed upregulations of the costimulatory molecules CD80, CD86, and major histocompatibility complex class II (MHC-II) (255) and increased CD8+ T cell expansion when cocultured with T cells (256). In vivo and in vitro experiments demonstrated that vitamin C regulated the isotype switching of mouse B cells (254). Vitamin C deficiency exaggerated inflammation and impaired its resolution in a murine model of sterile inflammation (257). Vitamin C administration attenuated acute lung, kidney, and liver injury in murine models of lethal LPS administration and intra-abdominal sepsis (257–259). The attenuated lung injury was accompanied by a reduced proinflammatory response, enhanced epithelial barrier function, increased alveolar fluid clearance, and reduced coagulopathy (257–259). The underlying mechanisms of this protective effect were attributed to reduced neutrophil NF-κB activation, endoplasmic reticulum stress, the induction of autophagy, and the generation of neutrophil extracellular traps (NETosis) (253). A phase 1 trial of intravenous ascorbic acid in adults with severe sepsis showed no evidence of ascorbic acid-induced toxicity and significantly reduced levels of biomarkers of both inflammation (CRP and procalcitonin) and vascular endothelial injury (thrombomodulin) (260). Subjects who received high-dose ascorbic acid also showed an attenuation of organ failure scores (260). There are no studies of the influence of vitamin C status on resistance or susceptibility to sepsis in malnourished children.
Vitamin D.
The primary role of vitamin D is in calcium homeostasis and bone metabolism, but it also has a number of effects that impact host defense. 25-Hydroxyvitamin D [25(OH)VD3] is the major circulating form and is metabolized by 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) to the primary active form 1,25-dihydroxyvitamin D [1,25(OH)2VD3], which induces signaling when it binds to its cognate nuclear receptor, the vitamin D receptor (VDR). Genetic variation in the VDR may modify the associations of vitamin D with human health and the interpretation of data from clinical studies (261). The optimal level of serum vitamin D has been fiercely debated. Individuals are considered to be vitamin D deficient when the serum 25(OH)VD3 level is <25 nmol/liter and vitamin D insufficient when the serum 25(OH)VD3 level is <50 to 75 nmol/liter (262). Vitamin D deficiency is estimated to affect 1 billion people worldwide. More than 40% of the elderly in the United States and Europe and more than 50% of postmenopausal women suffer from vitamin D deficiency (263). Vitamin D deficiency may also be common in children and young adults (264). There are few foods that are naturally rich in vitamin D, and therefore, its synthesis in the skin via exposure to UV light is of critical importance. A lack of adequate sun exposure is a common cause of vitamin D deficiency. Children with darker skin, which contains more of the pigment melanin, which blocks the effects of UV radiation, are at a greater risk for deficiency.
The effect of vitamin D on immunity and host defense is complex, having roles in both proinflammatory antimicrobial effector function and anti-inflammatory suppressive activity (Fig. 4). The role of vitamin D in innate immunity was recently reviewed (265, 266). A seminal observation by Liu et al. (267) identified Mycobacterium tuberculosis as a trigger for the TLR2-mediated induction of CYP27B1 and VDR in monocytes. Signaling through the TLR4/NF-κB and IFN-γ receptor (IFN-γR)/STAT1 pathways also induced the expression of CYP27B1 and VDR (268–270). The IFN-γ-mediated induction of CYP27B1 in human monocytes and macrophages was dependent on STAT1 and the induction of IL-15 and, in the presence of sufficient vitamin D, led to an antibacterial effect via the induction of autophagy, autophagolysosomal fusion, and the generation of the antimicrobial peptides cathelicidin (LL37) and β-defensin-2 (270). Mycobacterial killing was abrogated in the presence of vitamin D-deficient serum (270). Vitamin D-induced antituberculous autophagy was driven by cathelicidin and dependent on TLR1/2 signaling (271, 272). 1,25-Dihydroxyvitamin D enhanced the M. tuberculosis-induced expression of proinflammatory cytokines and chemokines in a human macrophage cell line via the NLRP3/caspase-1 inflammasome (273). In this in vitro model, augmented IL-1β secretion led to increased antimycobacterial activity in cocultured lung epithelial cells via the production of antimicrobial peptides (273). Other studies also identified a critical role for VDR signaling in the production of the antimicrobial peptides cathelicidin (LL37) and β-defensin-2, which mediate the growth restriction of M. tuberculosis in macrophages (267, 274–277). In addition to the IFN-γ-induced production of cathelicidin, IFN-γ/TNF-independent production via TLR signaling has been proposed (267, 276).
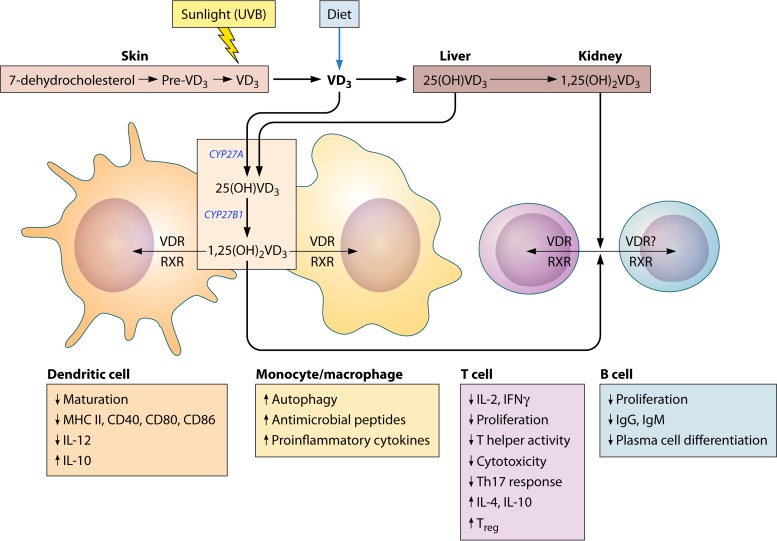
FIG 4.
Vitamin D metabolism and cells of the immune system. Vitamin D3 (VD3) (cholecalciferol) is primarily acquired preformed in the diet or synthesized in the skin through the action of UVB radiation in sunlight from 7-dehydrocholesterol. VD3 is metabolized first in the liver to 25-hydroxyvitamin D3 [25(OH)VD3] and then in the kidney to the most physiologically active metabolite, 1,25-dihydroxyvitamin D3 [1,25(OH)2VD3]. VD3 can also be metabolized by cells of the immune system (e.g., dendritic cells and macrophages) to 25(OH)VD3 and 1,25(OH)2VD3 through the action of the enzymes CYP27A and CYP27B1, respectively. 1,25(OH)2VD3 acts on immune cells in an autocrine or paracrine manner through binding to the nuclear vitamin D receptor (VDR). Upon binding with 1,25(OH)2VD3, VDR heterodimerizes with nuclear receptors of the retinoic X receptor (RXR) family, and the complex binds to VD3 response elements in the promoters of VD3 response genes. CYP27B1 and VDR are upregulated in cells activated through TLR2, TLR4/NF-κB, and IFN-γ/STAT1. VD3 has a largely suppressive effect on the adaptive immune system. Markers of dendritic cell maturation, activation, and antigen presentation are downregulated by exposure to 1,25(OH)2VD3. In particular, IL-12 production is diminished, leading to reduced Th1 differentiation, and suppressive cytokines such as IL-10 are upregulated. T lymphocytes show evidence of reduced proliferation, cytotoxic activity, and effector cytokine expression and increased regulatory function through increased regulatory T cell (Treg) and Th2 differentiation and IL-4 and IL-10 production. It is unclear if B cells express VDR or if their function is modulated indirectly through the reduced activity of antigen-presenting cells or reduced T cell help. B cells show reduced proliferation, differentiation to plasma cells, and immunoglobulin secretion. In contrast, monocytes and macrophages exposed to 1,25(OH)2VD3 have increased proinflammatory properties and produce antimicrobial peptides that are important for the innate immune response (211).
Clinical studies have investigated the role of vitamin D in tuberculosis. Most of these studies included primarily adult subjects. The seasonality of the prevalence of tuberculosis has long been known. Recent studies associated this with seasonal variations in vitamin D levels, presumably related to sun exposure, in individuals in South Africa and Peru (278, 279). Vitamin D deficiency was associated with an increased risk of active tuberculosis in a large number of studies (recently reviewed in references 278 and 280). The risk was influenced by polymorphisms in the VDR and vitamin D binding protein (281, 282). Vitamin D insufficiency was also associated with an increased risk of relapse following antituberculous therapy in both HIV-uninfected and -coinfected patients (283). Vitamin D was used to treat tuberculosis in the preantibiotic era (284), but recent clinical trials of adjunctive vitamin D therapy for active tuberculosis have reported conflicting results in clinical, bacteriological, and/or immunological outcomes (285–288). Vitamin D supplementation accelerated treatment-induced sputum smear conversion (285, 289), the resolution of lymphopenia and monocytosis, and the normalization of increased levels of serum inflammatory cytokines and chemokines (285). Significant clinical benefit may be achieved by the accelerated resolution of inflammation, which is clearly associated with increased tuberculosis mortality (290). In a multicenter, randomized, placebo-controlled trial of adjunctive vitamin D treatment for sputum smear-positive pulmonary tuberculosis patients in London, vitamin D3 (VD3) (cholecalciferol; three doses of 2.5 mg each) significantly improved the time to sputum conversion only in subjects that had the tt genotype of the TaqI vitamin D receptor polymorphism (286). However, a lower dose of oral cholecalciferol (100,000 IU) given 0, 5, and 8 months after the initiation of antituberculous treatment did not lead to improved sputum conversion, clinical outcomes, or 12-month mortality in adults with pulmonary tuberculosis compared to placebo (288). In contrast, two doses of 600,000 IU of intramuscular vitamin D3 accelerated clinical and radiographic improvement 12 weeks after the start of antituberculous therapy compared to placebo (291). In a study of children, most of whom had extrapulmonary tuberculosis, adjunctive vitamin D therapy improved clinical and radiological features (292).
A prospective cohort study showed a significant inverse association between vitamin D levels and the incidence of active tuberculosis disease among contacts of patients with pulmonary tuberculosis (293, 294). Vitamin D supplementation also reduced the incidence of latent tuberculosis infection (identified by tuberculin skin test conversion or a positive interferon gamma release assay) in contacts of patients with pulmonary tuberculosis (295, 296). In a double-blind, randomized, controlled trial with healthy adult tuberculosis contacts (94% of whom were either vitamin D deficient or insufficient), a single oral dose of vitamin D (ergocalciferol; 2.5 mg) enhanced the growth restriction of Mycobacterium bovis BCG in an ex vivo whole-blood assay (287). Collectively, data from these studies indicate that vitamin D modulates immune and inflammatory mechanisms that can enhance the control of infection and tissue damage. However, a beneficial effect has not been consistently demonstrated in clinical trials, possibly because the optimal dose and frequency of vitamin D supplementation remain to be determined. There is a need for further investigation of vitamin D in the management of children with tuberculosis.
The prophylactic or therapeutic effect of vitamin D supplementation on acute respiratory tract infection (ARI) was recently reviewed (297). A number of observational and cross-sectional studies have demonstrated an association of vitamin D deficiency with increased susceptibility to ARI, but randomized, controlled studies have inconsistently shown a benefit of vitamin D supplementation. This lack of consensus may arise from the variability in vitamin D dosing regimens, the variable prevalence of vitamin D deficiency in the study population, the failure to achieve or test for an effect on vitamin D levels, the use of endpoints that involved self-reported symptoms, the inclusion of diverse and unknown etiologies of ARI, and suboptimal power for subset analyses. In a randomized, controlled, double-blind trial of vitamin D-deficient school-age children in Mongolia in the winter, supplementation with vitamin D3-fortified milk (300 IU/day) versus nonfortified milk significantly reduced the frequency of ARI reported by mothers (rate ratio = 0.52) (298). In a randomized, placebo-controlled trial, 100,000 IU (2.5 mg) of vitamin D3 administered every 3 months for 18 months did not reduce the incidence of pneumonia in Afghan infants (299). The intermittent high dose of vitamin D used to achieve supraphysiological peaks followed by deficiency-level troughs may not be optimal (300). Indeed, high concentrations of vitamin D can impair adaptive immunity (301). In a large trial of adults (median age, 63 years) in Norway, vitamin D supplementation did not reduce the risk of influenza-like illness during a 6-month period, but vitamin D levels were not determined before or after the intervention (302). Similarly, in a randomized, controlled trial in New Zealand, vitamin D supplementation did not reduce the frequency or duration of upper respiratory tract symptoms (303).
In addition to the role of vitamin D in the activation of antimicrobial host defense, it has important anti-inflammatory activities. Vitamin D suppresses the proliferation and differentiation of B cells and blocks immunoglobulin secretion (304, 305). Through paracrine action, vitamin D leads to decreased expression levels of MHC class II on DCs, with consequent reductions in DC maturation, antigen presentation (306), and T cell priming (307). This is regulated by the balance of the activating (CYP27B1) and inactivating (CYP24A1) vitamin D hydroxylases and the consequent availability of active 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (308, 309). Vitamin D suppresses chronic T cell activation (reviewed in reference 310) and promotes Th2 and regulatory T cell expansion while blocking Th1 polarization (311–313). It also directly promotes the expression of the key transcription factor FoxP3 in regulatory T cells (314). In monocytes/macrophages, it leads to the decreased production of the proinflammatory mediators IL-1β, IL-6, IL-8, and TNF (315–317) and the increased production of anti-inflammatory mediators such as IL-10 (318). Not surprisingly, vitamin D has a significant effect on modulating the host inflammatory response to pathogens. In human airway epithelial cells, vitamin D restrained respiratory syncytial virus (RSV)-induced NF-κB-dependent inflammatory cytokine and chemokine production (319) and the activation of STAT1 and its downstream targets IRF1 and IRF7 (320), without compromising the antiviral effect. The Fok I polymorphism in the VDR, which predisposes patients to severe RSV bronchiolitis, was found to abrogate vitamin D-induced anti-inflammatory signaling (320). A similar anti-inflammatory effect was noted for influenza virus-infected lung epithelial cells (321). 1,25(OH)2D3 inhibited Th17 cytokine production in patients with severe asthma (322). In a murine model of cerebral malaria, vitamin D administration led to reduced neuropathology and improved survival. This was accompanied by reduced DC activation and pathogenic T cell infiltration but expanded regulatory T cells and IL-10 production (323). The vitamin D-dependent anti-inflammatory activity could also be detrimental for the control of some intracellular pathogens. The ablation of vitamin D signaling through receptor knockout or a block of vitamin D metabolism to the active form by CYP27B1 deletion led to an increased resistance of mice to the intracellular protozoan pathogen Leishmania major (324).
MALNUTRITION AND INFECTION
Malnutrition is a primary contributor to death in 60.7% of children with diarrheal diseases, 52.3% of children with pneumonia, 44.8% of children with measles, and 57.3% of children with malaria (325). The relationship between malnutrition and infection is bidirectional (326). Infection as a contributor to childhood growth faltering is most well documented for diarrhea and lower respiratory tract infection (LRTI), but other infections likely contribute on a more limited scale. Besides the direct organ-specific effect of infection (e.g., intestinal loss of nutrients during diarrhea), there is a metabolic cost to immune activation that contributes to the increased energy deficit in infected children (327). The majority of patient studies, particularly those that are cross-sectional and observational, identify the association between malnutrition and infection but are unable to clearly address risk and causality, especially in the case of chronic infections. Clinical studies that investigate an association between malnutrition and risk of infection must control for the many sociodemographic, environmental, and genetic confounders, such as seasonality, age, gender, household crowding, maternal education, and vaccination status, which influence the risk of infection. The risk of infection by specific pathogens is often undefined because the limited availability of sensitive, field-applicable diagnostic tests precludes the identification of etiologic agents in many resource-limited regions. Pathogens whose risk or severity of infection is associated with protein or protein-energy malnutrition are summarized in Table 3.
TABLE 3.
Malnutrition-associated susceptibility to specific pathogensd
| Pathogena | Modelb and/or study type | Infection outcome(s)c | Immunological outcome(s)c | Reference(s) |
|---|---|---|---|---|
| Viral pathogens | ||||
| Influenza virus | Mouse; 2% protein diet | Increased severity (viral persistence, pulmonary inflammation, mortality) | Decreased virus-specific antibody and CD8+ T cell responses | 126 |
| Influenza virus | Mouse; 40% energy restriction | Increased viral burden and lung pathology, decreased survival | Decreased no. and function of natural killer cells; decreased levels of type I interferons | 110 |
| Respiratory syncytial virus | Longitudinal study of birth cohort of rural Kenyan children | Stunting (HAZ of ≤−2) associated with higher incidence of RSV LRTI | Not evaluated | 339 |
| Respiratory syncytial virus | Longitudinal study of infants in Niger | Increased rate of severe RSV LRTI (hospitalization) in children with WAZ of ≤−2 and lower than median growth between 1st and 3rd vaccination visits | Not evaluated | 338 |
| Lymphocytic choriomeningitis virus | Mouse; isocaloric low-protein (0.6%) diet | Increased viral burden in liver | Decrease in no. of LCMV-specific CD8+ memory T cells; decreased homeostatic T cell proliferation; defects rescued by protein supplementation | 549 |
| Lymphocytic choriomeningitis virus | Mouse; isocaloric low-protein (0.6%) diet; adoptive transfer of virus-specific CD8+ T cells from normal to malnourished mice | Reduced viral clearance from serum | Reduced no. of total and LCMV-activated splenic CD8+ T cells; reduced frequency of virus-specific IFN-γ-producing T cells; adoptive transfer of virus-specific T cells showed that the malnourished microenvironment was a major determinant of impaired T cell activation | 131 |
| Norovirus | Mouse; 2% protein diet | Increased severity | Reduced antiviral antibody responses, loss of protective immunity | 349 |
| Rotavirus | Weanling mouse, dams of 3-day-old litters fed multideficient regional basic diet (5% fat, 7% protein, 88% carbohydrate) | Earlier peak intensity of infection and increased early viral shedding | No differences in rotavirus-specific serum IgG and stool IgA levels; higher rotavirus-specific serum IgA levels; rotavirus vaccine equally protective in the malnourished group | 69 |
| Bacterial pathogens | ||||
| Streptococcus pneumoniae | Mouse; protein-free diet for 21 days | Increased bacterial burden in lung and blood | Impaired macrophage and neutrophil responses, proinflammatory cytokines, granulopoiesis | 337 |
| Nontyphoidal Salmonella | Prospective hospital-based study of children (median age, 15 mo) | Increased severity of illness and increased mortality in children with severe acute malnutrition (WAZ of <−3) | Not evaluated | 550 |
| Shigella spp. | Prospective 1-yr study of children <15 yr of age in Dhaka, Bangladesh | Reduced wt for age associated with increased mortality | Not evaluated | 551 |
| Enteroaggregative E. coli | Mouse; 2% protein diet | Increased severity of disease (reduced wt gain); increased EAEC fecal shedding | Not evaluated | 345 |
| Enteroaggregative E. coli | Mouse; 7 % protein and reduced fat and micronutrients | Increased EAEC fecal shedding; increased intestinal tissue burden | Increased expression levels of IL-4, IL-12, IL-17, and TNF-α mRNAs in the ileum | 346 |
| Mycobacterium tuberculosis | Mouse; leptin-deficient mice | Higher bacterial loads in lungs | Reduced no. of well-shaped granulomas, no. of lung lymphocytes, and IFN-γ levels at the site of infection and delayed-type hypersensitivity | 552 |
| Mycobacterium tuberculosis | Population-based, retrospective cohort study of adult and child contacts of active TB cases | Increased incidence of active disease (hazard ratio, 37.5) in malnourished subjects (malnutrition not defined) | Not evaluated | 553 |
| Mycobacterium tuberculosis | Prospective study of children <5 yr of age who were household contacts of active adult TB cases | Increased incidence (odds ratio, 3.97) of active disease in children with severe malnutrition (wt <60% of that expected) | Not evaluated | 401 |
| BCG | Mouse; dietary restriction to 70% of controls | Increased bacterial dissemination | Dietary restriction blunted spleen cell production of IFN-γ, TNF-α, and IL-10 in an antigen-induced recall assay | 406 |
| Protozoal pathogens | ||||
| Leishmania donovani | Mouse; 3% protein, low Fe and Zn | Increased dissemination following cutaneous inoculation | Impaired LN barrier function; reduced no. of LN myeloid cells; increased LN conduit transit | 56, 57 |
| Leishmania chagasi | Mouse; 3% protein, low Fe and Zn | Increased parasite burden in liver and spleen during chronic infection | Reduced spleen IFN-γ levels | 554 |
| Leishmania chagasi | Mouse; 4% protein | Modest increase in parasite load in spleen but not liver | Decreased thymic and splenic cellularity; reduced no. of lymphoid follicles in spleen; increased no. of thymic CD4+ T cells and decreased no. of thymic CD4+ CD8+ T cells; decreased IL-12 production by thymus and spleen cells | 55 |
| Plasmodium spp. | Prospective longitudinal cohort study of young children in Uganda | Stunting (HAZ of ≤−1) associated with increased incidence of clinical malaria | Not evaluated | 415 |
| Plasmodium berghei | Pregnant mice; low protein | Early mortality; increased fetal loss | Lower plasma nitric oxide levels | 555 |
| Trypanosoma cruzi | Rats; 6% protein diet | Increased inflammatory process of Chagas disease; increased parasitemia | Reduced levels of cardiac CX3CL1, endothelin-1, and CD68 and CD163 macrophages | 556 |
| Cryptosporidium parvum | Mouse; 2% protein diet | Higher-level fecal C. parvum shedding; increased intestinal pathology (reduction in the villous ht/crypt depth ratio in the ileum) | Depressed TLR2 and -4 signaling and Th1 cytokine response | 361 |
| Cryptosporidium parvum | Mouse; 2% protein diet | Higher intensity of C. parvum infection (fecal oocyst counts) | Not evaluated | 360 |
| Cryptosporidium parvum | Mouse; undernutrition induced by daily separation of pups from lactating dams | Increased fecal oocyst shedding; increased ileal and colonic tissue infection; hyperplastic crypts and increased inflammation in the ileum | Increased TNF-α and IFN-γ levels in infected ileal tissues | 351 |
| Cryptosporidium parvum | Mouse; undernutrition induced by daily separation of pups from lactating dams | Increased fecal oocyst shedding; increased ileal and colonic tissue infection; hyperplastic crypts and increased inflammation in the ileum (all of which were improved by administration of l-arginine) | l-Arginine supplementation enhanced ileal nitric oxide production and decreased arginase 1 expression | 362 |
| Giardia lamblia | Mouse; 3 wk old; 2% protein diet | No difference in intensities of infection but enhanced infection-induced growth impairment; absence of infection-induced crypt hyperplasia; blunted villus architecture | Blunted increase in no. of B220+ cells in lamina propria; blunted mucosal eosinophil infiltration and expression of IL-4 and IL-5 mRNAs | 358 |
| Giardia lamblia | Gerbil; 4–6 wk old; 5% protein | No difference in intensities of infection; reduced villus ht | Not evaluated | 359 |
| Entamoeba histolytica | Prospective longitudinal study of children 2–5 yr of age | Not evaluated | Lower-level IFN-γ and higher-level IL-5 production by PBMCs in response to soluble amebic extract | 355 |
| Entamoeba histolytica | Prospective longitudinal study of children 2–5 yr of age | Increased incidence of amebiasis in malnourished children (WAZ of <2) | Not evaluated | 344 |
| Helminth pathogens | ||||
| Schistosoma japonicum | Cross-sectional study of people aged 7–30 yr | Intensity of infection inversely correlated with HAZ in children <12 yr of age | Not evaluated | 557 |
| Schistosoma mansoni | Mouse; neonatal malnutrition induced in pups by low-protein (8%) or calorie-restricted diet of lactating dams; mice infected as adults | Increased intensity of intestinal infection and increased liver pathology in low-protein group; smaller granulomas and increased liver regeneration in calorie-restricted group | Not evaluated | 373 |
| Heligmosomoides polygyrus | Mouse; low selenium and/or vitamin E | Delayed adult worm expulsion and increased fecundity during secondary infection | Blunted IL-4 response in selenium/vitamin E-deficient mice | 558 |
| Heligmosomoides bakeri | Mouse; 6% protein diet; refeeding with a protein-sufficient diet | Increased fecal egg output and worm burdens in protein-deficient animals; restored parasite clearance in protein-refed animals | Reduced IL-4 and IL-13 levels | 559, 560 |
| Heligmosomoides polygyrus | Mouse; 3% protein diet | Higher intestinal worm burdens | Decreased gut-associated IL-4 and increased IFN-γ levels; decreased serum IgE response; reduced intestinal eosinophilia and mucosal mast cell proliferation and activation | 372 |
| Heligmosomoides polygyrus | Mouse; low protein (3% or 7%) or low zinc | Higher intestinal worm burdens | Decreased eosinophilia in protein- and zinc-deficient mice | 561 |
Only studies that identified a specific pathogen were included.
