Summary
Antiphospholipid (aPL) antibodies are antibodies specific for anionic phospholipids. They are immunoglobulins that attack phospholipids, phospholipid-binding proteins, or phospholipid-protein complexes and are detected in anticardiolipin and lupus anticoagulant assays. aPL antibodies are often associated with antiphospholipid syndrome (APS) which can be idiopathic or from secondary causes such as systemic lupus erythematosus (SLE), infection or drugs. They have also been shown to be associated with Pulmonary Hypertension. We conducted a review of the literature that included all articles on PubMed with keywords ‘antiphospholipid antibody’ and ‘pulmonary hypertension’ between January 1980 and July 2017 and identified 217 articles. A total of 47 articles were found to be relevant to the topic and included as references. We ascertained that aPL antibodies have been implicated in the development of both idiopathic pulmonary arterial hypertension (PAH) and PAH associated with connective tissue disease (CTD). aPL antibodies were also noted to be associated with left-sided valvular heart disease that can lead to pulmonary venous hypertension (PVH). Patients with anitiphospholipid antibody syndrome (Diagnostic criteria incudes +aPL antibodies) were noted to have a high risk of developing chronic thromboembolic pulmonary hypertension (CTEPH). A recent study also found a positive association of aPL antibodies with ILD and PH in patients with systemic sclerosis. While association between autoimmune thyroid disease and PH (Group V PH), and autoimmune thyroid disease and aPL antibodies is established, no studies linked these three phenomena together. Thus, aPL antibodies had an association with all WHO groups of Pulmonary hypertension (PH). In this review article, we study the association and discuss the need for screening for PH in patients with positive aPL antibodies.
Keywords: Pulmonary hypertension, antiphospholipid antibodies, antiphospholipid antibody syndrome, chronic thromboembolic pulmonary hypertension, systemic lupus erythematosus
1. Introduction
Antiphospholipid antibodies (aPL) are antibodies specific for anionic phospholipids. They are immunoglobulins that attack phospholipids, phospholipid-binding proteins, or phospholipid-protein complexes (Figure 1) (1). They are usually detected in anticardiolipin assays. Due to their ability to prolong coagulation tests they are also identified in lupus anticoagulant assays (2). aPL antibodies are usually associated with antiphospholipid syndrome (APS). APS is an autoimmune disorder that manifests clinically as recurrent venous or arterial thrombosis and/or fetal loss associated with persistently detected aPL antibodies on two or more occasions at least 12 weeks apart. It can be idiopathic or from secondary causes such as systemic lupus erythematosus (SLE), infection and drugs. The criteria for definite APS classification were first proposed in 1999 and updated in 2005 (Figure 2) (3). APS is usually detected for the first time as a blood clot in an artery or vein, or as recurrent pregnancy loss. Catastrophic antiphospholipid syndrome (CAPS) is a rare, serious, and often fatal type of APS characterized by multi-organ failure in a span of a few days to weeks.
Figure 1.
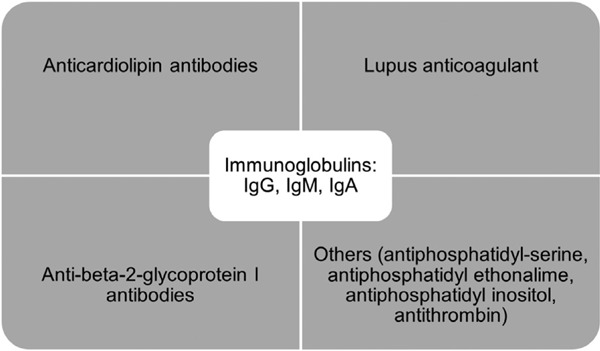
Different types of antiphospholipid antibodies.
Figure 2.
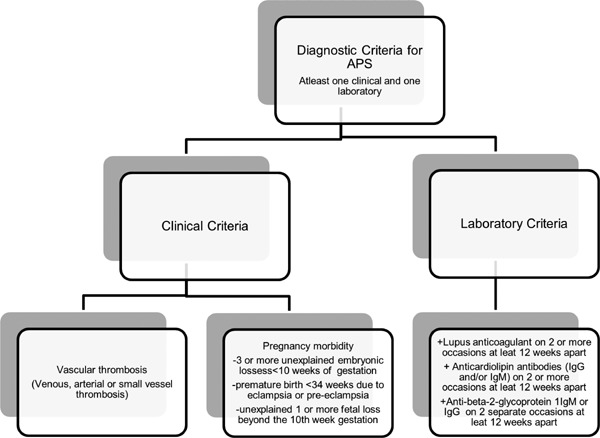
Diagnostic criteria of antiphospholipid syndrome (APS).