Human studies included prospective longitudinal studies unless noted otherwise.
Immunological outcomes related to malnutrition.
LN, lymph node; PBMCs, peripheral blood mononuclear cells.
Respiratory Infections
The increased susceptibility of the malnourished host to viral, bacterial, and mycobacterial respiratory infections is supported by data from both clinical and experimental animal studies (reviewed in references 328–330). Most studies have focused on syndromic case definitions without the identification of a microbial etiology. In a 1-year prospective study of children 5 to 12 years of age from low- and middle-income families in Bogota, Colombia, stunting was associated with an increased frequency of cough with fever (331). A prospective case-control study in India revealed that acute respiratory infection in children >1 month and <5 years of age was associated with an inadequate duration of breastfeeding (weaning at <4 months of age) (odds ration [OR], 3.01; 95% confidence interval [CI], 1.12 to 8.07) and a weight-for-age z score (WAZ) of <−2 (moderately to severely underweight) (OR, 1.75; 95% CI, 1.84 to 3.67) (332). An adequate duration of breastfeeding (for at least the first 6 months of life) was also associated with a reduced risk of LRTI in the United States (333). A low serum folate level, possibly as a consequence of inadequate breastfeeding, was identified as an independent risk factor for an increased risk of LRTI in young Indian children (334).
Streptococcus pneumoniae.
In a case-control study of children aged 0 to 10 years from indigenous people in Venezuela, malnutrition (WHZ or HAZ of <−2 for children aged <5 years and a body mass index [BMI]-for-age z score or a HAZ of <−2 for children aged 5 to 10 years) was significantly associated with increased nasopharyngeal or oropharyngeal colonization by Streptococcus pneumoniae (335). Alteration of upper respiratory mucosal immune function and/or the mucosal microbiota may facilitate this increased pathogen carriage, but this has not been investigated. No clinical studies have directly identified malnutrition as a risk factor for acute LRTI due to S. pneumoniae, but bacterial colonization is associated with a risk of acute LRTI. In mice, dietary protein deprivation was associated with more severe S. pneumoniae infection and impaired innate immune responses, including reduced leukocyte infiltration in the lung, impaired bactericidal activity of phagocytes, and diminished antipneumococcal mucosal IgA levels (336). The recovery of innate immune function and resistance to pneumococcal infection were accelerated in malnourished mice given a protein-replete diet supplemented with a Lactobacillus probiotic (336, 337).
Viral lower respiratory tract infections.
In a longitudinal cohort study, children in the Philippines (median age, 1.8 months) who were moderately underweight (WAZ of <−2) at the time of their first immunization, or who had reduced weight gain between their first and third immunizations, were at a higher risk of severe RSV infection (338). In a longitudinal study of a birth cohort conducted in rural Kenya across three successive RSV epidemics, stunting (HAZ of <−2) was a risk factor for all-cause LRTI and LRTI due to RSV, as were household crowding and the number of siblings (339). In a prospective study of a birth cohort in the Netherlands, neonates who had a cord blood 25-hydroxyvitamin D level of <50 nmol/liter had a 6-fold increased risk of RSV lower respiratory tract infection in their first year of life compared to neonates who had a vitamin D level of >75 nmol/liter (340). Surprisingly, there are no reported studies of the risk of influenza virus infection in malnourished children, but data from experimental animal studies suggest that protein and energy deficits increase the risk of influenza virus infection. Mice fed a low-protein diet (2%) and infected with influenza virus showed increased viral burdens, lung disease, and mortality. This was associated with impaired virus-specific antibody and CD8+ T cell responses (126). A 40% energy (calorie) deficit in mice resulted in an increased risk of severe influenza that was associated with impaired virus-specific type I interferon and NK cell responses (110).
Gastrointestinal Infections
Pooled data from multiple studies across several countries identified the relationship of early childhood diarrheal disease with subsequent stunting (341). Specifically, prolonged or persistent diarrheal illness carries a greater risk of subsequent growth faltering (342). Preexisting malnutrition also increases the risk and severity of gastrointestinal infection caused by some pathogens.
Bacterial gastroenteritis.
Malnutrition increases the risk of diarrheal diseases caused by some, but not all, enteropathogens. Impaired immune defenses, compromised gut integrity (81), and an altered intestinal microbiota (see below) are likely to influence defense against intestinal pathogens in the malnourished host. The Global Enteric Multicenter Study (GEMS), a large, 3-year, case-control study of moderate to severe diarrhea identified rotavirus, Cryptosporidium, enterotoxigenic Escherichia coli (ETEC), and Shigella as the most common pathogens across seven sites in sub-Saharan Africa and south Asia (343). Infection with each of these pathogens was associated with childhood malnutrition. In a prospective study of urban Bangladeshi children aged 2 to 5 years, a population that inherently has a high rate of exposure to enteric pathogens, only ETEC, Cryptosporidium sp., and Entamoeba histolytica were significantly more prevalent in malnourished (WHZ of <−2) children (344). Bacterial enteropathogens have also been studied in experimental models of malnutrition. Mice infected with enteroaggregative Escherichia coli (EAEC) demonstrated impaired growth that was proportional to the intensity of infection, and conversely, protein-malnourished mice showed an enhanced susceptibility to infection that further impaired their growth velocity (345). Similar results were found for mice fed a “regional basic diet” low in protein, fat, and micronutrients (346). Oral zinc supplementation in zinc-deficient mice resulted in improved weight gain and reduced bacterial shedding following challenge with EAEC (195). Interestingly, low zinc levels appeared to enhance EAEC virulence properties as well as alter the host inflammatory response. Vitamin A-deficient rats showed increased intestinal pathology following infection with Salmonella enterica serovar Typhimurium that was accompanied by increased numbers of mucosal DCs and the dysregulation of IL-12 and IFN-γ production (347).
Viral gastroenteritis.
It has long been held that malnutrition is a major contributor to the high mortality rates from viral gastroenteritis in low-income countries (343, 348). Surprisingly, there are few clinical studies that directly support this (344), and experimental animal studies have reported conflicting results. In the GEMS study noted above, diarrhea caused by rotavirus was associated with malnutrition (343). Protein-malnourished mice had increased weight loss, reduced antiviral mucosal IgA levels, high viral loads, and a delayed clearance of norovirus compared to normal controls (349). In contrast, malnourished mice infected with rotavirus or immunized with a rotavirus vaccine showed no deficit in virus-specific mucosal IgA, and disease severity or vaccine efficacy was not altered (69). Vitamin A supplementation reduced the prevalence of norovirus-associated diarrhea but increased the duration of viral shedding (350).
Infection with intestinal protozoa.
Intestinal infection with Cryptosporidium, Giardia, and Entamoeba histolytica is associated with growth faltering in children (344, 351–353). Limited human data also suggest that malnutrition has a role in increasing the risk or severity of infection by intestinal protozoa (344, 354). The GEMS study determined that moderate to severe diarrhea caused by Cryptosporidium was associated with childhood malnutrition (343). A community-based, prospective cohort study of infants from birth to 18 months of age determined that weight-for-age z scores at 6 months of age were inversely related to the risk of symptomatic giardiasis (354). Childhood malnutrition was also found to be strongly associated with intestinal amebiasis (344). Diarrheal illness due to Entamoeba histolytica within the preceding 3 years was associated with stunting in Bangladeshi children (353). In this cohort, antigen-induced IFN-γ production was linked to nutritional status and was associated with a reduced risk of subsequent infection by E. histolytica (355). Leptin signaling, which has a profound influence on innate and adaptive immunity (356), is involved in the mucosal defense against E. histolytica. A specific allelic amino acid substitution in the cytokine receptor homology domain of the leptin receptor was associated with increased risks of intestinal amebiasis in children and amebic liver abscess in adults in Bangladesh (357). Mice carrying a copy of this allele were also more susceptible to intestinal amebiasis (357).
Malnutrition as a risk factor for, and a consequence of, intestinal protozoal infection is well established through experimental animal studies. Infection of mice with Giardia led to impaired growth (358). Protein malnutrition in mice led to an increased severity of Giardia infection with evidence of increased mucous production, shortened intestinal villi, and a blunted host immune response (absence of crypt hyperplasia, decreased mucosal IL-4 and IL-5 levels, and reduced numbers of B cells in the lamina propria) (358, 359). Infection of protein-malnourished weanling mice with Cryptosporidium parvum oocysts led to increased weight loss and fecal shedding; increased attachment of the parasite to the ileum, cecum, and colon; a reduced ratio of villous height to crypt depth, and reduced proinflammatory signaling and levels of Th1 cytokines (351, 360–362). Supplementation with l-arginine, from which the antimicrobial molecule nitric oxide is generated by the action of inducible nitric oxide synthase (NOS2), in Cryptosporidium-infected protein-malnourished mice led to improved mucosal histology, weight gain, and decreased parasite burdens. The effect of l-arginine in this model was reversed by the inhibition of NOS2 (362).
Intestinal helminth infection.
Clinical studies have repeatedly demonstrated that chronic infection with helminths such as Schistosoma and soil-transmitted intestinal helminths contributes to underweight or stunting (363–368). Therefore, human studies to investigate nutritional deficiency as a cause of an increased risk or severity of gastrointestinal helminth infection are challenging. Plasma zinc levels were negatively correlated with the intensity of Trichuris trichiura infection in Jamaican children (369), but in Guatemalan children, the reinfection rate following antihelminthic treatment of zinc-deficient children was not reduced by zinc supplementation (10 mg Zn formulated as an amino acid chelate) (370). Experimental animal studies also demonstrated that zinc and protein deficiencies impair the host response and lead to more severe intestinal helminth infection (reviewed in reference 371). Protein-malnourished mice infected with Heligmosomoides polygyrus had an increased worm burden, which was associated with lower serum IgE levels, reduced numbers of intestinal eosinophils and activated mast cells, and reduced gut Th2 effector responses (372). Neonatal malnutrition, a consequence of a low level of protein in the diet of dams during lactation, led to increased egg output and liver damage in mice infected with Schistosoma mansoni (373).
Systemic Infections
Systemic bacterial infections.
Bacterial bloodstream infections are among the most dangerous complications of SAM. WHO guidelines for the management of malnourished children include evaluation for possible sepsis as part of the initial assessment. However, clinical assessment is poorly predictive of serious infections in these patients (374–376), and microbiology services are generally not available in low-resource health care facilities (377–380). Thus, routine observational data underestimate the true prevalence of bacteremia among malnourished patients. Epidemiological data regarding the prevalence of systemic bacterial infections in children with types of malnutrition other than complicated SAM are rare.
Reliable epidemiological data come from a few ad hoc research surveys in sub-Saharan Africa. In a study of 1,000 children treated as outpatients in Niger, 4% were bacteremic due to unspecified pathogens (381). However, most data come from hospitalized children with SAM. Studies conducted in Kenya, Ghana, and Mozambique found that hospitalized children with SAM had 2 to 3 times the risk of being bacteremic at admission compared with well-nourished children (380, 382–384). The prevalence of bacteremia in children admitted with complicated SAM ranged from 8% to 17%. Gram-negative enteric bacteria, particularly nontyphoidal Salmonella (NTS), E. coli, and Klebsiella spp., are common invasive pathogens in children with SAM, independent of HIV prevalence or the predominant type of malnutrition (marasmus versus kwashiorkor). A study of hospitalized children from Ghana found that underweight (low weight for age) but not stunting (low length/height for age) was associated with an increased risk of bacteremia (385). However, in a recent analysis of pooled data from 10 countries in Asia, Africa, and South America, severe stunting was associated with a 3-fold increased risk of death from sepsis, unspecified acute febrile illness, tuberculosis, or meningitis (386). Mixed infections are not rare, and the case-fatality rate ranges from 10% to 28% (374–376, 382).
A large study conducted in Uganda between 2003 and 2004 found that 17% of the 445 children admitted with SAM had bacteremia: of these children, 58% had at least one Gram-negative bacterium detected, with Salmonella Typhimurium and Salmonella enterica serovar Enteritidis being the most common species, followed by E. coli and Haemophilus influenzae. Among Gram-positive bacteria, Staphylococcus aureus was the most common pathogen, followed by Streptococcus pneumoniae. The odds ratio of being bacteremic was increased for children with oral thrush or hypoalbuminemia but not for children with HIV infection (diagnosed in 36% of the tested patients), focal infection, malaria, or severe anemia. The case-fatality rates were 28.9% overall and 43.5% in children with HIV (387).
Another study conducted in Niger between 2007 and 2008 found similar results: 17% of 311 children admitted with complicated SAM had a bloodstream infection. Sixty-eight percent of the bacteremic children had at least one Gram-negative bacterium detected in the blood, with NTS, Salmonella enterica serovar Typhi, E. coli, and Klebsiella pneumoniae being the most common. S. aureus and S. pneumoniae were the most frequently isolated Gram-positive microorganisms. Eight percent of the children had mixed infections. Another 7% of the admitted children were found to have blood cultures positive for opportunistic bacteria, such as Leuconostoc, Streptococcus equinus, Streptococcus infantarius, Staphylococcus epidermidis, or Enterococcus spp. The case-fatality rate was 16% in this study, which was carried out in a hospital with a dedicated research team and with a very low prevalence of HIV (1%). The only clinical sign associated with bacteremia was oral thrush (374). Fever was present only in 27.5% of children with bacteremia.
Septic shock in children with acute severe malnutrition is associated with high mortality rates and presents unique management challenges, especially in facilities where close hemodynamic monitoring is not possible (378). Children with SAM and septic shock often have severely impaired renal and cardiac function and multiple electrolyte derangements, which make them susceptible to fluid overload and congestive heart failure. The 2013 revised WHO guidelines suggest the use of 5% dextrose plus either half-strength Darrow's solution, Ringer lactate, or half-strength saline for the initial expansion of the circulating volume, in repeatable boluses of 10 to 15 ml/kg of body weight (378). However, the quality of evidence to support this recommendation is low. A large clinical trial aimed at defining the optimal fluid management for children presenting to African hospitals with severe infections (FEAST trial) found that the use of fluid boluses was associated with increased mortality. Severely malnourished children, however, were specifically excluded from the study (388, 389). The optimal management of septic shock in children with SAM therefore remains an area in great need of further research.
The high risk of invasive bacterial infections in children with malnutrition plausibly arises from a combination of factors. Beside the multiple immune cell dysfunctions discussed above, the relatively increased frequencies of mixed infections and sepsis due to enteric pathogens suggest that microbial translocation across a defective mucosal barrier is likely to play an important role. The structural and functional changes associated with environmental enteropathy (EE) are addressed above. In a murine model of EE, mice fed a low-fat, low-protein diet and infected with Salmonella Typhimurium had a significantly increased burden of bacteria in the jejunum compared with mice fed a normal diet (26). The increased load of potentially invasive bacteria colonizing permeable mucosae could explain the higher rates of bacteremia in malnourished children (390). A similar association between mucosal dysfunction and invasive infections is conceivable at the level of the respiratory system. Children in developing countries typically live in settings where crowding, inadequate room ventilation, and increased exposure to smoke from biomass fuel are common. Such risk factors have been associated with increased epithelial inflammation, pneumonia, and chronic lung disease (391). At the same time, epidemiological studies also showed that children in low-resource settings have high rates of nasopharyngeal carriage of S. pneumoniae and H. influenzae and a different immune response toward these bacteria than that of children living in industrialized countries (392). It is plausible that a disruption of the respiratory mucosal barrier coupled with an increased density of colonization facilitate invasive infections.
Tuberculosis.
The impacts of micronutrient and vitamin deficiencies on tuberculosis are discussed above. The association of malnutrition with tuberculosis and early tuberculosis-related mortality is well established in adults (393–395) and was recently reviewed (396). TB is also a common cause of pneumonia in children with acute malnutrition (397), and WHO guidelines for the management of malnourished children as well as guidelines for national TB programs highlight the importance of the association of malnutrition and TB in children (378, 398). However, few studies of children have been completed, and a causal role of malnutrition as a risk factor for TB is not definitively established. Active TB itself often causes wasting, so data from retrospective studies are difficult to interpret. Furthermore, diagnostic tests for TB in children lack sensitivity, so diagnosis is often based on clinical findings without bacteriological confirmation. Therefore, we are left to conclude that malnutrition places a child at risk for TB through inference and extrapolation from data from studies of adults, BCG-vaccinated children, children with latent TB infection, and animal models (reviewed in reference 329). Severe malnutrition is associated with a reduced rate of tuberculin skin test positivity in children who received BCG vaccination (399), suggesting impaired cellular immune function and an increased risk for developing active disease. Accordingly, adults with coexisting latent TB infection and low BMI had a reduced protective cytokine response (e.g., IFN-γ and TNF-α) and increased regulatory cytokine (e.g., IL-10, IL-13, and TGF-β) production compared to individuals with latent TB infection and normal BMI (400). Severely underweight children were more likely to acquire TB infection following exposure to a household contact with pulmonary TB (401), but no studies have specifically addressed malnutrition as a risk factor for TB disease in children. Studies of M. tuberculosis-challenged guinea pigs (402–404) and mice (405) demonstrated that protein malnutrition compromised resistance to TB through defects in both innate and adaptive immune functions. Compromised host defense was associated with impaired T cell trafficking and proliferation, reduced production of protective cytokines (IFN-γ and TNF-α), impaired granuloma maturation, and reduced macrophage effector function (e.g., generation of nitric oxide) (329). Undernourished mice challenged with BCG also had reduced IFN-γ and TNF-α production and an increased risk of bacterial dissemination (406), and BCG vaccination failed to protect protein-malnourished guinea pigs against M. tuberculosis challenge (404, 407).
Malaria.
Placental malaria is a major contributor to fetal malnutrition (408). Intermittent preventive antimalarial treatment during pregnancy can reduce the risk of low birth weight and increase the length of the infant at 4 weeks of age (409–411). Repeated episodes of clinical malaria in children can also cause underweight or growth faltering, which can be prevented by measures to reduce malaria transmission (412–414). On the other hand, there has been considerable controversy over the impact of malnutrition on the outcome of malaria infection. Unfortunately, most studies were not designed to determine causality (415). Prospective cohort studies found an insignificant increase in the malaria incidence in children with moderate to severe undernutrition (416). Stunting and underweight increase the risk of malaria mortality (5), and recent data show that deficiencies in protein-energy, zinc, and vitamin A contribute significantly to malaria morbidity and mortality (reviewed in reference 417). Data pooled from several large-cohort studies identified relative risks of malaria mortality of 9.5, 4.5, and 2.1 for severe (weight-for-age z score of <−3), moderate (z score of between −2 and −3), and mild (z score of between −1 and −2) undernutrition, respectively, compared to children with a z score of >−1 (416). Based on these relative risks and prevalence data, the fractions of clinical malaria episodes and deaths from malaria in children under 5 years of age that were attributable to malnutrition (underweight, zinc deficiency, or vitamin A deficiency) were substantial (416).
Visceral leishmaniasis.
The majority of people who are infected with the visceralizing Leishmania species Leishmania chagasi/L. infantum or L. donovani develop chronic latent infection without clinical disease. Both the innate immune response that occurs within a few days of infection and the long-term adaptive immune response play critical roles in preventing the development of active disease. Epidemiological studies have documented a greatly increased risk for visceral leishmaniasis (VL) in malnourished hosts (418–422). Malnutrition was identified as a risk factor for severe disease (419) and death from VL in both children (WFH value, <60%; odds ratio, 5.0) and adults (BMI, <13; odds ratio, 11.0) (423). Malnutrition-related VL is particularly evident in displaced and impoverished populations (424, 425), and the recently described movement of transmission into periurban slums is likely to lead to increased malnutrition-infection synergism (426). A murine model of polynutrient deficiency (deficient protein, energy, zinc, and iron) (93, 427, 428), which mimics moderate human malnutrition (429), recapitulated the epidemiological observations by demonstrating that polynutrient deficiency led to an increased rate of early dissemination following cutaneous infection with L. donovani (427). Subsequent studies using the polynutrient-deficient mouse model identified a defect in lymph node barrier function, likely related to fewer myeloid phagocytes and dysregulated cell trafficking, as a contributor to increased parasite dissemination (56, 57, 427). In a hamster model of progressive VL from L. infantum infection, protein malnutrition led to leukocyte depletion, impaired lymphoproliferation, and increased parasite burdens compared to well-nourished controls (430).
MALNUTRITION, HOST DEFENSE, AND THE INTESTINAL MICROBIOTA
The complex relationship between malnutrition and the commensal microbiota was recently reviewed (431–433). The composition of the microbiota that normally colonize the cutaneous and mucosal surfaces is determined by host age and gender and genetic, dietary, and other environmental factors. There are significant differences in the compositions of the microbiota between different populations (434–438). There is greater intestinal microbial diversity in people from resource-poor regions than in people from resource-rich regions of the world. It is thought that these differences are driven primarily by differences in diet (reviewed in reference 439). The limited diversity of the gut microbiota of newborn infants expands over the first 2 to 3 years of life to a more mature (adult-like) population (440–443). Initial colonization is likely influenced by the mother's diet and microbiota (444, 445) and whether the infant is breastfed or formula fed (446). There is a transient reduction of microbiota maturity in children during and following an episode of acute gastroenteritis compared to healthy controls (443, 447), and diarrhea-associated taxa, which have not been shown to be causal, have been identified (447, 448).
Recent evidence indicates that the diet-microbiota dyad is a major determinant of the nutritional status of the host. Indeed, the composition of the intestinal microbiota of malnourished children is different from that of healthy controls (443, 449–453). Using age-discriminatory bacterial taxa identified in a longitudinal cohort of healthy children from Bangladesh over the first 2 years of postnatal life, Subramanian et al. determined that the fecal microbiota in infants with SAM was significantly more immature (less diverse) than that of age-matched healthy controls (443). The proportional representation of a total of 220 bacterial taxa was significantly different in children with SAM. Gut microbiota immaturity was causally related to undernutrition (453), but the transition toward a more diverse (mature) microbiota following treatment with antibiotics and ready-to-use therapeutic food (RUTF) in children with severe malnutrition was short-lived (443). The level of maturity of the intestinal microbiota was also correlated with anthropometric measures in less-severely malnourished children. A secondary analysis of data from cohorts in Malawi and Bangladesh revealed that severe stunting was associated with a less diverse microbiota, and an increase in the relative abundance of Acidaminococcus spp. was associated with reduced future linear growth (454). It was postulated that the depletion of glutamate, an amino acid metabolite critical to the health of the intestinal epithelium, by Acidaminococcus spp. and possibly other glutamate-fermenting bacteria could account for this effect on growth. Another study in India found that the relative depletion of specific genera (Roseburia, Faecalibacterium, Butyrivibrio, Eubacterium, and Phascolarctobacterium) was associated with anthropometric indicators of malnutrition (450). In a cohort from Uganda, children with nonedematous SAM had less fecal microbiota diversity than did children with edematous SAM, but there were no clear differences in the abundances of individual genera (455). Some studies demonstrated the presence of pathogens or pathogenic virulence factors in the microbiota of malnourished children (450, 451), but this has not been the case in some larger studies (443, 452). Additional population studies to define enterotypes and their clinical relevance are needed. The contribution of an altered intestinal microbiota to growth faltering was elegantly shown in a study of identical twins discordant for kwashiorkor in Malawi. Transfer of the fecal microbiota from the well-nourished or malnourished twin to germfree mice conferred the corresponding phenotype (weight loss in the mice that received the microbiota from the malnourished twin) (452). The development of the phenotype required feeding the mice the nutrient-deficient Malawian diet. These data indicate that the combination of a nutrient-deficient diet and altered intestinal microbial flora contributes to the pathogenesis of kwashiorkor. A growth-faltering effect on mice that received microbiota from undernourished children could be abrogated by the cotransfer of two species, Ruminococcus gnavus and Clostridium symbiosum, found in the microbiota of healthy children (453). Chronically undernourished mice could also be protected from postnatal growth faltering by the microbiota-mediated activation of the somatotropic axis (growth hormone and insulin-like growth factor) (456). Breast milk sialylated oligosaccharides, the levels of which are reduced in mothers of severely stunted infants, also promoted the growth of undernourished mice through interactions with the intestinal microbiota (457).
The profound effects of the microbiota on mucosal immune development and homeostasis in normal hosts are well established (458–460). Germfree animals show reduced maturity of gut-associated lymphoid tissue, fewer intestinal lymphocytes, and reduced secretion of IgA and antimicrobial peptides (reviewed in reference 461). These abnormalities were corrected following the population of the gut with normal commensal flora. Intestinal epithelial cell (IEC) sensing of bacterial ligands and metabolites via pattern recognition receptors (e.g., TLRs and NLRP3) strengthens the epithelial barrier and resistance to pathogens and maintains immune cell homeostasis through the secretion of cytokines (462, 463). Signals from microbiota-triggered IECs delivered to DCs and follicular DCs lead to the differentiation of B cells into IgA-producing plasma cells (464, 465). Microbiota-derived metabolites, including short-chain fatty acids such as butyrate, signal through G-protein-coupled receptors (GPCR) to induce cytokines (e.g., IL-18) and regulatory T cells that restrain intestinal inflammation (466–469). Similarly, commensal bacteria maintain ILCs through direct stimulation or indirectly through cytokine synthesis by other cells. ILC3s are the primary source of IL-22, which stimulates antimicrobial peptide (Reg3γ and Reg3β) production to contain commensal bacteria in the intestinal lumen and prevent invasion by pathogenic bacteria (470, 471). The gut microbiome is regulated by vitamin D signaling, the absence of which leads to dysbiosis and greater susceptibility to inflammation-mediated intestinal injury (472). The mucus layer, besides being a physical barrier to commensal or pathogen invasion, also provides immunoregulatory signals that prevent pathological inflammation (473).
Considering this finely tuned interaction of the gut microbiota with host cells, and the dramatic alteration of the microbiota in the malnourished host, it is highly likely that the dysbiosis-related dysregulation of mucosal immune function leads to impaired intestinal function and an increased risk of infection. However, the role of the microbiota in shaping mucosal immunity in the malnourished host has received limited attention. Since dietary components modulate gut inflammation directly through ligand-receptor interactions or indirectly though the alteration of the microbiota and its metabolic products (474), specific nutrient deficiencies are likely to modulate the microbiota and mucosal immune homeostasis. For example, the transport of dietary tryptophan through angiotensin I-converting enzyme in the small intestinal epithelium regulates the intestinal epithelial barrier, the generation of antimicrobial peptides, the composition of the intestinal microbiota, and susceptibility to intestinal inflammation (475). Mechanistic studies are needed to determine how specific nutrient-related dysbiosis impacts nutrient absorption, mucosal immune function, and host defense.
The findings of malnutrition-related alterations in the gut microbiota suggest that probiotics, which promote a healthy microbiota, could be used in therapeutic interventions (476). Supplementation of the diet with a probiotic fermented milk product reduced the frequency and severity of diarrheal disease and improved growth recovery in a cohort of chronically malnourished (stunted) Indian children (477). The use of fermented milk products has the potential for an adverse effect if the milk product has a high lactose content and the child has reduced lactase production because of enteropathy or lactase nonpersistence. However, in a large, double-blind, randomized, placebo-controlled trial with children with severe acute malnutrition in Malawi (PRONUT study), the addition of a cocktail of four different probiotic lactic acid bacilli and four prebiotic fermentable fibers to RUTF (for a median of 33 days) did not improve the acute outcome (weight-for-height recovery) but showed a trend toward reduced mortality in the outpatient setting (478). The probiotics appeared to be safe in this cohort, in which >40% of the children were HIV seropositive. The delivery of a probiotic fermented milk product (containing lactobacilli and Streptococcus thermophilus) to protein-energy-malnourished mice led to improved systemic and gut immune functions and defense against enteric challenge with Salmonella Typhimurium (479, 480). Similarly, a probiotic (Lactobacillus reuteri) reduced rotavirus-induced diarrhea, but the effect was blunted in undernourished mice (481). Germfree mice, which have stunted growth because of the lack of microbiota-driven growth hormone sensitivity and IGF-1 signaling, showed no growth deficit when colonized with a single strain of Lactobacillus plantarum (456). Collectively, these data highlight the exciting potential for reshaping of the intestinal microbiota with probiotics and other therapies complementary to nutritional interventions. The identification of interventions that durably repair malnutrition-related dysbiosis is critically important (482).
NUTRITIONAL MANAGEMENT OF CHILDREN WITH MALNUTRITION
The WHO/United Nations Children's Emergency Fund (UNICEF) recommend exclusive breastfeeding for the first 6 months of life and nonexclusive breastfeeding for up to 24 months (483). The benefits of breastfeeding in the reduction of morbidity and mortality from respiratory and gastrointestinal infections has been discussed. Nevertheless, in resource-poor populations, there are high rates of early cessation of breastfeeding and early introduction of complementary foods, which increase the risk of malnutrition (483). Children with severe acute malnutrition are treated according to WHO guidelines (378). In the presence of clinical complications such as altered mental status, severe infection, hypothermia, hypoglycemia, severe anemia, or anorexia, children are admitted to hospitals and undergo nutritional rehabilitation implemented in 3 phases. During the initial intensive phase, patients with SAM are treated with liquid therapeutic “milk,” called F75, specially formulated to satisfy caloric (100 kcal/kg/day) and micronutrient requirements while avoiding overloads of proteins and sodium. Once the child is stabilized and the appetite has returned, the child is switched to the transition and maintenance phases, whereby F75 is gradually replaced with either F100, a more concentrated milk formula, or RUTF for a minimum caloric intake of ∼175 kcal/kg/day during the maintenance phase. Current protocols suggest a discontinuation of therapeutic feeding once the WHZ is greater than −2 or the MUAC is greater than 125 mm, although a longer period of treatment would probably reduce the risk of relapse. Children with SAM but no clinical complications can be effectively treated with RUTF from the start, provided that they pass the appetite test and are able to consume the recommended quantity (377–379, 484–487). RUTF is a high-energy, high-lipid, high-protein prepared-food supplement that is also fortified with vitamins and trace elements (488). It has several advantages over F100 infant formula in that it is not water based (most RUTF formulations have a peanut butter base), so it does not need to be reconstituted with potentially contaminated water, does not require preparation in the field or home, does not need refrigeration, is resistant to microbial colonization, is highly palatable, and is easily distributed on a mass scale. A recent review of pooled data from studies that included >20,000 severely malnourished children found that therapeutic dietary intervention with RUTF led to growth recovery in nearly 80% of children (489).
Treatment for moderate acute malnutrition is less defined. Current WHO guidelines include nutritional counseling, diagnosis and treatment of underlying infections, and, when feasible, the provision of supplementary food to guarantee a caloric intake of at least 75 kcal/kg/day, which is half of what is needed for catch-up growth. Several types of supplementary foods are used, with fortified spreads (ready-to-use supplemental food [RUSF]) being increasingly common (378, 379). Short-term intervention with RUSF led to the recovery of growth indicators in children with moderate acute malnutrition (490–495). Short-term preventive interventions within at-risk populations may also have short- and long-term growth benefits (491, 496, 497). A recent large trial of a corn-soy-blended flour supplement fortified with oil and dry skim milk demonstrated recovery (weight-for-height z score of ≥−2) within an average of 4 weeks after the initiation of RUSF in 85% of children (493).
Several interventions have been found effective in preventing or reducing the prevalence of stunting (498). However, there is no consensus for treatment, largely because the physiopathology of this condition is complex and poorly understood.
NUTRITIONAL INTERVENTIONS TO RESTORE HOST DEFENSE AND IMPROVE INFECTIOUS DISEASE OUTCOMES
Macronutrient Supplementation
The most severely malnourished children are at the greatest risk for morbidity and mortality due to infectious diseases. However, the higher prevalence of less severe forms of malnutrition leads to a greater global infectious disease impact. Therefore, intervention programs need to address all degrees of malnutrition in order to have a significant global effect. It is well established that in the setting of acute malnutrition, nutritional interventions effectively correct growth deficits. It is less clear, however, whether these interventions reduce the risk or severity of infections. Acute intervention by refeeding was effective in ameliorating the severity of tuberculosis (499), HIV, and respiratory infections (496), but the impact on long-term susceptibility and outcomes is less clear.
Only a single study has addressed the impact of RUTF on the prevalence of infection. In this study, children without acute malnutrition (weight for height >80% of the reference standard) who received RUTF had a statistically insignificant reduction in malaria prevalence and no reduction in the incidence of upper respiratory infection or diarrheal disease (496). The possible discordance between the growth-enhancing and infection-preventing effects of RUTF observed in this study is concerning, since infection is the major cause of morbidity and mortality in malnourished children. However, data from this study must be interpreted with caution because the older age of this cohort (median age, 30 months), the historical data collection methods related to infection, and the limited sample size may have contributed to the apparent lack of an effect. Additionally, no studies have addressed the impact of RUTF on the amelioration of malnutrition-related immunological deficits. This is a significant knowledge gap that needs to be investigated. In severely-protein-deficient mice, Taylor et al. found that the initiation of a protein-sufficient diet led to the recovery of CD8+ T cell and cytokine responses, increased viral clearance, and reduced mortality from influenza virus infection (126).
Multimicronutrient Supplementation
The role of dietary supplementation with a single micronutrient (e.g., a vitamin or mineral) is discussed above. A number of studies, most notably focused on tuberculosis, malaria, and diarrheal disease, have evaluated supplements of multiple micronutrients (vitamins and minerals), with both positive and negative outcomes. Comparison between studies to arrive at a consensus is challenging because they differed in study designs, micronutrient supplement compositions, and measured outcomes.
The impact of macro- and micronutrient nutritional supplements as adjunctive therapy in adults and children with tuberculosis was the subject of a recent Cochrane review (500). In general, macronutrient supplementation likely improved weight recovery and quality of life but had no proven effect on tuberculosis outcome. Similarly, consistent benefits of supplementation with single or multiple micronutrients have not been proven. Those studies were limited by small sample sizes, heterogeneous study populations, and undefined baseline nutrient deficits (500). Although vitamin A, zinc, and selenium deficiencies are common in patients with active tuberculosis (501, 502), few of the reported clinical trials included a baseline assessment of micronutrient deficiencies in the study population. A randomized, double-blind, placebo-controlled trial of a daily oral micronutrient supplement (mixture of vitamin A, B complex vitamins, vitamin C, vitamin E, and selenium) in 887 patients with active pulmonary tuberculosis (54% of whom were HIV seropositive) showed that the supplement decreased the risk of tuberculosis recurrence (median of 43 months of follow-up) and marginally reduced mortality in HIV-seronegative subjects without impacting the overall mortality of the cohort (208). The supplement had no effect on T cell responses to tuberculin antigens (207). A reduction in the rate of tuberculosis recurrence with multimicronutrient supplementation would be a significant advance. Other studies showed conflicting results. Increased sputum smear or culture conversion was identified in one study (503) but not in other studies (504–506) of supplementation with vitamin A plus zinc. Improved weight gain and short-term survival were identified in the subset of HIV-positive subjects in a large trial of subjects with pulmonary tuberculosis who received a supplement of zinc and a multivitamin-mineral mix (vitamins A, B, C, D, and E, with selenium and copper) (507). In that same study population, supplementation with zinc and the multivitamin-mineral mix had no effect on sputum conversion (508). In contrast, no mortality benefit was observed for tuberculosis patients (with or without HIV coinfection) who received a multivitamin-mineral supplement (509), nor was there an improved clinical response with the addition of a zinc-vitamin A supplement (504). A locally prepared supplement containing a cereal-lentil powder and a multivitamin-micronutrient mix showed a trend toward improved tuberculosis clinical outcomes, but the sample size was small (510). Children with intrathoracic tuberculosis who were given a supplement containing a micronutrient mix (vitamins with selenium and copper), with or without zinc, showed no improvement in radiological outcome or weight gain but had a significantly improved height-for-age z score (511). A similar effect of a multivitamin supplement on height, but not weight, was observed in a smaller trial (512).
Micronutrient deficiencies are common in patients with malaria, so micronutrient supplementation has been a target of investigation. A large population-based study (42,546 children aged 1 to 35 months at enrollment) of zinc supplementation (mean duration of supplementation, 485 days) in a region of Zanzibar where malaria is holoendemic showed a nonsignificant reduction (7%) in all-cause mortality. There was a marginally significant reduction in mortality (18%) in children aged 12 to 48 months (513). A randomized, placebo-controlled study of 6 months of zinc supplementation in children 6 to 31 months of age showed no effect on the incidence or severity of clinical malaria but reduced morbidity associated with diarrheal disease (194). Similarly, no protection from clinical malaria was seen in a randomized, controlled trial of malnourished children (HAZ of <−1.5; 60% with zinc deficiency) in an area of high malaria transmission in Tanzania who received a multinutrient supplement (micronutrients plus vitamins), multinutrients and zinc, or zinc alone (514). In contrast, in a randomized, controlled trial in children in Ghana with a high prevalence of stunting, vitamin A-zinc supplementation led to a significant reduction in the incidence of clinical malaria compared to supplementation with vitamin A alone during a 6-month follow-up period (515).
The positive impact of zinc supplementation on diarrheal disease has been well established (see above). Multimicronutrient supplementation, with or without zinc supplementation, has also received considerable attention. Conflicting findings of improved or worsened outcomes have led to questions regarding the inclusion of multiple micronutrients in a single supplement (reviewed in reference 516). The mechanistic underpinnings of the discordant results are unknown, but the etiological heterogeneity of diarrheal disease between different populations, differences in types of micronutrient supplements, variations in the underlying micronutrient status, and different effects of each micronutrient on pathogen-specific immunity are likely contributors. Several clinical trials demonstrated that the addition of a multimicronutrient supplement to zinc either had no effect or increased the frequency of diarrheal disease (517–519). Similarly, supplementation with zinc or a zinc-micronutrient mix with vitamin A showed no reduction in the prevalence of diarrheal disease compared to that with vitamin A alone in HIV-infected and -uninfected children starting at 6 months and continuing until 24 months of age (520). Also, supplementation of B complex vitamins, vitamin C, and vitamin E in HIV-exposed infants did not reduce the frequency of reported diarrhea or mortality (521).
In contrast, several studies reported a positive effect of multimicronutrient supplementation on diarrheal disease. The administration of a micronutrient-fortified seasoning powder through a school lunch program reduced the incidence of diarrheal disease in Thai children (522). In children who experienced multiple diarrheal episodes, a multimicronutrient supplement with zinc and vitamin A was more effective in preventing a decline in height for age than was vitamin A alone or vitamin A plus zinc (523). In a study of 2- to 6-year-old preschool children, supplementation with multimicronutrients, iron, and vitamin A led to a reduced incidence of diarrheal illness over 6 months compared to vitamin A alone or vitamin A plus iron (524). Treatment of diarrhea in children 6 to 24 months of age with an oral rehydration solution (ORS) plus adjunctive zinc, zinc plus vitamin A, or zinc plus vitamin A and a multimicronutrient supplement revealed that all three of the supplements led to a reduced duration of diarrhea, a reduced volume of stool output, and a reduced requirement for ORS compared to the placebo group (525). That study was not adequately powered to show a difference between the groups receiving supplements. Collectively, data from these studies indicate that the addition of a multimicronutrient supplement adds little or nothing to zinc or vitamin A supplementation. Any added benefit is likely to be incremental and will require large sample sizes to definitively show an effect. Additionally, studies have generally not stratified responses to supplementation by micronutrient status or other markers of malnutrition.
A couple of studies showed a benefit of amino acid supplementation in diarrheal disease. Lysine supplementation compared to placebo reduced the numbers of diarrheal episodes and days of diarrheal illness in a cohort of children (mean age, 8 years) in periurban Accra, Ghana (526). The administration of l-arginine to undernourished mice led to improved weight gain, gastrointestinal mucosal histology, and parasite burden following Cryptosporidium infection (362). Alanyl-glutamine supplementation improved weight gain and intestinal barrier function in Brazilian children with mild to moderate undernutrition and in mice with weanling malnutrition (527, 528).
Finally, the effect of micronutrient intake/supplementation may be different with regard to the prevention versus treatment of infection. Adequate micronutrient intake is likely protective with regard to pathogenic infections, but in a micronutrient-deficient host with a significant pathogen burden, supplementation may not always be effective, as the pathogen may “steal” micronutrients for its own use. In this case, treatment with antimicrobials to reduce the pathogen burden while simultaneously increasing micronutrient intake would be the best approach.
ANTIBIOTICS IN THE MANAGEMENT OF MALNUTRITION
Considering the high prevalence of bacterial infections among children with SAM, antibiotics have been traditionally part of treatment for these patients. The treatment guidelines issued by the WHO in 1999 recommended the use of broad-spectrum antibiotics as routine initial management: children with clinical complications would receive parenteral antibiotics, while uncomplicated cases would receive oral treatment, both for a minimum of 7 days (377). The recommendations regarding antibiotics remained in the revised guidelines of 2007 and 2014 (378, 484), although in 2014, it was recognized that there was little evidence to support universal antimicrobial treatment in cases of uncomplicated malnutrition. The one randomized, double-blind, controlled study available at that time had been conducted in Malawi between 2009 and 2011 among children with marasmus or kwashiorkor but no obvious clinical complications (529). In this study, 2,767 patients aged 6 to 59 months were randomly allocated to receive amoxicillin, cefdinir, or placebo for 7 days in combination with RUTF. The proportion of children with nutritional recovery was significantly higher among those who received antibiotics than among those who received placebo (89% for amoxicillin, 91% for cefdinir, and 85% for placebo; P < 0.002), and the mortality rate was significantly higher in the placebo group (5% for amoxicillin, 4% for cefdinir, and 7.4% for placebo; P < 0.0006). Furthermore, the rate of weight gain was increased in the groups who received antibiotics. No interaction between the type of malnutrition (edematous or nonedematous) and the intervention group was observed. Only 30% of the children were tested for HIV: among those children, 20% were seropositive and had higher rates of treatment failure or death. The high rates of edematous malnutrition and HIV infection, however, do not allow the generalization of these results to populations with different characteristics.
A second study was conducted in Niger between 2012 and 2013, this time enrolling only children with marasmus but not kwashiorkor (381). Only 1 out of 2,399 participants was HIV positive. Children were randomly allocated to receive either 7 days of amoxicillin or placebo in addition to RUTF. That study did not show differences in nutritional recovery at the end of treatment (8 weeks), but treatment with amoxicillin was associated with a lower risk of being transferred to inpatient care for clinical complications, mainly infections, during follow-up (7.5% versus 10.8%; P = 0.01). There were also trends toward reduced mortality in children older than 24 months of age and accelerated gains in weight and mid-upper-arm circumference in the antibiotic-treated group. The exclusion of children with kwashiorkor, the low prevalence of HIV infection, and the performance of the study by highly trained health care personnel limit the generalizability of the data from this study.
Thus, the available evidence suggests that a short course of antibiotics at the time of diagnosis benefits children with acute severe malnutrition if they show signs of clinical complications, but it is unclear if children without infections need the same treatment. The paucity of diagnostic tools available in resource-limited settings coupled with the low sensitivity of clinical evaluation for sepsis complicate the decision-making process. Importantly, several studies reported increasing prevalences of drug-resistant bacteria isolated from children living in low-resource countries. Resistance against not only antimicrobials routinely used in such settings but also newer antibiotics that have had limited use is common (374, 376, 380, 387, 530, 531). Thus, well-designed clinical trials and innovative point-of-care diagnostic tools are urgently needed to inform new guidelines, contextualize the treatment of SAM, and tailor the use of antibiotics to the specific needs of each child.
A second open issue concerns the use of antibiotics in the management of acute malnutrition beyond the first rehabilitation period. Even after initial nutritional recovery, in fact, children with acute malnutrition continue to show an increased risk of death, mainly due to infections (532, 533). Based on the observation that co-trimoxazole prophylaxis significantly decreased the rates of mortality of children with HIV infection, the effect of similar prophylaxis among children with SAM was evaluated in Kenya. Between 2009 and 2013, 1,778 HIV-negative children aged 2 to 59 months with complicated severe malnutrition were randomly assigned to 6 months of treatment with either oral co-trimoxazole or placebo, after the completion of standard initial treatment. That study achieved a remarkably efficient follow-up and had a very low attrition rate. Among the children surviving the first hospitalization, 11% died in the following 12 months, but no difference between children treated with antibiotics and those treated with placebo was observed, nor was there a difference in the numbers of children subsequently admitted to the hospital with bacteremia, pneumonia, or severe diarrhea.
Thus, current evidence indicates that children with acute malnutrition, albeit certainly immunosuppressed, should not be placed on long-term antibiotic prophylaxis. Not only are there no data to support such treatment, there is also growing evidence of the profound negative effect of antimicrobials on the diversity, functional profile, and abundance of antibiotic resistance genes in the host microbiota (534). This underscores the necessity for greater clarity and targeted approaches for the use of antimicrobials in acutely malnourished children heavily exposed to bacterial pathogens. Antibacterial drugs have no place in the treatment of chronic malnutrition in the absence of active infection.
RESEARCH PRIORITIES FOR THE FUTURE
Tremendous advances in our understanding of the roles of malnutrition in infection and host defense have been made over the past several decades. However, much remains to be learned, and there is a critical need for mechanistic studies that can lead to targeted clinical interventions. Research priorities related to childhood malnutrition have been identified and discussed (535, 536), but little attention has been given to mechanisms and interventions for malnutrition-related immune impairment. Clinical and immunological studies of malnourished children are challenging because of the vulnerability of this population and the limited opportunity for the collection of clinical samples. Knowledge can be gained from studies of plasma and peripheral blood leukocytes, but investigation of host defense at the tissue level is usually not possible. Furthermore, immunological studies of malnourished children in resource-poor settings is difficult because of the limited availability of research infrastructure and technology. Mechanistic studies are most easily conducted in experimental animals, but clinically and epidemiologically relevant animal models of malnutrition are needed. These models should include animals of an age representative of the childhood development period, should represent real-world dietary (often multinutrient) deficiencies, and should use natural routes of pathogen challenge. The reductionist approach often employed in mechanistic studies may not accurately represent the complex features of childhood malnutrition. New tools for unbiased transcriptomics, proteomics, metabolic profiling, and microbiome-metabolome analyses (537) have much to offer when applied to well-characterized clinically relevant cohorts (443, 452). In particular, the identification and validation of biomarkers, especially those that can be readily measured in resource-limited settings, will be critical for future clinical intervention studies. If the impact of malnutrition-infection synergism is to be lessened, we need to understand the risks for specific infections, the underlying immunological deficits, and the efficacy of nutritional interventions in correcting deficits and reducing risks. Table 4 identifies a number of future research needs and the types of studies that can address them.
TABLE 4.
Research priorities for host defense and risk of infection in childhood malnutrition
ACKNOWLEDGMENTS
This work was supported by funding from the Center for Tropical Diseases and the Institute for Human Infection and Immunity at the University of Texas Medical Branch and the National Institutes of Health (R21AI107419).
We thank Sarah Melby for excellent help with graphic design of the figures.
Biographies

Marwa K. Ibrahim received undergraduate training in biochemistry at Ain Shams University in Cairo, Egypt, and a master's degree in cancer and molecular biology at the National Research Center, Cairo, Egypt. Her Ph.D. studies at the University of Texas Health Science Center at San Antonio, TX, under the direction of Professor Peter Melby, focused on investigation of immune function of the lymph node in a murine model of polynutrient deficiency. Her work demonstrated a failure of barrier function and dysregulation of inflammation and leukocyte trafficking in the draining lymph node of malnourished weanling mice infected with the intracellular pathogen Leishmania. Her postgraduate studies as a researcher at the National Research Center in Cairo have focused on investigating the role of genetic factors, immunological mechanisms, and coinfection with other viruses in regulating the clinical outcome of hepatitis C virus infection.

Mara Zambruni obtained her medical degree and pediatrics specialization from the University of Padua, Italy, and a master's in Public Health at the London School of Hygiene and Tropical Medicine in London, UK. Between 2005 and 2010, she worked as pediatric attending physician in Mozambique in projects run by the Italian Government Agency for Aid. The interest in the short- and long-term effects of pediatric malnutrition arose from the contrast between the high prevalence of this condition in low-resource countries and the limited scientific knowledge available for clinicians providing care. In 2011, she moved to the United States, where she completed a fellowship in Pediatric Infectious Diseases at the University of Texas, Houston, and initiated a research project in Peru aimed at investigating the relationship between chronic malnutrition, enteric infections, and the gut microbiome. She is currently a postdoctoral fellow at the University of Texas Medical Branch in Galveston, TX.

Christopher L. Melby received his training (Dr.P.H., M.P.H.) in health science and nutrition at the Loma Linda University School of Public Health. He held a faculty position at Purdue University for 8 years and has been a professor of nutritional science at Colorado State University for the past 27 years, during which time he was department head for 10 years. He directs the Nutrition and Metabolic Fitness Laboratory with a research focus on the interaction of dietary and physical activity patterns on energy and macronutrient metabolism and on cardiometabolic risk factors in high-risk populations. His interest in public health nutrition issues in Latin America led to his work as a Fulbright Research and Teaching Fellow in Ecuador in 2015. There, he conducted research focused on better understanding the impact of nutrition and health status of rural and urban women transitioning from ancestral dietary patterns to greater reliance on commercially prepared foods.

Peter C. Melby received undergraduate training in microbiology at Colorado State University, where he became interested in tropical infectious diseases. He worked in clinical microbiology in Egypt before returning to complete his medical education at the University of Colorado. He received clinical and research training in infectious diseases and tropical medicine at the U.S. National Institutes of Health and the University of Texas Health Science Center in San Antonio. His research interests include immunity and pathogenesis of parasitic diseases and the interplay between nutrition and infection. He has studied the mechanisms of impaired host defense in the malnourished host using experimental animal models for the past 15 years. More recently, he has been involved in clinical care and studies of children with acute and chronic malnutrition in East Africa and Latin America. He is Director of the Division of Infectious Disease and Director of the Center for Tropical Diseases at the University of Texas Medical Branch in Galveston, TX.
REFERENCES
- 1.Canani RB, Costanzo MD, Leone L, Bedogni G, Brambilla P, Cianfarani S, Nobili V, Pietrobelli A, Agostoni C. 2011. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev 24:198–205. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 2.Palmer AC. 2011. Nutritionally mediated programming of the developing immune system. Adv Nutr 2:377–395. doi: 10.3945/an.111.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover Z, Ee LC. 2009. Protein energy malnutrition. Pediatr Clin North Am 56:1055–1068. doi: 10.1016/j.pcl.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Briend A, Khara T, Dolan C. 2015. Wasting and stunting—similarities and differences: policy and programmatic implications. Food Nutr Bull 36:S15–S23. doi: 10.1177/15648265150361S103. [DOI] [PubMed] [Google Scholar]
- 5.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 6.International Food Policy Research Institute. 2016. Global nutrition report 2016. International Food Policy Research Institute, Washington, DC. [Google Scholar]
- 7.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R, Maternal and Child Nutrition Study Group. 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 8.Martinsen TC, Bergh K, Waldum HL. 2005. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol 96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LW, Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 10.Prendergast A, Kelly P. 2012. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 86:756–763. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prendergast AJ, Humphrey JH. 2015. Stunting persists despite optimal feeding: are toilets part of the solution? Nestle Nutr Inst Workshop Ser 81:99–110. [DOI] [PubMed] [Google Scholar]
- 12.Ngure FM, Humphrey JH, Mbuya MN, Majo F, Mutasa K, Govha M, Mazarura E, Chasekwa B, Prendergast AJ, Curtis V, Boor KJ, Stoltzfus RJ. 2013. Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. Am J Trop Med Hyg 89:709–716. doi: 10.4269/ajtmh.12-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. 2014. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis 210:1296–1305. doi: 10.1093/infdis/jiu235. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Valenzano MC, Mercado JM, Zurbach EP, Mullin JM. 2013. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci 58:77–87. doi: 10.1007/s10620-012-2328-8. [DOI] [PubMed] [Google Scholar]
- 15.Ordiz MI, Shaikh N, Trehan I, Maleta K, Stauber J, Shulman R, Devaraj S, Tarr PI, Manary MJ. 2016. Environmental enteric dysfunction is associated with poor linear growth and can be identified by host fecal mRNAs. J Pediatr Gastroenterol Nutr 63:453–459. doi: 10.1097/MPG.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA Jr. 2012. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 54:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossain MI, Nahar B, Hamadani JD, Ahmed T, Roy AK, Brown KH. 2010. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J Pediatr Gastroenterol Nutr 51:638–644. doi: 10.1097/MPG.0b013e3181eb3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lykke M, Hother AL, Hansen CF, Friis H, Molgaard C, Michaelsen KF, Briend A, Larsen T, Sangild PT, Thymann T. 2013. Malnutrition induces gut atrophy and increases hepatic fat infiltration: studies in a pig model of childhood malnutrition. Am J Transl Res 5:543–554. [PMC free article] [PubMed] [Google Scholar]
- 19.Kasai A, Gama P, Alvares EP. 2012. Protein restriction inhibits gastric cell proliferation during rat postnatal growth in parallel to ghrelin changes. Nutrition 28:707–712. doi: 10.1016/j.nut.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, Jones A, Moulton LH, Stoltzfus RJ, Humphrey JH. 2014. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One 9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, Guerrant RL, Bhutta Z, Mason C, Kang G, Kabir M, Amour C, Bessong P, Turab A, Seidman J, Olortegui MP, Quetz J, Lang D, Gratz J, Miller M, Gottlieb M, MAL-ED Network. 2013. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JR, Sheng XY, Hu YQ, Yu XG, Westcott JE, Miller LV, Krebs NF, Hambidge KM. 2012. Fecal calprotectin levels are higher in rural than in urban Chinese infants and negatively associated with growth. BMC Pediatr 12:129. doi: 10.1186/1471-2431-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell DI, Elia M, Lunn PG. 2003. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr 133:1332–1338. [DOI] [PubMed] [Google Scholar]
- 24.Lunn PG, Northrop-Clewes CA, Downes RM. 1991. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338:907–910. doi: 10.1016/0140-6736(91)91772-M. [DOI] [PubMed] [Google Scholar]
- 25.Attia S, Versloot CJ, Voskuijl W, van Vliet SJ, Di Giovanni V, Zhang L, Richardson S, Bourdon C, Netea MG, Berkley JA, van Rheenen PF, Bandsma RH. 21 September 2016. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: an observational cohort study. Am J Clin Nutr doi: 10.3945/ajcn.116.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown EM, Wlodarska M, Willing BP, Vonaesch P, Han J, Reynolds LA, Arrieta MC, Uhrig M, Scholz R, Partida O, Borchers CH, Sansonetti PJ, Finlay BB. 2015. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun 6:7806. doi: 10.1038/ncomms8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heath ML, Sidbury R. 2006. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr 18:417–422. doi: 10.1097/01.mop.0000236392.87203.cc. [DOI] [PubMed] [Google Scholar]
- 28.Leite SN, Jordao AA Jr, Andrade TA, Masson DDS, Frade MA. 2011. Experimental models of malnutrition and its effect on skin trophism. An Bras Dermatol 86:681–688. doi: 10.1590/S0365-05962011000400009. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama A, Fujita Y, Kobayashi T, Ryu M, Suzuki Y, Masuda A, Ochi T, Takeuchi T. 2011. Effect of protein malnutrition on the skin epidermis of hairless mice. J Vet Med Sci 73:831–835. doi: 10.1292/jvms.10-0399. [DOI] [PubMed] [Google Scholar]
- 30.Mechanick JI. 2004. Practical aspects of nutritional support for wound-healing patients. Am J Surg 188:52–56. doi: 10.1016/S0002-9610(03)00291-5. [DOI] [PubMed] [Google Scholar]
- 31.Otranto M, Souza-Netto I, Aguila MB, Monte-Alto-Costa A. 2009. Male and female rats with severe protein restriction present delayed wound healing. Appl Physiol Nutr Metab 34:1023–1031. doi: 10.1139/H09-100. [DOI] [PubMed] [Google Scholar]
- 32.Lyra JS, Madi K, Maeda CT, Savino W. 1993. Thymic extracellular matrix in human malnutrition. J Pathol 171:231–236. doi: 10.1002/path.1711710312. [DOI] [PubMed] [Google Scholar]
- 33.Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. 2007. The thymus is a common target in malnutrition and infection. Br J Nutr 98(Suppl 1):S11–S16. doi: 10.1017/S0007114507832880. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz R, Cortes L, Cortes E, Medina H. 2009. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin Exp Immunol 155:96–106. doi: 10.1111/j.1365-2249.2008.03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsumori K, Takegawa K, Shimo T, Onodera H, Yasuhara K, Takahashi M. 1996. Morphometric and immunohistochemical studies on atrophic changes in lympho-hematopoietic organs of rats treated with piperonyl butoxide or subjected to dietary restriction. Arch Toxicol 70:809–814. doi: 10.1007/s002040050343. [DOI] [PubMed] [Google Scholar]
- 36.Nodera M, Yanagisawa H, Wada O. 2001. Increased apoptosis in a variety of tissues of zinc-deficient rats. Life Sci 69:1639–1649. doi: 10.1016/S0024-3205(01)01252-8. [DOI] [PubMed] [Google Scholar]
- 37.da Silva SV, Salama C, Renovato-Martins M, Helal-Neto E, Citelli M, Savino W, Barja-Fidalgo C. 2013. Increased leptin response and inhibition of apoptosis in thymocytes of young rats offspring from protein deprived dams during lactation. PLoS One 8:e64220. doi: 10.1371/journal.pone.0064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barone KS, O'Brien PC, Stevenson JR. 1993. Characterization and mechanisms of thymic atrophy in protein-malnourished mice: role of corticosterone. Cell Immunol 148:226–233. doi: 10.1006/cimm.1993.1105. [DOI] [PubMed] [Google Scholar]
- 39.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. 1999. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savino W. 2002. The thymus gland is a target in malnutrition. Eur J Clin Nutr 56(Suppl 3):S46–S49. doi: 10.1038/sj.ejcn.1601485. [DOI] [PubMed] [Google Scholar]
- 41.Ozkale M, Sipahi T. 2014. Hematologic and bone marrow changes in children with protein-energy malnutrition. Pediatr Hematol Oncol 31:349–358. doi: 10.3109/08880018.2013.813098. [DOI] [PubMed] [Google Scholar]
- 42.Xavier JG, Favero ME, Vinolo MA, Rogero MM, Dagli ML, Arana-Chavez VE, Borojevic R, Borelli P. 2007. Protein-energy malnutrition alters histological and ultrastructural characteristics of the bone marrow and decreases haematopoiesis in adult mice. Histol Histopathol 22:651–660. doi: 10.14670/HH-22.651. [DOI] [PubMed] [Google Scholar]
- 43.Borelli P, Barros FE, Nakajima K, Blatt SL, Beutler B, Pereira J, Tsujita M, Favero GM, Fock RA. 2009. Protein-energy malnutrition halts hemopoietic progenitor cells in the G0/G1 cell cycle stage, thereby altering cell production rates. Braz J Med Biol Res 42:523–530. doi: 10.1590/S0100-879X2009000600008. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima K, Crisma AR, Silva GB, Rogero MM, Fock RA, Borelli P. 2014. Malnutrition suppresses cell cycle progression of hematopoietic progenitor cells in mice via cyclin D1 down-regulation. Nutrition 30:82–89. doi: 10.1016/j.nut.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 45.Borelli P, Blatt S, Pereira J, de Maurino BB, Tsujita M, de Souza AC, Xavier JG, Fock RA. 2007. Reduction of erythroid progenitors in protein-energy malnutrition. Br J Nutr 97:307–314. doi: 10.1017/S0007114507172731. [DOI] [PubMed] [Google Scholar]
- 46.Vinolo MA, Crisma AR, Nakajima K, Rogero MM, Fock RA, Borelli P. 2008. Malnourished mice display an impaired hematologic response to granulocyte colony-stimulating factor administration. Nutr Res 28:791–797. doi: 10.1016/j.nutres.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Fock RA, Vinolo MA, Blatt SL, Borelli P. 2012. Impairment of the hematological response and interleukin-1beta production in protein-energy malnourished mice after endotoxemia with lipopolysaccharide. Braz J Med Biol Res 45:1163–1171. doi: 10.1590/S0100-879X2012007500151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fock RA, Blatt SL, Beutler B, Pereira J, Tsujita M, de Barros FE, Borelli P. 2010. Study of lymphocyte subpopulations in bone marrow in a model of protein-energy malnutrition. Nutrition 26:1021–1028. doi: 10.1016/j.nut.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 49.Cunha MC, Lima FDS, Vinolo MA, Hastreiter A, Curi R, Borelli P, Fock RA. 2013. Protein malnutrition induces bone marrow mesenchymal stem cells commitment to adipogenic differentiation leading to hematopoietic failure. PLoS One 8:e58872. doi: 10.1371/journal.pone.0058872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Najera O, Gonzalez C, Toledo G, Lopez L, Ortiz R. 2004. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin Diagn Lab Immunol 11:577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes SM, Amadi B, Mwiya M, Nkamba H, Tomkins A, Goldblatt D. 2009. Dendritic cell anergy results from endotoxemia in severe malnutrition. J Immunol 183:2818–2826. doi: 10.4049/jimmunol.0803518. [DOI] [PubMed] [Google Scholar]
- 52.Mello AS, de Oliveira DC, Bizzarro B, Sa-Nunes A, Hastreiter AA, de Oliveira Beltran JS, Xavier JG, Borelli P, Fock RA. 2014. Protein malnutrition alters spleen cell proliferation and IL-2 and IL-10 production by affecting the STAT-1 and STAT-3 balance. Inflammation 37:2125–2138. doi: 10.1007/s10753-014-9947-5. [DOI] [PubMed] [Google Scholar]
- 53.Manhart N, Vierlinger K, Bergmeister H, Boltz-Nitulescu G, Spittler A, Roth E. 2000. Influence of short-term protein malnutrition of mice on the phenotype and costimulatory signals of lymphocytes from spleen and Peyer's patches. Nutrition 16:197–201. doi: 10.1016/S0899-9007(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 54.Cortes-Barberena E, Ceballos-Olvera I, Gonzalez-Marquez H, Ortiz-Muniz R. 2013. Moderate and severe malnutrition alters proliferation of spleen cells in rats. Cell Prolif 46:164–171. doi: 10.1111/cpr.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuervo-Escobar S, Losada-Barragan M, Umana-Perez A, Porrozzi R, Saboia-Vahia L, Miranda LH, Morgado FN, Menezes RC, Sanchez-Gomez M, Cuervo P. 2014. T-cell populations and cytokine expression are impaired in thymus and spleen of protein malnourished BALB/c mice infected with Leishmania infantum. PLoS One 9:e114584. doi: 10.1371/journal.pone.0114584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim MK, Barnes JL, Osorio EY, Anstead GM, Jimenez F, Osterholzer JJ, Travi BL, Ahuja SS, White AC Jr, Melby PC. 2014. Deficiency of lymph node-resident dendritic cells (DCs) and dysregulation of DC chemoattractants in a malnourished mouse model of Leishmania donovani infection. Infect Immun 82:3098–3112. doi: 10.1128/IAI.01778-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibrahim MK, Barnes JL, Anstead GM, Jimenez F, Travi BL, Peniche AG, Osorio EY, Ahuja SS, Melby PC. 2013. The malnutrition-related increase in early visceralization of Leishmania donovani is associated with a reduced number of lymph node phagocytes and altered conduit system flow. PLoS Negl Trop Dis 7:e2329. doi: 10.1371/journal.pntd.0002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green F, Heyworth B. 1980. Immunoglobulin-containing cells in jejunal mucosa of children with protein-energy malnutrition and gastroenteritis. Arch Dis Child 55:380–383. doi: 10.1136/adc.55.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reddy V, Raghuramulu N, Bhaskaram C. 1976. Secretory IgA in protein-calorie malnutrition. Arch Dis Child 51:871–874. doi: 10.1136/adc.51.11.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amaral JF, Foschetti DA, Assis FA, Menezes JS, Vaz NM, Faria AM. 2006. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplementation. Braz J Med Biol Res 39:1581–1586. doi: 10.1590/S0100-879X2006001200009. [DOI] [PubMed] [Google Scholar]
- 61.Chang SY, Cha HR, Chang JH, Ko HJ, Yang H, Malissen B, Iwata M, Kweon MN. 2010. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology 138:1468–1478, 1478.e1–1478.e6. doi: 10.1053/j.gastro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Flo J, Elias F, Massouh E, Roux ME. 1994. Impairment of B and T cell maturation in gut associated lymphoid tissues due to malnutrition during lactation. Dev Comp Immunol 18:543–555. doi: 10.1016/S0145-305X(06)80008-X. [DOI] [PubMed] [Google Scholar]
- 63.Flo J, Elias F, Benedetti R, Massouh E. 1996. Reversible effects on B and T cells of the gut-associated lymphoid tissues in rats malnourished during suckling: impaired induction of the immune response to intra-Peyer patches immunization with cholera toxin. Clin Immunol Immunopathol 80:147–154. doi: 10.1006/clin.1996.0108. [DOI] [PubMed] [Google Scholar]
- 64.Czerkinsky C, Holmgren J. 2009. Enteric vaccines for the developing world: a challenge for mucosal immunology. Mucosal Immunol 2:284–287. doi: 10.1038/mi.2009.22. [DOI] [PubMed] [Google Scholar]
- 65.Levine MM. 2010. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Coia ML, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schodel F, Ciarlet M, Neuzil KM. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 67.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schodel F, Steele AD, Neuzil KM, Ciarlet M. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 68.Perez-Schael I, Salinas B, Tomat M, Linhares AC, Guerrero ML, Ruiz-Palacios GM, Bouckenooghe A, Yarzabal JP. 2007. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis 196:537–540. doi: 10.1086/519687. [DOI] [PubMed] [Google Scholar]
- 69.Maier EA, Weage KJ, Guedes MM, Denson LA, McNeal MM, Bernstein DI, Moore SR. 2013. Protein-energy malnutrition alters IgA responses to rotavirus vaccination and infection but does not impair vaccine efficacy in mice. Vaccine 32:48–53. doi: 10.1016/j.vaccine.2013.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Netea MG, Wijmenga C, O'Neill LA. 2012. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol 13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 71.Misch EA, Hawn TR. 2008. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 72.Stettler N, Schutz Y, Whitehead R, Jequier E. 1992. Effect of malaria and fever on energy metabolism in Gambian children. Pediatr Res 31:102–106. doi: 10.1203/00006450-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Benhariz M, Goulet O, Salas J, Colomb V, Ricour C. 1997. Energy cost of fever in children on total parenteral nutrition. Clin Nutr 16:251–255. doi: 10.1016/S0261-5614(97)80037-4. [DOI] [PubMed] [Google Scholar]
- 74.Doherty JF, Golden MH, Remick DG, Griffin GE. 1994. Production of interleukin-6 and tumour necrosis factor-alpha in vitro is reduced in whole blood of severely malnourished children. Clin Sci (Lond) 86:347–351. doi: 10.1042/cs0860347. [DOI] [PubMed] [Google Scholar]
- 75.Jahoor F, Badaloo A, Reid M, Forrester T. 2008. Protein metabolism in severe childhood malnutrition. Ann Trop Paediatr 28:87–101. doi: 10.1179/146532808X302107. [DOI] [PubMed] [Google Scholar]
- 76.Reid M, Badaloo A, Forrester T, Morlese JF, Heird WC, Jahoor F. 2002. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am J Clin Nutr 76:1409–1415. [DOI] [PubMed] [Google Scholar]
- 77.Manary MJ, Yarasheski KE, Berger R, Abrams ET, Hart CA, Broadhead RL. 2004. Whole-body leucine kinetics and the acute phase response during acute infection in marasmic Malawian children. Pediatr Res 55:940–946. doi: 10.1203/01.pdr.0000127017.44938.6d. [DOI] [PubMed] [Google Scholar]
- 78.Page AL, de Rekeneire N, Sayadi S, Aberrane S, Janssens AC, Dehoux M, Baron E. 2014. Diagnostic and prognostic value of procalcitonin and C-reactive protein in malnourished children. Pediatrics 133:e363–e370. doi: 10.1542/peds.2013-2112. [DOI] [PubMed] [Google Scholar]
- 79.Sauerwein RW, Mulder JA, Mulder L, Lowe B, Peshu N, Demacker PN, van der Meer JW, Marsh K. 1997. Inflammatory mediators in children with protein-energy malnutrition. Am J Clin Nutr 65:1534–1539. [DOI] [PubMed] [Google Scholar]
- 80.Azevedo ZM, Luz RA, Victal SH, Kurdian B, Fonseca VM, Fitting C, Camara FP, Haeffner-Cavaillon N, Cavaillon JM, Gaspar Elsas MI, Xavier Elsas P. 2005. Increased production of tumor necrosis factor-alpha in whole blood cultures from children with primary malnutrition. Braz J Med Biol Res 38:171–183. doi: 10.1590/S0100-879X2005000200005. [DOI] [PubMed] [Google Scholar]
- 81.Weisz AJ, Manary MJ, Stephenson K, Agapova S, Manary FG, Thakwalakwa C, Shulman RJ, Manary MJ. 2012. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J Pediatr Gastroenterol Nutr 55:747–750. doi: 10.1097/MPG.0b013e3182650a4d. [DOI] [PubMed] [Google Scholar]
- 82.Lotfy OA, Saleh WA, el-Barbari M. 1998. A study of some changes of cell-mediated immunity in protein energy malnutrition. J Egypt Soc Parasitol 28:413–428. [PubMed] [Google Scholar]
- 83.Pennec Y, Garre M, Youinou P, Jouquan J, Miossec P, Boles JM, Le Menn G. 1984. Serum immunoglobulins and complement fractions in protein malnutrition. Pathol Biol (Paris) 32:49–52. (In French.) [PubMed] [Google Scholar]
- 84.Guerrant RL, Leite AM, Pinkerton R, Medeiros PH, Cavalcante PA, DeBoer M, Kosek M, Duggan C, Gewirtz A, Kagan JC, Gauthier AE, Swann J, Mayneris-Perxachs J, Bolick DT, Maier EA, Guedes MM, Moore SR, Petri WA, Havt A, Lima IF, Prata MM, Michaleckyj JC, Scharf RJ, Sturgeon C, Fasano A, Lima AA. 2016. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One 11:e0158772. doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeBoer MD, Scharf RJ, Leite AM, Ferrer A, Havt A, Pinkerton R, Lima AA, Guerrant RL. 2017. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition 33:248–253. doi: 10.1016/j.nut.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCormick BJ, Lee GO, Seidman JC, Haque R, Mondal D, Quetz J, Lima AA, Babji S, Kang G, Shrestha SK, Mason CJ, Qureshi S, Bhutta ZA, Olortegui MP, Yori PP, Samie A, Bessong P, Amour C, Mduma E, Patil CL, Guerrant RL, Lang DR, Gottlieb M, Caulfield LE, Kosek MN, MAL-ED Network. 2017. Dynamics and trends in fecal biomarkers of gut function in children from 1-24 months in the MAL-ED study. Am J Trop Med Hyg 96:465–472. doi: 10.4269/ajtmh.16-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prata MM, Havt A, Bolick DT, Pinkerton R, Lima A, Guerrant RL. 2016. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J Transl Sci 2:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nassar MF, El-Batrawy SR, Nagy NM. 2009. CD95 expression in white blood cells of malnourished infants during hospitalization and catch-up growth. East Mediterr Health J 15:574–583. [PubMed] [Google Scholar]
- 89.Morris HJ, Carrillo OV, Llaurado G, Alonso ME, Bermudez RC, Lebeque Y, Fontaine R, Soria NE, Venet G. 2011. Effect of starvation and refeeding on biochemical and immunological status of Balb/c mice: an experimental model of malnutrition. Immunopharmacol Immunotoxicol 33:438–446. doi: 10.3109/08923973.2010.531732. [DOI] [PubMed] [Google Scholar]
- 90.de Melo JF, da Costa TB, da Costa Lima TD, Chaves ME, Vayssade M, Nagel MD, de Castro CM. 2013. Long-term effects of a neonatal low-protein diet in rats on the number of macrophages in culture and the expression/production of fusion proteins. Eur J Nutr 52:1475–1482. doi: 10.1007/s00394-012-0453-y. [DOI] [PubMed] [Google Scholar]
- 91.Teshima S, Rokutan K, Takahashi M, Nikawa T, Kido Y, Kishi K. 1995. Alteration of the respiratory burst and phagocytosis of macrophages under protein malnutrition. J Nutr Sci Vitaminol (Tokyo) 41:127–137. doi: 10.3177/jnsv.41.127. [DOI] [PubMed] [Google Scholar]
- 92.Redmond HP, Leon P, Lieberman MD, Hofmann K, Shou J, Reynolds JV, Goldfine J, Johnston RB Jr, Daly JM. 1991. Impaired macrophage function in severe protein-energy malnutrition. Arch Surg 126:192–196. doi: 10.1001/archsurg.1991.01410260080011. [DOI] [PubMed] [Google Scholar]
- 93.Anstead GM, Chandrasekar B, Zhang Q, Melby PC. 2003. Multinutrient undernutrition dysregulates the resident macrophage proinflammatory cytokine network, nuclear factor-kappaB activation, and nitric oxide production. J Leukoc Biol 74:982–991. doi: 10.1189/jlb.0203064. [DOI] [PubMed] [Google Scholar]
- 94.Schaible UE, Kaufmann SH. 2007. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fock RA, Rogero MM, Vinolo MA, Curi R, Borges MC, Borelli P. 2010. Effects of protein-energy malnutrition on NF-kappaB signalling in murine peritoneal macrophages. Inflammation 33:101–109. doi: 10.1007/s10753-009-9163-x. [DOI] [PubMed] [Google Scholar]
- 96.Fock RA, Vinolo MA, de Moura Sa Rocha V, de Sa Rocha LC, Borelli P. 2007. Protein-energy malnutrition decreases the expression of TLR-4/MD-2 and CD14 receptors in peritoneal macrophages and reduces the synthesis of TNF-alpha in response to lipopolysaccharide (LPS) in mice. Cytokine 40:105–114. doi: 10.1016/j.cyto.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 97.de Oliveira DC, Hastreiter AA, Mello AS, de Oliveira Beltran JS, Oliveira Santos EW, Borelli P, Fock RA. 2014. The effects of protein malnutrition on the TNF-RI and NF-kappaB expression via the TNF-alpha signaling pathway. Cytokine 69:218–225. doi: 10.1016/j.cyto.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Nayak KC, Sethi AS, Aggarwal TD, Chadda VS, Kumar KK. 1989. Bactericidal power of neutrophils in protein calorie malnutrition. Indian J Pediatr 56:371–377. doi: 10.1007/BF02722303. [DOI] [PubMed] [Google Scholar]
- 99.Jose DG, Shelton M, Tauro GP, Belbin R, Hosking CS. 1975. Deficiency of immunological and phagocytic function in aboriginal children with protein-calorie malnutrition. Med J Aust 2:699–705. [DOI] [PubMed] [Google Scholar]
- 100.Tuck R, Burke V, Gracey M, Malajczuk A, Sunoto. 1979. Defective Candida killing in childhood malnutrition. Arch Dis Child 54:445–447. doi: 10.1136/adc.54.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Twining SS, Schulte DP, Wilson PM, Fish BL, Moulder JE. 1997. Vitamin A deficiency alters rat neutrophil function. J Nutr 127:558–565. [DOI] [PubMed] [Google Scholar]
- 102.Jimenez C, Leets I, Puche R, Anzola E, Montilla R, Parra C, Aguilera A, Garcia-Casal MN. 2010. A single dose of vitamin A improves haemoglobin concentration, retinol status and phagocytic function of neutrophils in preschool children. Br J Nutr 103:798–802. doi: 10.1017/S0007114509992765. [DOI] [PubMed] [Google Scholar]
- 103.Abe I, Shirato K, Hashizume Y, Mitsuhashi R, Kobayashi A, Shiono C, Sato S, Tachiyashiki K, Imaizumi K. 2013. Folate-deficiency induced cell-specific changes in the distribution of lymphocytes and granulocytes in rats. Environ Health Prev Med 18:78–84. doi: 10.1007/s12199-012-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Someya Y, Tanihata J, Sato S, Kawano F, Shirato K, Sugiyama M, Kawashima Y, Nomura S, Tachiyashiki K, Imaizumi K. 2009. Zinc-deficiency induced changes in the distribution of rat white blood cells. J Nutr Sci Vitaminol (Tokyo) 55:162–169. doi: 10.3177/jnsv.55.162. [DOI] [PubMed] [Google Scholar]
- 105.Sakakibara Y, Sato S, Kawashima Y, Someya Y, Shirato K, Tachiyashiki K, Imaizumi K. 2011. Different recovery responses from dietary zinc-deficiency in the distribution of rat granulocytes. J Nutr Sci Vitaminol (Tokyo) 57:197–201. doi: 10.3177/jnsv.57.197. [DOI] [PubMed] [Google Scholar]
- 106.Vissers MC, Wilkie RP. 2007. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha. J Leukoc Biol 81:1236–1244. doi: 10.1189/jlb.0806541. [DOI] [PubMed] [Google Scholar]
- 107.Rikimaru T, Taniguchi K, Yartey JE, Kennedy DO, Nkrumah FK. 1998. Humoral and cell-mediated immunity in malnourished children in Ghana. Eur J Clin Nutr 52:344–350. doi: 10.1038/sj.ejcn.1600560. [DOI] [PubMed] [Google Scholar]
- 108.Salimonu LS, Ojo-Amaize E, Williams AI, Johnson AO, Cooke AR, Adekunle FA, Alm GV, Wigzell H. 1982. Depressed natural killer cell activity in children with protein-calorie malnutrition. Clin Immunol Immunopathol 24:1–7. doi: 10.1016/0090-1229(82)90082-4. [DOI] [PubMed] [Google Scholar]
- 109.Salimonu LS, Ojo-Amaize E, Johnson AO, Laditan AA, Akinwolere OA, Wigzell H. 1983. Depressed natural killer cell activity in children with protein-calorie malnutrition. II. Correction of the impaired activity after nutritional recovery. Cell Immunol 82:210–215. doi: 10.1016/0008-8749(83)90154-5. [DOI] [PubMed] [Google Scholar]
- 110.Ritz BW, Aktan I, Nogusa S, Gardner EM. 2008. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr 138:2269–2275. doi: 10.3945/jn.108.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowman TA, Goonewardene IM, Pasatiempo AM, Ross AC, Taylor CE. 1990. Vitamin A deficiency decreases natural killer cell activity and interferon production in rats. J Nutr 120:1264–1273. [DOI] [PubMed] [Google Scholar]
- 112.Kim YI, Hayek M, Mason JB, Meydani SN. 2002. Severe folate deficiency impairs natural killer cell-mediated cytotoxicity in rats. J Nutr 132:1361–1367. [DOI] [PubMed] [Google Scholar]
- 113.Hillyer L, Whitley C, Olver A, Webster M, Steevels T, Woodward B. 2008. Adoptively transferred dendritic cells restore primary cell-mediated inflammatory competence to acutely malnourished weanling mice. Am J Pathol 172:378–385. doi: 10.2353/ajpath.2008.070456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, Hillyer LM, Woodward BD. 2002. The capacity of noninflammatory (steady-state) dendritic cells to present antigen in the primary response is preserved in acutely protein- or energy-deficient weanling mice. J Nutr 132:2748–2756. [DOI] [PubMed] [Google Scholar]
- 115.Abe M, Akbar F, Matsuura B, Horiike N, Onji M. 2003. Defective antigen-presenting capacity of murine dendritic cells during starvation. Nutrition 19:265–269. doi: 10.1016/S0899-9007(02)00854-7. [DOI] [PubMed] [Google Scholar]
- 116.Niiya T, Akbar SM, Yoshida O, Miyake T, Matsuura B, Murakami H, Abe M, Hiasa Y, Onji M. 2007. Impaired dendritic cell function resulting from chronic undernutrition disrupts the antigen-specific immune response in mice. J Nutr 137:671–675. [DOI] [PubMed] [Google Scholar]
- 117.Beijer MR, Kraal G, den Haan JM. 2014. Vitamin A and dendritic cell differentiation. Immunology 142:39–45. doi: 10.1111/imm.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, Prentice AM. 2009. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr 139:2154S–2218S. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- 119.Prendergast AJ. 2015. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci 370:20140141. doi: 10.1098/rstb.2014.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Najera O, Gonzalez C, Toledo G, Lopez L, Cortes E, Betancourt M, Ortiz R. 2001. CD45RA and CD45RO isoforms in infected malnourished and infected well-nourished children. Clin Exp Immunol 126:461–465. doi: 10.1046/j.1365-2249.2001.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Najera O, Gonzalez C, Cortes E, Toledo G, Ortiz R. 2007. Effector T lymphocytes in well-nourished and malnourished infected children. Clin Exp Immunol 148:501–506. doi: 10.1111/j.1365-2249.2007.03369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Azevedo Paiva A, Rondo PH, Rehder Vaz-de-Lima L, de Freitas Oliveira C, Ueda M, Goncalves-Carvalho C, Reinaldo LG. 2010. The impact of vitamin A supplementation on the immune system of vitamin A-deficient children. Int J Vitam Nutr Res 80:188–196. doi: 10.1024/0300-9831/a000017. [DOI] [PubMed] [Google Scholar]
- 123.Badr G, Sayed D, Alhazza IM, Elsayh KI, Ahmed EA, Alwasel SH. 2011. T lymphocytes from malnourished infants are short-lived and dysfunctional cells. Immunobiology 216:309–315. doi: 10.1016/j.imbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez-Torres C, Gonzalez-Martinez H, Miliar A, Najera O, Graniel J, Firo V, Alvarez C, Bonilla E, Rodriguez L. 2013. Effect of malnutrition on the expression of cytokines involved in Th1 cell differentiation. Nutrients 5:579–593. doi: 10.3390/nu5020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gonzalez-Martinez H, Rodriguez L, Najera O, Cruz D, Miliar A, Dominguez A, Sanchez F, Graniel J, Gonzalez-Torres MC. 2008. Expression of cytokine mRNA in lymphocytes of malnourished children. J Clin Immunol 28:593–599. doi: 10.1007/s10875-008-9204-5. [DOI] [PubMed] [Google Scholar]
- 126.Taylor AK, Cao W, Vora KP, De La Cruz J, Shieh WJ, Zaki SR, Katz JM, Sambhara S, Gangappa S. 2013. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis 207:501–510. doi: 10.1093/infdis/jis527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Procaccini C, De Rosa V, Galgani M, Carbone F, Cassano S, Greco D, Qian K, Auvinen P, Cali G, Stallone G, Formisano L, La Cava A, Matarese G. 2012. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol 189:2941–2953. doi: 10.4049/jimmunol.1200935. [DOI] [PubMed] [Google Scholar]
- 128.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. 2014. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sakai T, Mitsuya K, Kogiso M, Ono K, Komatsu T, Yamamoto S. 2006. Protein deficiency impairs DNA vaccine-induced antigen-specific T cell but not B cell response in C57BL/6 mice. J Nutr Sci Vitaminol (Tokyo) 52:376–382. doi: 10.3177/jnsv.52.376. [DOI] [PubMed] [Google Scholar]
- 130.Steevels TA, Hillyer LM, Monk JM, Fisher ME, Woodward BD. 2010. Effector/memory T cells of the weanling mouse exhibit type 2 cytokine polarization in vitro and in vivo in the advanced stages of acute energy deficit. J Nutr Biochem 21:504–511. doi: 10.1016/j.jnutbio.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 131.Chatraw JH, Wherry EJ, Ahmed R, Kapasi ZF. 2008. Diminished primary CD8 T cell response to viral infection during protein energy malnutrition in mice is due to changes in microenvironment and low numbers of viral-specific CD8 T cell precursors. J Nutr 138:806–812. [DOI] [PubMed] [Google Scholar]
- 132.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. 2008. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol 180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cham CM, Gajewski TF. 2005. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol 174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 134.Neyestani TR, Woodward B. 2005. Blood concentrations of Th2-type immunoglobulins are selectively increased in weanling mice subjected to acute malnutrition. Exp Biol Med (Maywood) 230:128–134. [DOI] [PubMed] [Google Scholar]
- 135.Shimosato T, Tomida K, Otani H. 2011. Effect of Lactobacillus pentosus ONRIC b0240 on intestinal IgA production in mice fed differing levels of protein. J Agric Food Chem 59:2646–2651. doi: 10.1021/jf104240d. [DOI] [PubMed] [Google Scholar]
- 136.Chun TY, Carman JA, Hayes CE. 1992. Retinoid repletion of vitamin A-deficient mice restores IgG responses. J Nutr 122:1062–1069. [DOI] [PubMed] [Google Scholar]
- 137.Bjersing JL, Telemo E, Dahlgren U, Hanson LA. 2002. Loss of ileal IgA+ plasma cells and of CD4+ lymphocytes in ileal Peyer's patches of vitamin A deficient rats. Clin Exp Immunol 130:404–408. doi: 10.1046/j.1365-2249.2002.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.King LE, Osati-Ashtiani F, Fraker PJ. 1995. Depletion of cells of the B lineage in the bone marrow of zinc-deficient mice. Immunology 85:69–73. [PMC free article] [PubMed] [Google Scholar]
- 139.Michaelsen KF, Hoppe C, Roos N, Kaestel P, Stougaard M, Lauritzen L, Molgaard C, Girma T, Friis H. 2009. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr Bull 30:S343–S404. doi: 10.1177/15648265090303S303. [DOI] [PubMed] [Google Scholar]
- 140.Calder PC. 2013. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc 72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 141.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. 2004. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol 173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 142.Fan YY, McMurray DN, Ly LH, Chapkin RS. 2003. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr 133:1913–1920. [DOI] [PubMed] [Google Scholar]
- 143.Damsgaard CT, Lauritzen L, Kjaer TM, Holm PM, Fruekilde MB, Michaelsen KF, Frokiaer H. 2007. Fish oil supplementation modulates immune function in healthy infants. J Nutr 137:1031–1036. [DOI] [PubMed] [Google Scholar]
- 144.Lauritzen L, Kjaer TM, Fruekilde MB, Michaelsen KF, Frokiaer H. 2005. Fish oil supplementation of lactating mothers affects cytokine production in 2 1/2-year-old children. Lipids 40:669–676. doi: 10.1007/s11745-005-1429-6. [DOI] [PubMed] [Google Scholar]
- 145.Brenna JT, Akomo P, Bahwere P, Berkley JA, Calder PC, Jones KD, Liu L, Manary M, Trehan I, Briend A. 2015. Balancing omega-6 and omega-3 fatty acids in ready-to-use therapeutic foods (RUTF). BMC Med 13:117. doi: 10.1186/s12916-015-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, Holzgreve W, Lapillonne A, Putet G, Secher NJ, Symonds M, Szajewska H, Willatts P, Uauy R, World Association of Perinatal Medicine Dietary Guidelines Working Group. 2008. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36:5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- 147.Jones K, Ali R, Khasira MA, Odera D, West AL, Koster G, Akomo P, Talbert AW, Goss VM, Ngari M, Thitiri J, Ndoro S, Knight MA, Omollo K, Ndungu A, Mulongo MM, Bahwere P, Fegan G, Warner JO, Postle AD, Collins S, Calder PC, Berkley JA. 2015. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: a randomized controlled trial. BMC Med 13:93. doi: 10.1186/s12916-015-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hsieh JC, Liu L, Zeilani M, Ickes S, Trehan I, Maleta K, Craig C, Thakwalakwa C, Singh L, Brenna JT, Manary MJ. 2015. High oleic ready-to-use therapeutic food maintains docosahexaenoic acid status in severe malnutrition: a randomized, blinded trial. J Pediatr Gastroenterol Nutr 61:138–143. doi: 10.1097/MPG.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bonilla DL, Ly LH, Fan YY, Chapkin RS, McMurray DN. 2010. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to Mycobacterium tuberculosis. PLoS One 5:e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wintergerst ES, Maggini S, Hornig DH. 2007. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab 51:301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 151.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. 2009. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr 12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 152.Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs BA. 2013. Control of iron deficiency anemia in low- and middle-income countries. Blood 121:2607–2617. doi: 10.1182/blood-2012-09-453522. [DOI] [PubMed] [Google Scholar]
- 153.Prasad AS. 2009. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 154.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 155.Cherayil BJ. 2010. Iron and immunity: immunological consequences of iron deficiency and overload. Arch Immunol Ther Exp (Warsz) 58:407–415. doi: 10.1007/s00005-010-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bubici C, Papa S, Dean K, Franzoso G. 2006. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene 25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 157.Chen L, Xiong S, She H, Lin SW, Wang J, Tsukamoto H. 2007. Iron causes interactions of TAK1, p21ras, and phosphatidylinositol 3-kinase in caveolae to activate IkappaB kinase in hepatic macrophages. J Biol Chem 282:5582–5588. doi: 10.1074/jbc.M609273200. [DOI] [PubMed] [Google Scholar]
- 158.Nizet V, Johnson RS. 2009. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bergman M, Bessler H, Salman H, Siomin D, Straussberg R, Djaldetti M. 2004. In vitro cytokine production in patients with iron deficiency anemia. Clin Immunol 113:340–344. doi: 10.1016/j.clim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 160.Kuvibidila SR, Gardner R, Velez M, Yu L. 2010. Iron deficiency, but not underfeeding reduces the secretion of interferon-gamma by mitogen-activated murine spleen cells. Cytokine 52:230–237. doi: 10.1016/j.cyto.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 161.Ned RM, Swat W, Andrews NC. 2003. Transferrin receptor 1 is differentially required in lymphocyte development. Blood 102:3711–3718. doi: 10.1182/blood-2003-04-1086. [DOI] [PubMed] [Google Scholar]
- 162.Vanoaica L, Richman L, Jaworski M, Darshan D, Luther SA, Kuhn LC. 2014. Conditional deletion of ferritin H in mice reduces B and T lymphocyte populations. PLoS One 9:e89270. doi: 10.1371/journal.pone.0089270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. 2008. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol 181:2723–2731. doi: 10.4049/jimmunol.181.4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kasvosve I, Debebe Z, Nekhai S, Gordeuk VR. 2010. Ferroportin (SLC40A1) Q248H mutation is associated with lower circulating plasma tumor necrosis factor-alpha and macrophage migration inhibitory factor concentrations in African children. Clin Chim Acta 411:1248–1252. doi: 10.1016/j.cca.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. 2006. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 167.Oppenheimer SJ. 2001. Iron and its relation to immunity and infectious disease. J Nutr 131:616S–633S; discussion 633S–635S. [DOI] [PubMed] [Google Scholar]
- 168.Baker HM, Baker EN. 2012. A structural perspective on lactoferrin function. Biochem Cell Biol 90:320–328. doi: 10.1139/o11-071. [DOI] [PubMed] [Google Scholar]
- 169.Lindenmayer GW, Stoltzfus RJ, Prendergast AJ. 2014. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv Nutr 5:1–6. doi: 10.3945/an.113.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gautam B, Deb K, Banerjee M, Ali MS, Akhter S, Shahidullah SM, Hoque MR. 2008. Serum zinc and copper level in children with protein energy malnutrition. Mymensingh Med J 17:S12–S15. [PubMed] [Google Scholar]
- 171.Jain A, Varma M, Agrawal BK, Jadhav AA. 2008. Serum zinc and malondialdehyde concentrations and their relation to total antioxidant capacity in protein energy malnutrition. J Nutr Sci Vitaminol (Tokyo) 54:392–395. doi: 10.3177/jnsv.54.392. [DOI] [PubMed] [Google Scholar]
- 172.Amesty-Valbuena A, Pereira-Medero N, Nunez-Gonzalez JR, Garcia D, de Villaroel MV, Granadillo V, Manzanilla J, Fernandez D. 2006. Serum levels of Zn in children with different degress of nutritional deficiency. Invest Clin 47:349–359. (In Spanish.) [PubMed] [Google Scholar]
- 173.Filteau SM, Woodward B. 1982. The effect of severe protein deficiency on serum zinc concentration of mice fed a requirement level or a very high level of dietary zinc. J Nutr 112:1974–1977. [DOI] [PubMed] [Google Scholar]
- 174.Cunningham-Rundles S, McNeeley DF, Moon A. 2005. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 115:1119–1128; quiz 1129. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 175.Prasad AS. 2007. Zinc: mechanisms of host defense. J Nutr 137:1345–1349. [DOI] [PubMed] [Google Scholar]
- 176.Gastinel LN, Dardenne M, Pleau JM, Bach JF. 1984. Studies on the zinc binding site to the serum thymic factor. Biochim Biophys Acta 797:147–155. doi: 10.1016/0304-4165(84)90116-8. [DOI] [PubMed] [Google Scholar]
- 177.Chen J, Qu N, Xia YM. 2005. Effect of zinc on thymulin level in mice. Wei Sheng Yan Jiu 34:430–432. (In Chinese.) [PubMed] [Google Scholar]
- 178.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. 2006. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 179.Bao B, Prasad AS, Beck FW, Bao GW, Singh T, Ali S, Sarkar FH. 2011. Intracellular free zinc up-regulates IFN-gamma and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. Biochem Biophys Res Commun 407:703–707. doi: 10.1016/j.bbrc.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Honscheid A, Dubben S, Rink L, Haase H. 2012. Zinc differentially regulates mitogen-activated protein kinases in human T cells. J Nutr Biochem 23:18–26. doi: 10.1016/j.jnutbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 181.Mayer LS, Uciechowski P, Meyer S, Schwerdtle T, Rink L, Haase H. 2014. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics 6:1288–1295. doi: 10.1039/c4mt00051j. [DOI] [PubMed] [Google Scholar]
- 182.Brough D, Pelegrin P, Rothwell NJ. 2009. Pannexin-1-dependent caspase-1 activation and secretion of IL-1beta is regulated by zinc. Eur J Immunol 39:352–358. doi: 10.1002/eji.200838843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Prasad AS. 2008. Zinc in human health: effect of zinc on immune cells. Mol Med 14:353–357. doi: 10.1007/s00894-008-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gavella M, Lipovac V, Car A. 2000. In vitro effect of zinc on superoxide anion production by polymorphonuclear leukocytes of diabetic patients. Acta Diabetol 37:135–137. doi: 10.1007/s005920070016. [DOI] [PubMed] [Google Scholar]
- 185.Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. 2008. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr 138:1664–1670. [DOI] [PubMed] [Google Scholar]
- 186.Duggal S, Chugh TD, Duggal AK. 2012. HIV and malnutrition: effects on immune system. Clin Dev Immunol 2012:784740. doi: 10.1155/2012/784740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Foster M, Samman S. 2012. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 4:676–694. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Das JK, Kumar R, Salam RA, Bhutta ZA. 2013. Systematic review of zinc fortification trials. Ann Nutr Metab 62(Suppl 1):S44–S56. doi: 10.1159/000348262. [DOI] [PubMed] [Google Scholar]
- 189.Garenne M, Becher H, Ye Y, Kouyate B, Muller O. 2007. Sex-specific responses to zinc supplementation in Nouna, Burkina Faso. J Pediatr Gastroenterol Nutr 44:619–628. doi: 10.1097/MPG.0b013e31802c695e. [DOI] [PubMed] [Google Scholar]
- 190.Bhandari N, Bahl R, Taneja S, Strand T, Molbak K, Ulvik RJ, Sommerfelt H, Bhan MK. 2002. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 109:e86. doi: 10.1542/peds.109.6.e86. [DOI] [PubMed] [Google Scholar]
- 191.Makonnen B, Venter A, Joubert G. 2003. A randomized controlled study of the impact of dietary zinc supplementation in the management of children with protein-energy malnutrition in Lesotho. II. Special investigations. J Trop Pediatr 49:353–360. doi: 10.1093/tropej/49.6.353. [DOI] [PubMed] [Google Scholar]
- 192.Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, Rosado JL, Roy SK, Ruel M, Sazawal S, Shankar A. 2000. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 72:1516–1522. [DOI] [PubMed] [Google Scholar]
- 193.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, Rosado JL, Roy SK, Ruel M, Sazawal S, Shankar A. 1999. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. J Pediatr 135:689–697. doi: 10.1016/S0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 194.Muller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, Gbangou A, Kouyate B, Garenne M. 2001. Effect of zinc supplementation on malaria and other causes of morbidity in West African children: randomised double blind placebo controlled trial. BMJ 322:1567. doi: 10.1136/bmj.322.7302.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Medeiros P, Bolick DT, Roche JK, Noronha F, Pinheiro C, Kolling GL, Lima A, Guerrant RL. 2013. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence 4:624–633. doi: 10.4161/viru.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sempertegui F, Estrella B, Vallejo W, Tapia L, Herrera D, Moscoso F, Ceron G, Griffiths JK, Hamer DH. 2003. Selenium serum concentrations in malnourished Ecuadorian children: a case-control study. Int J Vitam Nutr Res 73:181–186. doi: 10.1024/0300-9831.73.3.181. [DOI] [PubMed] [Google Scholar]
- 197.Chadha VD, Sood A, Dhawan DK. 2014. Sodium selenite enhances thyroid uptake of iodine-131 and regulates thyroid function in rats. Hell J Nucl Med 17:27–30. [DOI] [PubMed] [Google Scholar]
- 198.Kohrle J. 2013. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes 20:441–448. doi: 10.1097/01.med.0000433066.24541.88. [DOI] [PubMed] [Google Scholar]
- 199.Joshi D, Mittal DK, Shukla S, Srivastav AK, Srivastav SK. 2014. N-Acetyl cysteine and selenium protects mercuric chloride-induced oxidative stress and antioxidant defense system in liver and kidney of rats: a histopathological approach. J Trace Elem Med Biol 28:218–226. doi: 10.1016/j.jtemb.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 200.Vunta H, Belda BJ, Arner RJ, Channa Reddy C, Vanden Heuvel JP, Sandeep Prabhu K. 2008. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol Nutr Food Res 52:1316–1323. doi: 10.1002/mnfr.200700346. [DOI] [PubMed] [Google Scholar]
- 201.Gandhi UH, Kaushal N, Ravindra KC, Hegde S, Nelson SM, Narayan V, Vunta H, Paulson RF, Prabhu KS. 2011. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J Biol Chem 286:27471–27482. doi: 10.1074/jbc.M111.260547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carlson BA, Yoo MH, Shrimali RK, Irons R, Gladyshev VN, Hatfield DL, Park JM. 2010. Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc 69:300–310. doi: 10.1017/S002966511000176X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shrimali RK, Irons RD, Carlson BA, Sano Y, Gladyshev VN, Park JM, Hatfield DL. 2008. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J Biol Chem 283:20181–20185. doi: 10.1074/jbc.M802559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR. 2011. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 186:2127–2137. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huang Z, Rose AH, Hoffmann PR. 2012. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR. 2010. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr 140:1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kawai K, Meydani SN, Urassa W, Wu D, Mugusi FM, Saathoff E, Bosch RJ, Villamor E, Spiegelman D, Fawzi WW. 2014. Micronutrient supplementation and T cell-mediated immune responses in patients with tuberculosis in Tanzania. Epidemiol Infect 142:1505–1509. doi: 10.1017/S0950268813002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, Meydani SN, Fawzi WW. 2008. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis 197:1499–1505. doi: 10.1086/587846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Iannotti LL, Trehan I, Manary MJ. 2013. Review of the safety and efficacy of vitamin A supplementation in the treatment of children with severe acute malnutrition. Nutr J 12:125. doi: 10.1186/1475-2891-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim CH. 2011. Retinoic acid, immunity, and inflammation. Vitam Horm 86:83–101. doi: 10.1016/B978-0-12-386960-9.00004-6. [DOI] [PubMed] [Google Scholar]
- 211.Mora JR, Iwata M, von Andrian UH. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labao-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O'Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H. 2014. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. 2003. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 23:229–261. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 214.Gray T, Koo JS, Nettesheim P. 2001. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology 160:35–46. doi: 10.1016/S0300-483X(00)00455-8. [DOI] [PubMed] [Google Scholar]
- 215.Rudraraju R, Jones BG, Surman SL, Sealy RE, Thomas PG, Hurwitz JL. 2014. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One 9:e86554. doi: 10.1371/journal.pone.0086554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Seo GY, Jang YS, Kim HA, Lee MR, Park MH, Park SR, Lee JM, Choe J, Kim PH. 2013. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-beta1 to enhance the overall IgA response. J Leukoc Biol 94:325–335. doi: 10.1189/jlb.0313128. [DOI] [PubMed] [Google Scholar]
- 217.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF Jr, Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. 2014. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ross AC. 2012. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr 96:1166S–1172S. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, Ferreyra GA, Leonardi AJ, Borman ZA, Wang J, Palmer DC, Wilhelm C, Cai R, Sun J, Napoli JL, Danner RL, Gattinoni L, Belkaid Y, Restifo NP. 2013. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med 210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N, Kuge Y, Tamaki N, Nakagawa K, Hamada J, Moriuchi T, Kobayashi M. 2007. All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J Immunol 179:4616–4625. doi: 10.4049/jimmunol.179.7.4616. [DOI] [PubMed] [Google Scholar]
- 221.Luo XM, Ross AC. 2005. Physiological and receptor-selective retinoids modulate interferon gamma signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1. J Biol Chem 280:36228–36236. doi: 10.1074/jbc.M505749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Raverdeau M, Mills KH. 2014. Modulation of T cell and innate immune responses by retinoic acid. J Immunol 192:2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 223.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 224.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. 2014. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol 8:265–278. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 225.Menning A, Loddenkemper C, Westendorf AM, Szilagyi B, Buer J, Siewert C, Hamann A, Huehn J. 2010. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol 40:2539–2548. doi: 10.1002/eji.200939938. [DOI] [PubMed] [Google Scholar]
- 226.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 227.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. 2011. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Allie SR, Zhang W, Tsai CY, Noelle RJ, Usherwood EJ. 2013. Critical role for all-trans retinoic acid for optimal effector and effector memory CD8 T cell differentiation. J Immunol 190:2178–2187. doi: 10.4049/jimmunol.1201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. 2011. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol 85:8316–8327. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ma Y, Chen Q, Ross AC. 2005. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol 174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stephensen CB, Jiang X, Freytag T. 2004. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr 134:2660–2666. [DOI] [PubMed] [Google Scholar]
- 233.Iwata M, Eshima Y, Kagechika H. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 234.Yamada H, Mizuno S, Ross AC, Sugawara I. 2007. Retinoic acid therapy attenuates the severity of tuberculosis while altering lymphocyte and macrophage numbers and cytokine expression in rats infected with Mycobacterium tuberculosis. J Nutr 137:2696–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. 2009. Markers of innate immune function are associated with vitamin A stores in men. J Nutr 139:377–385. doi: 10.3945/jn.108.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dawson HD, Li NQ, DeCicco KL, Nibert JA, Ross AC. 1999. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr 129:1510–1517. [DOI] [PubMed] [Google Scholar]
- 237.Duriancik DM, Hoag KA. 2010. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8(+)Gr-1(+) memory T lymphocytes in C57BL/6J mice. Cell Immunol 265:156–163. doi: 10.1016/j.cellimm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu X, Li Y, Wang Y, Wang Q, Li X, Bi Y, Liu L, Wei X, Li T, Chen J. 2014. Gestational vitamin A deficiency reduces the intestinal immune response by decreasing the number of immune cells in rat offspring. Nutrition 30:350–357. doi: 10.1016/j.nut.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 239.Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. 2010. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol 184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- 240.Ross AC, Chen Q, Ma Y. 2009. Augmentation of antibody responses by retinoic acid and costimulatory molecules. Semin Immunol 21:42–50. doi: 10.1016/j.smim.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sudfeld CR, Navar AM, Halsey NA. 2010. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol 39(Suppl 1):i48–i55. doi: 10.1093/ije/dyq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mayo-Wilson E, Imdad A, Herzer K, Bhutta ZA. 2013. Vitamin A supplementation in Indian children. Lancet 382:594. doi: 10.1016/S0140-6736(13)61739-0. [DOI] [PubMed] [Google Scholar]
- 243.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. 2011. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. 2010. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev 2010:CD008524. doi: 10.1002/14651858.CD008524.pub2. [DOI] [PubMed] [Google Scholar]
- 245.Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D, DEVTA Team. 2013. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 381:1469–1477. doi: 10.1016/S0140-6736(12)62125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ravindran RD, Vashist P, Gupta SK, Young IS, Maraini G, Camparini M, Jayanthi R, John N, Fitzpatrick KE, Chakravarthy U, Ravilla TD, Fletcher AE. 2011. Prevalence and risk factors for vitamin C deficiency in north and south India: a two centre population based study in people aged 60 years and over. PLoS One 6:e28588. doi: 10.1371/journal.pone.0028588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cahill L, Corey PN, El-Sohemy A. 2009. Vitamin C deficiency in a population of young Canadian adults. Am J Epidemiol 170:464–471. doi: 10.1093/aje/kwp156. [DOI] [PubMed] [Google Scholar]
- 248.Schleicher RL, Carroll MD, Ford ES, Lacher DA. 2009. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 249.Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S. 2014. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Chem 14:444–452. doi: 10.2174/1389557514666140428112602. [DOI] [PubMed] [Google Scholar]
- 250.Hartel C, Strunk T, Bucsky P, Schultz C. 2004. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine 27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 251.Hartel C, Puzik A, Gopel W, Temming P, Bucsky P, Schultz C. 2007. Immunomodulatory effect of vitamin C on intracytoplasmic cytokine production in neonatal cord blood cells. Neonatology 91:54–60. doi: 10.1159/000096972. [DOI] [PubMed] [Google Scholar]
- 252.Perez-Cruz I, Carcamo JM, Golde DW. 2003. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood 102:336–343. doi: 10.1182/blood-2002-11-3559. [DOI] [PubMed] [Google Scholar]
- 253.Mohammed BM, Fisher BJ, Kraskauskas D, Farkas D, Brophy DF, Fowler AA III, Natarajan R. 2013. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients 5:3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Woo A, Kim JH, Jeong YJ, Maeng HG, Lee YT, Kang JS, Lee WJ, Hwang YI. 2010. Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat Cell Biol 43:25–35. doi: 10.5115/acb.2010.43.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kim HW, Cho SI, Bae S, Kim H, Kim Y, Hwang YI, Kang JS, Lee WJ. 2012. Vitamin C up-regulates expression of CD80, CD86 and MHC class II on dendritic cell line, DC-1 via the activation of p38 MAPK. Immune Netw 12:277–283. doi: 10.4110/in.2012.12.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jeong YJ, Kim JH, Hong JM, Kang JS, Kim HR, Lee WJ, Hwang YI. 2014. Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology 219:554–564. doi: 10.1016/j.imbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 257.Mohammed BM, Fisher BJ, Huynh QK, Wijesinghe DS, Chalfant CE, Brophy DF, Fowler AA III, Natarajan R. 2014. Resolution of sterile inflammation: role for vitamin C. Mediators Inflamm 2014:173403. doi: 10.1155/2014/173403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA III, Natarajan R. 2012. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol 303:L20–L32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 259.Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA III, Natarajan R. 2011. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med 39:1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- 260.Fowler AA III, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Medical Respiratory Intensive Care Unit Nursing, Fisher BJ, Natarajan R. 2014. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, Kritchevsky SB, Cauley JA, Tanaka T, Ferrucci L, Bandinelli S, Patel KV, Hagstrom E, Michaelsson K, Melhus H, Wang T, Wolf M, Psaty BM, Siscovick D, Kestenbaum B. 2012. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 308:1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lips P. 2010. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 263.Holick MF. 2006. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 264.Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 265.Lagishetty V, Liu NQ, Hewison M. 2011. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol 347:97–105. doi: 10.1016/j.mce.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.White JH. 2012. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch Biochem Biophys 523:58–63. doi: 10.1016/j.abb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 267.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 268.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. 2009. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. 2006. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res 21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 270.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zugel U, Cheng G, Jo EK, Bloom BR, Modlin RL. 2011. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med 3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. 2009. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 272.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. 2010. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol 12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Verway M, Bouttier M, Wang TT, Carrier M, Calderon M, An BS, Devemy E, McIntosh F, Divangahi M, Behr MA, White JH. 2013. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog 9:e1003407. doi: 10.1371/journal.ppat.1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gombart AF, Borregaard N, Koeffler HP. 2005. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 275.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. 2009. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One 4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ. 2007. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 277.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. 2004. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 278.Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, Bangani N, Tsekela R, Bashe L, de Azevedo V, Caldwell J, Venton TR, Timms PM, Wilkinson KA, Wilkinson RJ. 2011. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A 108:19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wingfield T, Schumacher SG, Sandhu G, Tovar MA, Zevallos K, Baldwin MR, Montoya R, Ramos ES, Jongkaewwattana C, Lewis JJ, Gilman RH, Friedland JS, Evans CA. 2014. The seasonality of tuberculosis, sunlight, vitamin D, and household crowding. J Infect Dis 210:774–783. doi: 10.1093/infdis/jiu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Martineau AR. 2012. Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosis. Proc Nutr Soc 71:84–89. doi: 10.1017/S0029665111003326. [DOI] [PubMed] [Google Scholar]
- 281.Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, Pienaar SM, Skolimowska KH, Rocha MA, Rolla VC, Levin M, Davidson RN, Bremner SA, Griffiths CJ, Eley BS, Bonecini-Almeida MG, Wilkinson RJ. 2010. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J 35:1106–1112. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. 2000. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 283.Mehta S, Mugusi FM, Bosch RJ, Aboud S, Urassa W, Villamor E, Fawzi WW. 2013. Vitamin D status and TB treatment outcomes in adult patients in Tanzania: a cohort study. BMJ Open 3:e003703. doi: 10.1136/bmjopen-2013-003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. 2007. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol 103:793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 285.Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, Timms PM, Venton TR, Bothamley GH, Packe GE, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Mein CA, Bhaw-Rosun L, Nuamah R, Young DB, Drobniewski FA, Griffiths CJ, Martineau AR. 2012. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A 109:15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ. 2011. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. 2007. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 288.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. 2009. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 289.Nursyam EW, Amin Z, Rumende CM. 2006. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones 38:3–5. [PubMed] [Google Scholar]
- 290.Barnes PF, Leedom JM, Chan LS, Wong SF, Shah J, Vachon LA, Overturf GD, Modlin RL. 1988. Predictors of short-term prognosis in patients with pulmonary tuberculosis. J Infect Dis 158:366–371. doi: 10.1093/infdis/158.2.366. [DOI] [PubMed] [Google Scholar]
- 291.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. 2013. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT study [supplementary cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis. BMC Infect Dis 13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, Michail RR. 1998. Vitamin D administration to tuberculous children and its value. Boll Chim Farm 137:157–164. [PubMed] [Google Scholar]
- 293.Arnedo-Pena A, Juan-Cerdan JV, Romeu-Garcia A, Garcia-Ferrer D, Holguin-Gomez R, Iborra-Millet J, Gil-Fortuno M, Gomila-Sard B, Roach-Poblete F. 2015. Vitamin D status and incidence of tuberculosis among contacts of pulmonary tuberculosis patients. Int J Tuberc Lung Dis 19:65–69. doi: 10.5588/ijtld.14.0348. [DOI] [PubMed] [Google Scholar]
- 294.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. 2010. Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis 16:853–855. doi: 10.3201/eid1605.091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Arnedo-Pena A, Juan-Cerdan JV, Romeu-Garcia MA, Garcia-Ferrer D, Holguin-Gomez R, Iborra-Millet J, Pardo-Serrano F. 2015. Vitamin D status and incidence of tuberculosis infection conversion in contacts of pulmonary tuberculosis patients: a prospective cohort study. Epidemiol Infect 143:1731–1741. doi: 10.1017/S0950268814002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, Batbaatar D, Sumberzul N, Holick MF, Willett WC. 2012. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr 96:391–396. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Jolliffe DA, Griffiths CJ, Martineau AR. 2013. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 136:321–329. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 298.Camargo CA Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, Sumberzul N, Rich-Edwards JW. 2012. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 130:e561–e567. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 299.Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, Walraven G, Chandramohan D. 2012. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 379:1419–1427. doi: 10.1016/S0140-6736(11)61650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Martineau AR. 2012. Bolus-dose vitamin D and prevention of childhood pneumonia. Lancet 379:1373–1375. doi: 10.1016/S0140-6736(12)60405-X. [DOI] [PubMed] [Google Scholar]
- 301.Nielsen NO, Skifte T, Andersson M, Wohlfahrt J, Soborg B, Koch A, Melbye M, Ladefoged K. 2010. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case-control study in Greenland. Br J Nutr 104:1487–1491. doi: 10.1017/S0007114510002333. [DOI] [PubMed] [Google Scholar]
- 302.Jorde R, Witham M, Janssens W, Rolighed L, Borchhardt K, de Boer IH, Grimnes G, Hutchinson MS. 2012. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis 44:126–132. doi: 10.3109/00365548.2011.621446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, Florkowski CM, Livesey JH, Camargo CA, Scragg R. 2012. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 308:1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 304.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. 2007. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 305.Lemire JM, Adams JS, Sakai R, Jordan SC. 1984. 1α,25-Dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest 74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Szeles L, Keresztes G, Torocsik D, Balajthy Z, Krenacs L, Poliska S, Steinmeyer A, Zuegel U, Pruenster M, Rot A, Nagy L. 2009. 1,25-Dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol 182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 307.Penna G, Adorini L. 2000. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 308.Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, Kaur S, Raza K, Sansom DM. 2012. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol 189:5155–5164. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kundu R, Chain BM, Coussens AK, Khoo B, Noursadeghi M. 2014. Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur J Immunol 44:1781–1790. doi: 10.1002/eji.201344157. [DOI] [PubMed] [Google Scholar]
- 310.Cantorna MT, Waddell A. 2014. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci 1317:70–75. doi: 10.1111/nyas.12408. [DOI] [PubMed] [Google Scholar]
- 311.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 2001. 1α,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 312.Aranow C. 2011. Vitamin D and the immune system. J Investig Med 59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mocanu V, Oboroceanu T, Zugun-Eloae F. 2013. Current status in vitamin D and regulatory T cells—immunological implications. Rev Med Chir Soc Med Nat Iasi 117:965–973. [PubMed] [Google Scholar]
- 314.Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, Lee SH, Kim WU, Kang I. 2012. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol 188:5276–5282. doi: 10.4049/jimmunol.1101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Villaggio B, Soldano S, Cutolo M. 2012. 1,25-Dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin Exp Rheumatol 30:934–938. [PubMed] [Google Scholar]
- 316.Barker T, Martins TB, Hill HR, Kjeldsberg CR, Dixon BM, Schneider ED, Henriksen VT, Weaver LK. 2013. Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur J Appl Physiol 113:1523–1534. doi: 10.1007/s00421-012-2582-7. [DOI] [PubMed] [Google Scholar]
- 317.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 2009. 1α,25-Dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine 45:190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 318.Spach KM, Nashold FE, Dittel BN, Hayes CE. 2006. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol 177:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 319.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. 2010. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Stoppelenburg AJ, von Hegedus JH, Huis in't Veld R, Bont L, Boes M. 2014. Defective control of vitamin D receptor-mediated epithelial STAT1 signalling predisposes to severe respiratory syncytial virus bronchiolitis. J Pathol 232:57–64. doi: 10.1002/path.4267. [DOI] [PubMed] [Google Scholar]
- 321.Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S, Kumar D, Dhole TN, Godbole MM. 2013. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr 52:1405–1415. doi: 10.1007/s00394-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 322.Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. 2013. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1alpha,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol 132:297.e3–304.e3. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 323.He X, Yan J, Zhu X, Wang Q, Pang W, Qi Z, Wang M, Luo E, Parker DM, Cantorna MT, Cui L, Cao Y. 2014. Vitamin D inhibits the occurrence of experimental cerebral malaria in mice by suppressing the host inflammatory response. J Immunol 193:1314–1323. doi: 10.4049/jimmunol.1400089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Whitcomb JP, Deagostino M, Ballentine M, Fu J, Tenniswood M, Welsh J, Cantorna M, McDowell MA. 2012. The role of vitamin D and vitamin D receptor in immunity to Leishmania major infection. J Parasitol Res 2012:134645. doi: 10.1155/2012/134645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. 2005. WHO estimates of the causes of death in children. Lancet 365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 326.Schlaudecker EP, Steinhoff MC, Moore SR. 2011. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr Opin Infect Dis 24:496–502. doi: 10.1097/QCO.0b013e328349287d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Muehlenbein MP, Hirschtick JL, Bonner JZ, Swartz AM. 2010. Toward quantifying the usage costs of human immunity: altered metabolic rates and hormone levels during acute immune activation in men. Am J Hum Biol 22:546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- 328.Victora CG, Kirkwood BR, Ashworth A, Black RE, Rogers S, Sazawal S, Campbell H, Gove S. 1999. Potential interventions for the prevention of childhood pneumonia in developing countries: improving nutrition. Am J Clin Nutr 70:309–320. [DOI] [PubMed] [Google Scholar]
- 329.Cegielski JP, McMurray DN. 2004. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 8:286–298. [PubMed] [Google Scholar]
- 330.Jaganath D, Mupere E. 2012. Childhood tuberculosis and malnutrition. J Infect Dis 206:1809–1815. doi: 10.1093/infdis/jis608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dekker LH, Mora-Plazas M, Marin C, Baylin A, Villamor E. 2010. Stunting associated with poor socioeconomic and maternal nutrition status and respiratory morbidity in Colombian schoolchildren. Food Nutr Bull 31:242–250. doi: 10.1177/156482651003100207. [DOI] [PubMed] [Google Scholar]
- 332.Bhat RY, Manjunath N. 2013. Correlates of acute lower respiratory tract infections in children under 5 years of age in India. Int J Tuberc Lung Dis 17:418–422. doi: 10.5588/ijtld.12.0117. [DOI] [PubMed] [Google Scholar]
- 333.Chantry CJ, Howard CR, Auinger P. 2006. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics 117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 334.Strand TA, Taneja S, Bhandari N, Refsum H, Ueland PM, Gjessing HK, Bahl R, Schneede J, Bhan MK, Sommerfelt H. 2007. Folate, but not vitamin B-12 status, predicts respiratory morbidity in north Indian children. Am J Clin Nutr 86:139–144. [DOI] [PubMed] [Google Scholar]
- 335.Verhagen LM, Gomez-Castellano K, Snelders E, Rivera-Olivero I, Pocaterra L, Melchers WJ, de Waard JH, Hermans PW. 2013. Respiratory infections in Enepa Amerindians are related to malnutrition and Streptococcus pneumoniae carriage. J Infect 67:273–281. doi: 10.1016/j.jinf.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Villena J, Racedo S, Aguero G, Bru E, Medina M, Alvarez S. 2005. Lactobacillus casei improves resistance to pneumococcal respiratory infection in malnourished mice. J Nutr 135:1462–1469. [DOI] [PubMed] [Google Scholar]
- 337.Herrera M, Salva S, Villena J, Barbieri N, Marranzino G, Alvarez S. 2014. Dietary supplementation with lactobacilli improves emergency granulopoiesis in protein-malnourished mice and enhances respiratory innate immune response. PLoS One 9:e90227. doi: 10.1371/journal.pone.0090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Paynter S, Ware RS, Lucero MG, Tallo V, Nohynek H, Weinstein P, Williams G, Sly PD, Simoes EA. 2014. Malnutrition: a risk factor for severe respiratory syncytial virus infection and hospitalization. Pediatr Infect Dis J 33:267–271. doi: 10.1097/INF.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 339.Okiro EA, Ngama M, Bett A, Cane PA, Medley GF, Nokes DJ. 2008. Factors associated with increased risk of progression to respiratory syncytial virus-associated pneumonia in young Kenyan children. Trop Med Int Health 13:914–926. doi: 10.1111/j.1365-3156.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, Rovers M, Bont L. 2011. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 127:e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 341.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Molbak K, Valentiner-Branth P, Lanata CF, Black RE, Childhood Malnutrition and Infection Network. 2008. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Moore SR, Lima NL, Soares AM, Oria RB, Pinkerton RC, Barrett LJ, Guerrant RL, Lima AA. 2010. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology 139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 344.Petri WA Jr, Mondal D, Peterson KM, Duggal P, Haque R. 2009. Association of malnutrition with amebiasis. Nutr Rev 67(Suppl 2):S207–S215. doi: 10.1111/j.1753-4887.2009.00242.x. [DOI] [PubMed] [Google Scholar]
- 345.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. 2010. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J Infect Dis 202:506–514. doi: 10.1086/654894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Bolick DT, Roche JK, Hontecillas R, Bassaganya-Riera J, Nataro JP, Guerrant RL. 2013. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. J Med Microbiol 62:896–905. doi: 10.1099/jmm.0.046300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yang Y, Yuan Y, Tao Y, Wang W. 2011. Effects of vitamin A deficiency on mucosal immunity and response to intestinal infection in rats. Nutrition 27:227–232. doi: 10.1016/j.nut.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 348.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-Coordinated Global Rotavirus Surveillance Network. 2012. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 349.Hickman D, Jones MK, Zhu S, Kirkpatrick E, Ostrov DA, Wang X, Ukhanova M, Sun Y, Mai V, Salemi M, Karst SM. 2014. The effect of malnutrition on norovirus infection. mBio 5:e01032-13. doi: 10.1128/mBio.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Long KZ, Garcia C, Santos JI, Rosado JL, Hertzmark E, Dupont HL, Ko G. 2007. Vitamin A supplementation has divergent effects on norovirus infections and clinical symptoms among Mexican children. J Infect Dis 196:978–985. doi: 10.1086/521195. [DOI] [PubMed] [Google Scholar]
- 351.Coutinho BP, Oria RB, Vieira CM, Sevilleja JE, Warren CA, Maciel JG, Thompson MR, Pinkerton RC, Lima AA, Guerrant RL. 2008. Cryptosporidium infection causes undernutrition and, conversely, weanling undernutrition intensifies infection. J Parasitol 94:1225–1232. doi: 10.1645/GE-1411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ignatius R, Gahutu JB, Klotz C, Steininger C, Shyirambere C, Lyng M, Musemakweri A, Aebischer T, Martus P, Harms G, Mockenhaupt FP. 2012. High prevalence of Giardia duodenalis assemblage B infection and association with underweight in Rwandan children. PLoS Negl Trop Dis 6:e1677. doi: 10.1371/journal.pntd.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Mondal D, Petri WA Jr, Sack RB, Kirkpatrick BD, Haque R. 2006. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg 100:1032–1038. doi: 10.1016/j.trstmh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 354.Coles CL, Levy A, Dagan R, Deckelbaum RJ, Fraser D. 2009. Risk factors for the initial symptomatic giardia infection in a cohort of young Arab-Bedouin children. Ann Trop Paediatr 29:291–300. doi: 10.1179/027249309X12547917869041. [DOI] [PubMed] [Google Scholar]
- 355.Haque R, Mondal D, Shu J, Roy S, Kabir M, Davis AN, Duggal P, Petri WA Jr. 2007. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg 76:340–344. [PubMed] [Google Scholar]
- 356.Procaccini C, Jirillo E, Matarese G. 2012. Leptin as an immunomodulator. Mol Aspects Med 33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 357.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC Jr, Myers MG Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA Jr. 2011. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest 121:1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, Hoffman P, Warren C, Singer S, Guerrant R. 2013. Persistent G. lamblia impairs growth in a murine malnutrition model. J Clin Invest 123:2672–2684. doi: 10.1172/JCI67294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ventura LL, Oliveira DR, Viana JC, Santos JF, Caliari MV, Gomes MA. 2013. Impact of protein malnutrition on histological parameters of experimentally infected animals with Giardia lamblia. Exp Parasitol 133:391–395. doi: 10.1016/j.exppara.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 360.Costa LB, Noronha FJ, Roche JK, Sevilleja JE, Warren CA, Oria R, Lima A, Guerrant RL. 2012. Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of Cryptosporidium infection and malnutrition. J Infect Dis 205:1464–1471. doi: 10.1093/infdis/jis216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Costa LB, JohnBull EA, Reeves JT, Sevilleja JE, Freire RS, Hoffman PS, Lima AA, Oria RB, Roche JK, Guerrant RL, Warren CA. 2011. Cryptosporidium-malnutrition interactions: mucosal disruption, cytokines, and TLR signaling in a weaned murine model. J Parasitol 97:1113–1120. doi: 10.1645/GE-2848.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Castro IC, Oliveira BB, Slowikowski JJ, Coutinho BP, Siqueira FJ, Costa LB, Sevilleja JE, Almeida CA, Lima AA, Warren CA, Oria RB, Guerrant RL. 2012. Arginine decreases Cryptosporidium parvum infection in undernourished suckling mice involving nitric oxide synthase and arginase. Nutrition 28:678–685. doi: 10.1016/j.nut.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.McGarvey ST, Aligui G, Graham KK, Peters P, Olds GR, Olveda R. 1996. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. Am J Trop Med Hyg 54:498–502. doi: 10.4269/ajtmh.1996.54.498. [DOI] [PubMed] [Google Scholar]
- 364.Assis AM, Prado MS, Barreto ML, Reis MG, Conceicao Pinheiro SM, Parraga IM, Blanton RE. 2004. Childhood stunting in Northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr 58:1022–1029. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- 365.Parraga IM, Assis AM, Prado MS, Barreto ML, Reis MG, King CH, Blanton RE. 1996. Gender differences in growth of school-aged children with schistosomiasis and geohelminth infection. Am J Trop Med Hyg 55:150–156. doi: 10.4269/ajtmh.1996.55.150. [DOI] [PubMed] [Google Scholar]
- 366.Papier K, Williams GM, Luceres-Catubig R, Ahmed F, Olveda RM, McManus DP, Chy D, Chau TN, Gray DJ, Ross AG. 2014. Childhood malnutrition and parasitic helminth interactions. Clin Infect Dis 59:234–243. doi: 10.1093/cid/ciu211. [DOI] [PubMed] [Google Scholar]
- 367.Verhagen LM, Incani RN, Franco CR, Ugarte A, Cadenas Y, Sierra Ruiz CI, Hermans PW, Hoek D, Campos Ponce M, de Waard JH, Pinelli E. 2013. High malnutrition rate in Venezuelan Yanomami compared to Warao Amerindians and Creoles: significant associations with intestinal parasites and anemia. PLoS One 8:e77581. doi: 10.1371/journal.pone.0077581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. 2001. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol 30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 369.Bundy DA, Golden MH. 1987. The impact of host nutrition on gastrointestinal helminth populations. Parasitology 95(Part 3):623–635. doi: 10.1017/S0031182000058042. [DOI] [PubMed] [Google Scholar]
- 370.Grazioso CF, Isalgue M, de Ramirez I, Ruz M, Solomons NW. 1993. The effect of zinc supplementation on parasitic reinfestation of Guatemalan schoolchildren. Am J Clin Nutr 57:673–678. [DOI] [PubMed] [Google Scholar]
- 371.Scott ME, Koski KG. 2000. Zinc deficiency impairs immune responses against parasitic nematode infections at intestinal and systemic sites. J Nutr 130:1412S–1420S. [DOI] [PubMed] [Google Scholar]
- 372.Ing R, Su Z, Scott ME, Koski KG. 2000. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proc Natl Acad Sci U S A 97:7078–7083. doi: 10.1073/pnas.97.13.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Correa CL, Lisboa PC, Oliveira E, Moura EG, Oliveira RM, Gomes AC, Machado-Silva JR. 2011. The outcome of acute schistosomiasis infection in adult mice with postnatal exposure to maternal malnutrition. Mem Inst Oswaldo Cruz 106:584–593. doi: 10.1590/S0074-02762011000500011. [DOI] [PubMed] [Google Scholar]
- 374.Page AL, de Rekeneire N, Sayadi S, Aberrane S, Janssens AC, Rieux C, Djibo A, Manuguerra JC, Ducou-le-Pointe H, Grais RF, Schaefer M, Guerin PJ, Baron E. 2013. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One 8:e68699. doi: 10.1371/journal.pone.0068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bachou H, Tumwine JK, Mwadime RK, Tylleskar T. 2006. Risk factors in hospital deaths in severely malnourished children in Kampala, Uganda. BMC Pediatr 6:7. doi: 10.1186/1471-2431-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Okomo UA, Garba D, Fombah AE, Secka O, Ikumapayi UN, Udo JJ, Ota MO. 2011. Bacterial isolates and antibiotic sensitivity among Gambian children with severe acute malnutrition. Int J Pediatr 2011:825123. doi: 10.1155/2011/825123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.WHO. 1999. Management of malnutrition: a manual for physicians and senior health workers. WHO, Geneva, Switzerland. [Google Scholar]
- 378.WHO. 2013. Guideline: updates on the management of severe acute malnutrition in infants and children. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 379.Manary MJ, Sandige HL. 2008. Management of acute moderate and severe childhood malnutrition. BMJ 337:a2180. doi: 10.1136/bmj.a2180. [DOI] [PubMed] [Google Scholar]
- 380.Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardaji A, Nhalungo D, Soriano-Gabarro M, Flannery B, Menendez C, Levine MM, Alonso PL. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 381.Isanaka S, Langendorf C, Berthe F, Gnegne S, Li N, Ousmane N, Harouna S, Hassane H, Schaefer M, Adehossi E, Grais RF. 2016. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med 374:444–453. doi: 10.1056/NEJMoa1507024. [DOI] [PubMed] [Google Scholar]
- 382.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 383.Brent AJ, Ahmed I, Ndiritu M, Lewa P, Ngetsa C, Lowe B, Bauni E, English M, Berkley JA, Scott JA. 2006. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet 367:482–488. doi: 10.1016/S0140-6736(06)68180-4. [DOI] [PubMed] [Google Scholar]
- 384.Hill PC, Onyeama CO, Ikumapayi UN, Secka O, Ameyaw S, Simmonds N, Donkor SA, Howie SR, Tapgun M, Corrah T, Adegbola RA. 2007. Bacteraemia in patients admitted to an urban hospital in West Africa. BMC Infect Dis 7:2. doi: 10.1186/1471-2334-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Nielsen MV, Sarpong N, Krumkamp R, Dekker D, Loag W, Amemasor S, Agyekum A, Marks F, Huenger F, Krefis AC, Hagen RM, Adu-Sarkodie Y, May J, Schwarz NG. 2012. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One 7:e44063. doi: 10.1371/journal.pone.0044063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, Caulfield LE, Danaei G, Nutrition Impact Model Study. 2013. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One 8:e64636. doi: 10.1371/journal.pone.0064636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bachou H, Tylleskar T, Kaddu-Mulindwa DH, Tumwine JK. 2006. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis 6:160. doi: 10.1186/1471-2334-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, Reyburn H, Brent B, Nteziyaremye J, Mpoya A, Prevatt N, Dambisya CM, Semakula D, Ddungu A, Okuuny V, Wokulira R, Timbwa M, Otii B, Levin M, Crawley J, Babiker AG, Gibb DM, FEAST Trial Group. 2013. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med 11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM, FEAST Trial Group. 2011. Mortality after fluid bolus in African children with severe infection. N Engl J Med 364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 390.Shahunja KM, Leung DT, Ahmed T, Bardhan PK, Ahmed D, Qadri F, Ryan ET, Chisti MJ. 2015. Factors associated with non-typhoidal Salmonella bacteremia versus typhoidal Salmonella bacteremia in patients presenting for care in an urban diarrheal disease hospital in Bangladesh. PLoS Negl Trop Dis 9:e0004066. doi: 10.1371/journal.pntd.0004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. 2008. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ 86:390C–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Glennie SJ, Banda D, Mulwafu W, Nkhata R, Williams NA, Heyderman RS. 2012. Regulation of naturally acquired mucosal immunity to Streptococcus pneumoniae in healthy Malawian adults and children. PLoS One 7:e51425. doi: 10.1371/journal.pone.0051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lonnroth K, Williams BG, Cegielski P, Dye C. 2010. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 39:149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 394.Cegielski JP, Arab L, Cornoni-Huntley J. 2012. Nutritional risk factors for tuberculosis among adults in the United States, 1971-1992. Am J Epidemiol 176:409–422. doi: 10.1093/aje/kws007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. 2002. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg 96:291–294. doi: 10.1016/S0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 396.Koethe JR, von Reyn CF. 2016. Protein-calorie malnutrition, macronutrient supplements, and tuberculosis. Int J Tuberc Lung Dis 20:857–863. doi: 10.5588/ijtld.15.0936. [DOI] [PubMed] [Google Scholar]
- 397.Chisti MJ, Graham SM, Duke T, Ahmed T, Ashraf H, Faruque AS, La Vincente S, Banu S, Raqib R, Salam MA. 2014. A prospective study of the prevalence of tuberculosis and bacteraemia in Bangladeshi children with severe malnutrition and pneumonia including an evaluation of Xpert MTB/RIF assay. PLoS One 9:e93776. doi: 10.1371/journal.pone.0093776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.WHO. 2014. Guidance for national tuberculosis programmes on the management of tuberculosis in children, 2nd ed WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 399.Satyanarayana K, Bhaskaram P, Seshu VC, Reddy V. 1980. Influence of nutrition on postvaccinial tuberculin sensitivity. Am J Clin Nutr 33:2334–2337. [DOI] [PubMed] [Google Scholar]
- 400.Anuradha R, Munisankar S, Bhootra Y, Kumar NP, Dolla C, Kumaran P, Babu S. 2016. Coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin Vaccine Immunol 23:339–345. doi: 10.1128/CVI.00009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Singh M, Mynak ML, Kumar L, Mathew JL, Jindal SK. 2005. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child 90:624–628. doi: 10.1136/adc.2003.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Dai G, McMurray DN. 1998. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect Immun 66:3562–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Mainali ES, McMurray DN. 1998. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect Immun 66:927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.McMurray DN, Carlomagno MA, Mintzer CL, Tetzlaff CL. 1985. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect Immun 50:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Chan J, Tian Y, Tanaka KE, Tsang MS, Yu K, Salgame P, Carroll D, Kress Y, Teitelbaum R, Bloom BR. 1996. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci U S A 93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ishikawa LL, da Rosa LC, Franca TG, Peres RS, Chiuso-Minicucci F, Zorzella-Pezavento SF, Sartori A. 2012. Is the BCG vaccine safe for undernourished individuals? Clin Dev Immunol 2012:673186. doi: 10.1155/2012/673186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Cohen MK, Bartow RA, Mintzer CL, McMurray DN. 1987. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect Immun 55:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Adebami OJ, Owa JA, Oyedeji GA, Oyelami OA, Omoniyi-Esan GO. 2007. Associations between placental and cord blood malaria infection and fetal malnutrition in an area of malaria holoendemicity. Am J Trop Med Hyg 77:209–213. [PubMed] [Google Scholar]
- 409.Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. 2013. The effect of antenatal monthly sulphadoxine-pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Trop Med Int Health 18:386–397. doi: 10.1111/tmi.12074. [DOI] [PubMed] [Google Scholar]
- 410.Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. 2010. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am J Trop Med Hyg 83:1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ter Kuile FO, Steketee RW. 2007. Intermittent preventive therapy with sulfadoxine-pyrimethamine during pregnancy: seeking information on optimal dosing frequency. J Infect Dis 196:1574–1576. doi: 10.1086/522233. [DOI] [PubMed] [Google Scholar]
- 412.Lee G, Yori P, Olortegui MP, Pan W, Caulfield L, Gilman RH, Sanders JW, Delgado HS, Kosek M. 2012. Comparative effects of vivax malaria, fever and diarrhoea on child growth. Int J Epidemiol 41:531–539. doi: 10.1093/ije/dyr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.ter Kuile FO, Terlouw DJ, Kariuki SK, Phillips-Howard PA, Mirel LB, Hawley WA, Friedman JF, Shi YP, Kolczak MS, Lal AA, Vulule JM, Nahlen BL. 2003. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg 68:68–77. [PubMed] [Google Scholar]
- 414.ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kolczak MS, Kariuki SK, Shi YP, Kwena AM, Vulule JM, Nahlen BL. 2003. Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: cross-sectional survey. Am J Trop Med Hyg 68:100–107. [PubMed] [Google Scholar]
- 415.Arinaitwe E, Gasasira A, Verret W, Homsy J, Wanzira H, Kakuru A, Sandison TG, Young S, Tappero JW, Kamya MR, Dorsey G. 2012. The association between malnutrition and the incidence of malaria among young HIV-infected and -uninfected Ugandan children: a prospective study. Malar J 11:90. doi: 10.1186/1475-2875-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Fishman S, Caulfield L, de Onis M, Blossner M, Hyder A, Mullany L, Black RE. 2004. Childhood and maternal underweight, p 63–161. In Ezzati M, Lopez AD, Rodgers A, Murray CJL (ed), Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. WHO, Geneva, Switzerland. [Google Scholar]
- 417.Caulfield LE, Richard SA, Black RE. 2004. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am J Trop Med Hyg 71:55–63. [PubMed] [Google Scholar]
- 418.Badaro R, Jones TC, Lorenco R, Cerf BJ, Sampaio D, Carvalho EM, Rocha H, Teixeira R, Johnson WD Jr. 1986. A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis 154:639–649. doi: 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- 419.Cerf BJ, Jones TC, Badaro R, Sampaio D, Teixeira R, Johnson WD Jr. 1987. Malnutrition as a risk factor for severe visceral leishmaniasis. J Infect Dis 156:1030–1033. doi: 10.1093/infdis/156.6.1030. [DOI] [PubMed] [Google Scholar]
- 420.Dye C, Vidor E, Dereure J. 1993. Serological diagnosis of leishmaniasis: on detecting infection as well as disease. Epidemiol Infect 110:647–656. doi: 10.1017/S0950268800051074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Harrison LH, Naidu TG, Drew JS, de Alencar JE, Pearson RD. 1986. Reciprocal relationships between undernutrition and the parasitic disease visceral leishmaniasis. Rev Infect Dis 8:447–453. doi: 10.1093/clinids/8.3.447. [DOI] [PubMed] [Google Scholar]
- 422.Malafaia G. 2009. Protein-energy malnutrition as a risk factor for visceral leishmaniasis: a review. Parasite Immunol 31:587–596. doi: 10.1111/j.1365-3024.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 423.Collin S, Davidson R, Ritmeijer K, Keus K, Melaku Y, Kipngetich S, Davies C. 2004. Conflict and kala-azar: determinants of adverse outcomes of kala-azar among patients in southern Sudan. Clin Infect Dis 38:612–619. doi: 10.1086/381203. [DOI] [PubMed] [Google Scholar]
- 424.Kolaczinski JH, Hope A, Ruiz JA, Rumunu J, Richer M, Seaman J. 2008. Kala-azar epidemiology and control, southern Sudan. Emerg Infect Dis 14:664–666. doi: 10.3201/eid1404.071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Marlet MV, Sang DK, Ritmeijer K, Muga RO, Onsongo J, Davidson RN. 2003. Emergence or re-emergence of visceral leishmaniasis in areas of Somalia, north-eastern Kenya, and south-eastern Ethiopia in 2000-01. Trans R Soc Trop Med Hyg 97:515–518. doi: 10.1016/S0035-9203(03)80012-3. [DOI] [PubMed] [Google Scholar]
- 426.Maciel BL, Lacerda HG, Queiroz JW, Galvao J, Pontes NN, Dimenstein R, McGowan SE, Pedrosa LF, Jeronimo SM. 2008. Association of nutritional status with the response to infection with Leishmania chagasi. Am J Trop Med Hyg 79:591–598. [PubMed] [Google Scholar]
- 427.Anstead GM, Chandrasekar B, Zhao W, Yang J, Perez LE, Melby PC. 2001. Malnutrition alters the innate immune response and increases early visceralization following Leishmania donovani infection. Infect Immun 69:4709–4718. doi: 10.1128/IAI.69.8.4709-4718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Anstead GM, Zhang Q, Melby PC. 2009. Malnutrition promotes prostaglandin over leukotriene production and dysregulates eicosanoid-cytokine crosstalk in activated resident macrophages. Prostaglandins Leukot Essent Fatty Acids 81:41–51. doi: 10.1016/j.plefa.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 429.Gomez F, Galvan RR, Frerenk S, Munoz JC, Chavez R, Vasquez J. 1956. Mortality in second and third degree malnutrition. J Trop Pediatr 2:77–83. doi: 10.1093/oxfordjournals.tropej.a057419. [DOI] [PubMed] [Google Scholar]
- 430.Carrillo E, Jimenez MA, Sanchez C, Cunha J, Martins CM, da Paixao Seva A, Moreno J. 2014. Protein malnutrition impairs the immune response and influences the severity of infection in a hamster model of chronic visceral leishmaniasis. PLoS One 9:e89412. doi: 10.1371/journal.pone.0089412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Kane AV, Dinh DM, Ward HD. 2015. Childhood malnutrition and the intestinal microbiome. Pediatr Res 77:256–262. doi: 10.1038/pr.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Voreades N, Kozil A, Weir TL. 2014. Diet and the development of the human intestinal microbiome. Front Microbiol 5:494. doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ahmed T, Auble D, Berkley JA, Black R, Ahern PP, Hossain M, Hsieh A, Ireen S, Arabi M, Gordon JI. 2014. An evolving perspective about the origins of childhood undernutrition and nutritional interventions that includes the gut microbiome. Ann N Y Acad Sci 1332:22–38. doi: 10.1111/nyas.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN. 2014. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Lin A, Bik EM, Costello EK, Dethlefsen L, Haque R, Relman DA, Singh U. 2013. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS One 8:e53838. doi: 10.1371/journal.pone.0053838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Grzeskowiak L, Collado MC, Mangani C, Maleta K, Laitinen K, Ashorn P, Isolauri E, Salminen S. 2012. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr 54:812–816. doi: 10.1097/MPG.0b013e318249039c. [DOI] [PubMed] [Google Scholar]
- 439.Xu Z, Knight R. 2015. Dietary effects on human gut microbiome diversity. Br J Nutr 113(Suppl):S1–S5. doi: 10.1017/S0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol 5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108(Suppl 1):S4578–S4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, Molgaard C, Michaelsen KF, Licht TR. 2014. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol 80:2889–2900. doi: 10.1128/AEM.00342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA Jr, Ahmed T, Gordon JI. 2014. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. 2014. The placenta harbors a unique microbiome. Sci Transl Med 6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. 2012. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 96:544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 446.Bezirtzoglou E, Tsiotsias A, Welling GW. 2011. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 17:478–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 447.Pop M, Walker AW, Paulson J, Lindsay B, Antonio M, Hossain MA, Oundo J, Tamboura B, Mai V, Astrovskaya I, Corrada Bravo H, Rance R, Stares M, Levine MM, Panchalingam S, Kotloff K, Ikumapayi UN, Ebruke C, Adeyemi M, Ahmed D, Ahmed F, Alam MT, Amin R, Siddiqui S, Ochieng JB, Ouma E, Juma J, Mailu E, Omore R, Morris JG, Breiman RF, Saha D, Parkhill J, Nataro JP, Stine OC. 2014. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol 15:R76. doi: 10.1186/gb-2014-15-6-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Lindsay B, Oundo J, Hossain MA, Antonio M, Tamboura B, Walker AW, Paulson JN, Parkhill J, Omore R, Faruque AS, Das SK, Ikumapayi UN, Adeyemi M, Sanogo D, Saha D, Sow S, Farag TH, Nasrin D, Li S, Panchalingam S, Levine MM, Kotloff K, Magder LS, Hungerford L, Sommerfelt H, Pop M, Nataro JP, Stine OC. 2015. Microbiota that affect risk for shigellosis in children in low-income countries. Emerg Infect Dis 21:242–250. doi: 10.3201/eid2101.140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Monira S, Nakamura S, Gotoh K, Izutsu K, Watanabe H, Alam NH, Endtz HP, Cravioto A, Ali SI, Nakaya T, Horii T, Iida T, Alam M. 2011. Gut microbiota of healthy and malnourished children in bangladesh. Front Microbiol 2:228. doi: 10.3389/fmicb.2011.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Ghosh TS, Gupta SS, Bhattacharya T, Yadav D, Barik A, Chowdhury A, Das B, Mande SS, Nair GB. 2014. Gut microbiomes of Indian children of varying nutritional status. PLoS One 9:e95547. doi: 10.1371/journal.pone.0095547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Gupta SS, Mohammed MH, Ghosh TS, Kanungo S, Nair GB, Mande SS. 2011. Metagenome of the gut of a malnourished child. Gut Pathog 3:7. doi: 10.1186/1757-4749-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, Liu J, Houpt E, Li JV, Holmes E, Nicholson J, Knights D, Ursell LK, Knight R, Gordon JI. 2013. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. 2016. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351:aad3311. doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Gough EK, Stephens DA, Moodie EE, Prendergast AJ, Stoltzfus RJ, Humphrey JH, Manges AR. 2015. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome 3:24. doi: 10.1186/s40168-015-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Kristensen KH, Wiese M, Rytter MJ, Ozcam M, Hansen LH, Namusoke H, Friis H, Nielsen DS. 2016. Gut microbiota in children hospitalized with oedematous and non-oedematous severe acute malnutrition in Uganda. PLoS Negl Trop Dis 10:e0004369. doi: 10.1371/journal.pntd.0004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. 2016. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 457.Charbonneau MR, O'Donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JR, Muehlbauer MJ, Ilkayeva O, Wu C, Struckmeyer T, Barile D, Mangani C, Jorgensen J, Fan YM, Maleta K, Dewey KG, Ashorn P, Newgard CB, Lebrilla C, Mills DA, Gordon JI. 2016. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164:859–871. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Chinen T, Rudensky AY. 2012. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev 245:45–55. doi: 10.1111/j.1600-065X.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 459.Hill DA, Artis D. 2010. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol 28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Kabat AM, Srinivasan N, Maloy KJ. 2014. Modulation of immune development and function by intestinal microbiota. Trends Immunol 35:507–517. doi: 10.1016/j.it.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 463.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. 2010. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R III, McCoy KD, Macpherson AJ. 2010. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. 2010. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 466.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 468.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. 2014. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 472.Ooi JH, Li Y, Rogers CJ, Cantorna MT. 2013. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr 143:1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. 2013. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Tilg H, Moschen AR. 2015. Food, immunity, and the microbiome. Gastroenterology 148:1107–1119. doi: 10.1053/j.gastro.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 475.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. 2012. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Sheridan PO, Bindels LB, Saulnier DM, Reid G, Nova E, Holmgren K, O'Toole PW, Bunn J, Delzenne N, Scott KP. 2014. Can prebiotics and probiotics improve therapeutic outcomes for undernourished individuals? Gut Microbes 5:74–82. doi: 10.4161/gmic.27252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Saran S, Gopalan S, Krishna TP. 2002. Use of fermented foods to combat stunting and failure to thrive. Nutrition 18:393–396. doi: 10.1016/S0899-9007(01)00790-0. [DOI] [PubMed] [Google Scholar]
- 478.Kerac M, Bunn J, Seal A, Thindwa M, Tomkins A, Sadler K, Bahwere P, Collins S. 2009. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet 374:136–144. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 479.Maldonado Galdeano C, Novotny Nunez I, de Moreno de LeBlanc A, Carmuega E, Weill R, Perdigon G. 2011. Impact of a probiotic fermented milk in the gut ecosystem and in the systemic immunity using a non-severe protein-energy-malnutrition model in mice. BMC Gastroenterol 11:64. doi: 10.1186/1471-230X-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Nunez IN, Galdeano CM, Carmuega E, Weill R, de Moreno de LeBlanc A, Perdigon G. 2013. Effect of a probiotic fermented milk on the thymus in Balb/c mice under non-severe protein-energy malnutrition. Br J Nutr 110:500–508. doi: 10.1017/S0007114512005302. [DOI] [PubMed] [Google Scholar]
- 481.Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Barrish JP, Finegold MJ, Petrosino JF, Guerrant RL, Conner ME, Versalovic J. 2012. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J Pediatr Gastroenterol Nutr 55:299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. 2016. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 352:1533. doi: 10.1126/science.aad9359. [DOI] [PubMed] [Google Scholar]
- 483.WHO. 2016. Infant and young child feeding; fact sheet no. 342. WHO, Geneva, Switzerland. [Google Scholar]
- 484.WHO. 2007. Community-based management of severe acute malnutrition: a joint statement of the World Health Organization, World Food Programme, the United Nations System Standing Commitee on Nutrition, and the United Nations Children's Fund. WHO, Geneva, Switzerland. [Google Scholar]
- 485.Diop EHI, Dossou NI, Ndour MM, Briend A, Wade S. 2003. Comparison of the efficacy of a solid ready-to-use food and a liquid, milk-based diet for the rehabilitation of severely malnourished children: a randomized trial. Am J Clin Nutr 78:302–307. [DOI] [PubMed] [Google Scholar]
- 486.Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. 2004. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child 89:557–561. doi: 10.1136/adc.2003.034306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Sandige H, Ndekha MJ, Briend A, Ashorn P, Manary MJ. 2004. Home-based treatment of malnourished Malawian children with locally produced or imported ready-to-use food. J Pediatr Gastroenterol Nutr 39:141–146. doi: 10.1097/00005176-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 488.Hendricks KM. 2010. Ready-to-use therapeutic food for prevention of childhood undernutrition. Nutr Rev 68:429–435. doi: 10.1111/j.1753-4887.2010.00302.x. [DOI] [PubMed] [Google Scholar]
- 489.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, Shekar M, Maternal and Child Undernutrition Study Group. 2008. What works? Interventions for maternal and child undernutrition and survival. Lancet 371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 490.Thakwalakwa C, Ashorn P, Phuka J, Cheung YB, Briend A, Puumalainen T, Maleta K. 2010. A lipid-based nutrient supplement but not corn-soy blend modestly increases weight gain among 6- to 18-month-old moderately underweight children in rural Malawi. J Nutr 140:2008–2013. doi: 10.3945/jn.110.122499. [DOI] [PubMed] [Google Scholar]
- 491.Phuka J, Thakwalakwa C, Maleta K, Cheung YB, Briend A, Manary M, Ashorn P. 2009. Supplementary feeding with fortified spread among moderately underweight 6-18-month-old rural Malawian children. Matern Child Nutr 5:159–170. doi: 10.1111/j.1740-8709.2008.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Lagrone L, Cole S, Schondelmeyer A, Maleta K, Manary MJ. 2010. Locally produced ready-to-use supplementary food is an effective treatment of moderate acute malnutrition in an operational setting. Ann Trop Paediatr 30:103–108. doi: 10.1179/146532810X12703901870651. [DOI] [PubMed] [Google Scholar]
- 493.LaGrone LN, Trehan I, Meuli GJ, Wang RJ, Thakwalakwa C, Maleta K, Manary MJ. 2012. A novel fortified blended flour, corn-soy blend “plus-plus,” is not inferior to lipid-based ready-to-use supplementary foods for the treatment of moderate acute malnutrition in Malawian children. Am J Clin Nutr 95:212–219. doi: 10.3945/ajcn.111.022525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Matilsky DK, Maleta K, Castleman T, Manary MJ. 2009. Supplementary feeding with fortified spreads results in higher recovery rates than with a corn/soy blend in moderately wasted children. J Nutr 139:773–778. doi: 10.3945/jn.108.104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Nackers F, Broillet F, Oumarou D, Djibo A, Gaboulaud V, Guerin PJ, Rusch B, Grais RF, Captier V. 2010. Effectiveness of ready-to-use therapeutic food compared to a corn/soy-blend-based pre-mix for the treatment of childhood moderate acute malnutrition in Niger. J Trop Pediatr 56:407–413. doi: 10.1093/tropej/fmq019. [DOI] [PubMed] [Google Scholar]
- 496.Isanaka S, Nombela N, Djibo A, Poupard M, Van Beckhoven D, Gaboulaud V, Guerin PJ, Grais RF. 2009. Effect of preventive supplementation with ready-to-use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 301:277–285. doi: 10.1001/jama.2008.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Isanaka S, Roederer T, Djibo A, Luquero FJ, Nombela N, Guerin PJ, Grais RF. 2010. Reducing wasting in young children with preventive supplementation: a cohort study in Niger. Pediatrics 126:e442–e450. doi: 10.1542/peds.2009-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE, Lancet Nutrition Interventions Review Group, Maternal and Child Nutrition Study Group. 2013. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382:452–477. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 499.Wang Q, Ma A, Bygbjerg IC, Han X, Liu Y, Zhao S, Cai J. 2013. Rationale and design of a randomized controlled trial of the effect of retinol and vitamin D supplementation on treatment in active pulmonary tuberculosis patients with diabetes. BMC Infect Dis 13:104. doi: 10.1186/1471-2334-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Sinclair D, Abba K, Grobler L, Sudarsanam TD. 2011. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2011:CD006086. doi: 10.1002/14651858.CD006086.pub3. [DOI] [PubMed] [Google Scholar]
- 501.Karyadi E, Schultink W, Nelwan RH, Gross R, Amin Z, Dolmans WM, van der Meer JW, Hautvast JG, West CE. 2000. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. J Nutr 130:2953–2958. [DOI] [PubMed] [Google Scholar]
- 502.van Lettow M, Harries AD, Kumwenda JJ, Zijlstra EE, Clark TD, Taha TE, Semba RD. 2004. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis 4:61. doi: 10.1186/1471-2334-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Karyadi E, West CE, Schultink W, Nelwan RH, Gross R, Amin Z, Dolmans WM, Schlebusch H, van der Meer JW. 2002. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr 75:720–727. [DOI] [PubMed] [Google Scholar]
- 504.Lawson L, Thacher TD, Yassin MA, Onuoha NA, Usman A, Emenyonu NE, Shenkin A, Davies PD, Cuevas LE. 2010. Randomized controlled trial of zinc and vitamin A as co-adjuvants for the treatment of pulmonary tuberculosis. Trop Med Int Health 15:1481–1490. doi: 10.1111/j.1365-3156.2010.02638.x. [DOI] [PubMed] [Google Scholar]
- 505.Pakasi TA, Karyadi E, Suratih NM, Salean M, Darmawidjaja N, Bor H, van der Velden K, Dolmans WM, van der Meer JW. 2010. Zinc and vitamin A supplementation fails to reduce sputum conversion time in severely malnourished pulmonary tuberculosis patients in Indonesia. Nutr J 9:41. doi: 10.1186/1475-2891-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Visser ME, Grewal HM, Swart EC, Dhansay MA, Walzl G, Swanevelder S, Lombard C, Maartens G. 2011. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. Am J Clin Nutr 93:93–100. doi: 10.3945/ajcn.110.001784. [DOI] [PubMed] [Google Scholar]
- 507.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. 2006. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr 95:762–770. doi: 10.1079/BJN20051684. [DOI] [PubMed] [Google Scholar]
- 508.Range N, Andersen AB, Magnussen P, Mugomela A, Friis H. 2005. The effect of micronutrient supplementation on treatment outcome in patients with pulmonary tuberculosis: a randomized controlled trial in Mwanza, Tanzania. Trop Med Int Health 10:826–832. doi: 10.1111/j.1365-3156.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 509.Semba RD, Kumwenda J, Zijlstra E, Ricks MO, van Lettow M, Whalen C, Clark TD, Jorgensen L, Kohler J, Kumwenda N, Taha TE, Harries AD. 2007. Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. Int J Tuberc Lung Dis 11:854–859. [PubMed] [Google Scholar]
- 510.Sudarsanam TD, John J, Kang G, Mahendri V, Gerrior J, Franciosa M, Gopal S, John KR, Wanke CA, Muliyil J. 2011. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV-tuberculosis coinfection receiving directly observed short-course chemotherapy for tuberculosis. Trop Med Int Health 16:699–706. doi: 10.1111/j.1365-3156.2011.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Lodha R, Mukherjee A, Singh V, Singh S, Friis H, Faurholt-Jepsen D, Bhatnagar S, Saini S, Kabra SK, Grewal HM, Delhi Pediatric TB Study Group. 2014. Effect of micronutrient supplementation on treatment outcomes in children with intrathoracic tuberculosis: a randomized controlled trial. Am J Clin Nutr 100:1287–1297. doi: 10.3945/ajcn.113.082255. [DOI] [PubMed] [Google Scholar]
- 512.Mehta S, Mugusi FM, Bosch RJ, Aboud S, Chatterjee A, Finkelstein JL, Fataki M, Kisenge R, Fawzi WW. 2011. A randomized trial of multivitamin supplementation in children with tuberculosis in Tanzania. Nutr J 10:120. doi: 10.1186/1475-2891-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Sazawal S, Black RE, Ramsan M, Chwaya HM, Dutta A, Dhingra U, Stoltzfus RJ, Othman MK, Kabole FM. 2007. Effect of zinc supplementation on mortality in children aged 1-48 months: a community-based randomised placebo-controlled trial. Lancet 369:927–934. doi: 10.1016/S0140-6736(07)60452-8. [DOI] [PubMed] [Google Scholar]
- 514.Veenemans J, Milligan P, Prentice AM, Schouten LR, Inja N, van der Heijden AC, de Boer LC, Jansen EJ, Koopmans AE, Enthoven WT, Kraaijenhagen RJ, Demir AY, Uges DR, Mbugi EV, Savelkoul HF, Verhoef H. 2011. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med 8:e1001125. doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Owusu-Agyei S, Newton S, Mahama E, Febir LG, Ali M, Adjei K, Tchum K, Alhassan L, Moleah T, Tanumihardjo SA. 2013. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr J 12:131. doi: 10.1186/1475-2891-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Long KZ, Rosado JL, Fawzi W. 2007. The comparative impact of iron, the B-complex vitamins, vitamins C and E, and selenium on diarrheal pathogen outcomes relative to the impact produced by vitamin A and zinc. Nutr Rev 65:218–232. doi: 10.1111/j.1753-4887.2007.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 517.Baqui AH, Zaman K, Persson LA, El Arifeen S, Yunus M, Begum N, Black RE. 2003. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhea and acute lower respiratory infection in Bangladeshi infants. J Nutr 133:4150–4157. [DOI] [PubMed] [Google Scholar]
- 518.Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lonnerdal B, Black RE, Brown KH. 2004. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. Am J Clin Nutr 79:457–465. [DOI] [PubMed] [Google Scholar]
- 519.Veenemans J, Schouten LR, Ottenhof MJ, Mank TG, Uges DR, Mbugi EV, Demir AY, Kraaijenhagen RJ, Savelkoul HF, Verhoef H. 2012. Effect of preventive supplementation with zinc and other micronutrients on non-malarial morbidity in Tanzanian pre-school children: a randomized trial. PLoS One 7:e41630. doi: 10.1371/journal.pone.0041630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Luabeya KK, Mpontshane N, Mackay M, Ward H, Elson I, Chhagan M, Tomkins A, Van den Broeck J, Bennish ML. 2007. Zinc or multiple micronutrient supplementation to reduce diarrhea and respiratory disease in South African children: a randomized controlled trial. PLoS One 2:e541. doi: 10.1371/journal.pone.0000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Duggan C, Manji KP, Kupka R, Bosch RJ, Aboud S, Kisenge R, Okuma J, Fawzi WW. 2012. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo-controlled clinical trial. Am J Clin Nutr 96:1437–1446. doi: 10.3945/ajcn.112.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Manger MS, McKenzie JE, Winichagoon P, Gray A, Chavasit V, Pongcharoen T, Gowachirapant S, Ryan B, Wasantwisut E, Gibson RS. 2008. A micronutrient-fortified seasoning powder reduces morbidity and improves short-term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. Am J Clin Nutr 87:1715–1722. [DOI] [PubMed] [Google Scholar]
- 523.Chhagan MK, Van den Broeck J, Luabeya KK, Mpontshane N, Tomkins A, Bennish ML. 2010. Effect on longitudinal growth and anemia of zinc or multiple micronutrients added to vitamin A: a randomized controlled trial in children aged 6-24 months. BMC Public Health 10:145. doi: 10.1186/1471-2458-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Chen K, Zhang X, Li TY, Chen L, Wei XP, Qu P, Liu YX. 2011. Effect of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on infectious morbidity of preschool children. Nutrition 27:428–434. doi: 10.1016/j.nut.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 525.Dutta P, Mitra U, Dutta S, Naik TN, Rajendran K, Chatterjee MK. 2011. Zinc, vitamin A, and micronutrient supplementation in children with diarrhea: a randomized controlled clinical trial of combination therapy versus monotherapy. J Pediatr 159:633–637. doi: 10.1016/j.jpeds.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 526.Ghosh S, Smriga M, Vuvor F, Suri D, Mohammed H, Armah SM, Scrimshaw NS. 2010. Effect of lysine supplementation on health and morbidity in subjects belonging to poor peri-urban households in Accra, Ghana. Am J Clin Nutr 92:928–939. doi: 10.3945/ajcn.2009.28834. [DOI] [PubMed] [Google Scholar]
- 527.Lima NL, Soares AM, Mota RM, Monteiro HS, Guerrant RL, Lima AA. 2007. Wasting and intestinal barrier function in children taking alanyl-glutamine-supplemented enteral formula. J Pediatr Gastroenterol Nutr 44:365–374. doi: 10.1097/MPG.0b013e31802eecdd. [DOI] [PubMed] [Google Scholar]
- 528.Ueno PM, Oria RB, Maier EA, Guedes M, de Azevedo OG, Wu D, Willson T, Hogan SP, Lima AA, Guerrant RL, Polk DB, Denson LA, Moore SR. 2011. Alanyl-glutamine promotes intestinal epithelial cell homeostasis in vitro and in a murine model of weanling undernutrition. Am J Physiol Gastrointest Liver Physiol 301:G612–G622. doi: 10.1152/ajpgi.00531.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, Manary MJ. 2013. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 368:425–435. doi: 10.1056/NEJMoa1202851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Mandomando I, Macete E, Sigauque B, Morais L, Quinto L, Sacarlal J, Espasa M, Valles X, Bassat Q, Aide P, Nhampossa T, Machevo S, Ruiz J, Nhacolo A, Menendez C, Kotloff KL, Roca A, Levine MM, Alonso PL. 2009. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 14:1467–1474. doi: 10.1111/j.1365-3156.2009.02399.x. [DOI] [PubMed] [Google Scholar]
- 531.Ndir A, Diop A, Faye PM, Cisse MF, Ndoye B, Astagneau P. 2016. Epidemiology and burden of bloodstream infections caused by extended-spectrum beta-lactamase producing Enterobacteriaceae in a pediatric hospital in Senegal. PLoS One 11:e0143729. doi: 10.1371/journal.pone.0143729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kerac M, Bunn J, Chagaluka G, Bahwere P, Tomkins A, Collins S, Seal A. 2014. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PLoS One 9:e96030. doi: 10.1371/journal.pone.0096030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Chisti MJ, Graham SM, Duke T, Ahmed T, Faruque AS, Ashraf H, Bardhan PK, Shahid AS, Shahunja KM, Salam MA. 2014. Post-discharge mortality in children with severe malnutrition and pneumonia in Bangladesh. PLoS One 9:e107663. doi: 10.1371/journal.pone.0107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, Lander ES, Knip M, DIABIMMUNE Study Group, Xavier RJ. 2016. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8:343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Angood C, Khara T, Dolan C, Berkley JA, WaSt Technical Interest Group. 2016. Research priorities on the relationship between wasting and stunting. PLoS One 11:e0153221. doi: 10.1371/journal.pone.0153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Angood C, McGrath M, Mehta S, Mwangome M, Lung'aho M, Roberfroid D, Perry A, Wilkinson C, Israel AD, Bizouerne C, Haider R, Seal A, Berkley JA, Kerac M, MAMI Working Group Collaborators. 2015. Research priorities to improve the management of acute malnutrition in infants aged less than six months (MAMI). PLoS Med 12:e1001812. doi: 10.1371/journal.pmed.1001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Kinross J, Li JV, Muirhead LJ, Nicholson J. 2014. Nutritional modulation of the metabonome: applications of metabolic phenotyping in translational nutritional research. Curr Opin Gastroenterol 30:196–207. doi: 10.1097/MOG.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 538.Gomez F, Galvan RR, Cravioto J, Frenk S. 1955. Malnutrition in infancy and childhood, with special reference to kwashiorkor. Adv Pediatr 7:131–169. [PubMed] [Google Scholar]
- 539.Venturi S, Venturi M. 2009. Iodine, thymus, and immunity. Nutrition 25:977–979. doi: 10.1016/j.nut.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 540.Percival SS. 1998. Copper and immunity. Am J Clin Nutr 67:1064S–1068S. [DOI] [PubMed] [Google Scholar]
- 541.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. 2007. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 98(Suppl 1):S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 542.Meydani SN, Han SN, Wu D. 2005. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev 205:269–284. doi: 10.1111/j.0105-2896.2005.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Checker R, Sharma D, Sandur SK, Khan NM, Patwardhan RS, Kohli V, Sainis KB. 2011. Vitamin K3 suppressed inflammatory and immune responses in a redox-dependent manner. Free Radic Res 45:975–985. doi: 10.3109/10715762.2011.585647. [DOI] [PubMed] [Google Scholar]
- 544.Schramm M, Wiegmann K, Schramm S, Gluschko A, Herb M, Utermohlen O, Kronke M. 2014. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur J Immunol 44:728–741. doi: 10.1002/eji.201343940. [DOI] [PubMed] [Google Scholar]
- 545.Kuroishi T. 2015. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol 93:1091–1096. doi: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- 546.Agrawal S, Agrawal A, Said HM. 2016. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol 311:C386–C391. doi: 10.1152/ajpcell.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, Saitoh T, Kurabayshi H, Naruse T. 1999. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol 116:28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Fata FT, Herzlich BC, Schiffman G, Ast AL. 1996. Impaired antibody responses to pneumococcal polysaccharide in elderly patients with low serum vitamin B12 levels. Ann Intern Med 124:299–304. doi: 10.7326/0003-4819-124-3-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 549.Iyer SS, Chatraw JH, Tan WG, Wherry EJ, Becker TC, Ahmed R, Kapasi ZF. 2012. Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells. J Immunol 188:77–84. doi: 10.4049/jimmunol.1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. 2006. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J 25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 551.Khan WA, Griffiths JK, Bennish ML. 2013. Gastrointestinal and extra-intestinal manifestations of childhood shigellosis in a region where all four species of Shigella are endemic. PLoS One 8:e64097. doi: 10.1371/journal.pone.0064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, Fantuzzi G, van der Poll T. 2005. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol 17:1399–1408. doi: 10.1093/intimm/dxh317. [DOI] [PubMed] [Google Scholar]
- 553.Moran-Mendoza O, Marion SA, Elwood K, Patrick D, FitzGerald JM. 2010. Risk factors for developing tuberculosis: a 12-year follow-up of contacts of tuberculosis cases. Int J Tuberc Lung Dis 14:1112–1119. [PubMed] [Google Scholar]
- 554.Serafim TD, Malafaia G, Silva ME, Pedrosa ML, Rezende SA. 2010. Immune response to Leishmania (Leishmania) chagasi infection is reduced in malnourished BALB/c mice. Mem Inst Oswaldo Cruz 105:811–817. doi: 10.1590/S0074-02762010000600014. [DOI] [PubMed] [Google Scholar]
- 555.Rabiu OR, Arinola OG, Odaibo AB, Ademowo OG. 2012. Effects of low protein diet and pregnancy on course of Plasmodium berghei infection in mice. Afr J Med Med Sci 41(Suppl):139–144. [PubMed] [Google Scholar]
- 556.Martins RF, Martinelli PM, Guedes PM, da Cruz Padua B, Dos Santos FM, Silva ME, Bahia MT, Talvani A. 2013. Protein deficiency alters CX3CL1 and endothelin-1 in experimental Trypanosoma cruzi-induced cardiomyopathy. Trop Med Int Health 18:466–476. doi: 10.1111/tmi.12071. [DOI] [PubMed] [Google Scholar]
- 557.Friedman JF, Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Wu H, Olveda RM, McGarvey ST, Kurtis JD. 2005. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, the Philippines. Am J Trop Med Hyg 72:527–533. [PubMed] [Google Scholar]
- 558.Smith A, Madden KB, Yeung KJ, Zhao A, Elfrey J, Finkelman F, Levander O, Shea-Donohue T, Urban JF Jr. 2005. Deficiencies in selenium and/or vitamin E lower the resistance of mice to Heligmosomoides polygyrus infections. J Nutr 135:830–836. [DOI] [PubMed] [Google Scholar]
- 559.Tu T, Koski KG, Scott ME. 2008. Mechanisms underlying reduced expulsion of a murine nematode infection during protein deficiency. Parasitology 135:81–93. doi: 10.1017/S0031182007003617. [DOI] [PubMed] [Google Scholar]
- 560.Tu T, Koski KG, Wykes LJ, Scott ME. 2007. Re-feeding rapidly restores protection against Heligmosomoides bakeri (Nematoda) in protein-deficient mice. Parasitology 134:899–909. doi: 10.1017/S0031182007002314. [DOI] [PubMed] [Google Scholar]
- 561.Boulay M, Scott ME, Conly SL, Stevenson MM, Koski KG. 1998. Dietary protein and zinc restrictions independently modify a Heligmosomoides polygyrus (Nematoda) infection in mice. Parasitology 116(Part 5):449–462. doi: 10.1017/S0031182098002431. [DOI] [PubMed] [Google Scholar]