Pulmonary manifestations of APS with positive aPL antibodies include pulmonary embolism and infarction, acute respiratory distress syndrome (ARDS), intra-alveolar bleeding, primary thrombosis of lung vessels, pulmonary capillaritis or fibrosing alveolitis (4–6). aPL antibodies have also been shown to be associated with pulmonary hypertension (Figure 3). In this article we review the existing literature regarding the association of aPL antibodies with various groups of PH and discuss the possible implications in clinical practice.
Figure 3.
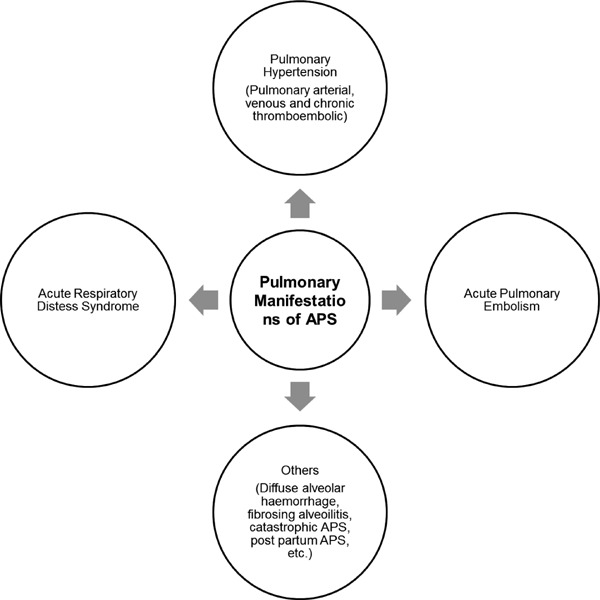
Pulmonary manifestations of antiphospholipid syndrome (APS).
2. Methodology
We conducted a review of the literature that included all articles on PubMed with keywords “antiphospholipid antibody” and “pulmonary hypertension” between January 1980 and July 2017. These articles were independently examined by 2 reviewers AA and RP. We largely selected publications from the last 20 years, but did not exclude any older publications that were widely referenced and highly regarded. A total of 217 articles were identified from PubMed for review by this strategy. All pertinent reports were retrieved and the relative reference lists were systematically searched in order to identify any potential additional studies that could be included. For less researched topics, we included case reports for purposes of our review. For topics with available retrospective and prospective studies and metanalysis, we excluded case series and case reports. Thus a total of 47 articles were found to be relevant to the topic with pertinent information and were included as references in this review.
3. aPL antibodies and pulmonary hypertension
Pulmonary hypertension is a chronic and progressive condition defined by a mean pulmonary artery pressure > 25 mm Hg at rest (7,8). The World Health Organization (WHO currently classifies PH into 5 groups (Table 1) (9). Recent evidence suggests the association of PH with the presence of aPL antibodies (10). Hypotheses regarding the pathophysiology include large vessel and small vessel thrombosis, chronic thromboemboli and associated endothelial remodeling (10). Recent findings of exuberant inflammation and lymphoid neogenesis in remodeled vessels have suggested a role of inflammation in PH, especially pulmonary arterial hypertension (PAH) (11,12). Pioneering work from Soon et al. demonstrated significantly increased levels of a broad range of inflammatory cytokines in patients with PAH. It has been well established that aPL antibodies have proinflammatory cellular effects. Thus we hypothesize that pro-inflammatory effects of aPL antibodies may also be contributing to the pathogenesis of PH in such patients.
Table 1. Classification of pulmonary hypertension based on etiology (9).
| Group 1 |
|
| Group 2 |
|
| Group 3 |
|
| Group 4 |
|
Asherson et al. first reported the association of aPL antibodies with PH in 1985 (13). Karmochkine et al. went on to document a high prevalence of aPL antibodies in pre-capillary PH. In this study of 38 patients with PH, 4/9 (44%) patients with primary pulmonary hypertension (PPH) and 7/29 (24%) patients with secondary pulmonary hypertension (SPH) were found to have positive aPL antibodies. Most patients had IgG alone (22/33). Multiple studies since then have studied the association of apL antibodies with PH. A thorough review of the literature suggested that aPL antibodies are associated with PH across all five WHO groups. aPL antibodies have been noted in patients with WHO Group I PH including idiopathic pulmonary arterial hypertension, pulmonary arterial hypertension associated with connective tissue disorders and rarely PH associated with pulmonary veno-occlusive disease. aPL antibodies can be associated with cardiac valvular disease and thus can lead to pulmonary venous hypertension (WHO Group II PH). These antibodies have also been noted in patients with interstitial lung disease associated with connective tissue disorders like SLE and scleroderma (WHO Group III PH). It is well known that patients with positive aPL antibodies and APS have an increased incidence of recurrent venous thromboembolism leading to CTEPH (Group IV PH). While association between autoimmune thyroid disease and PH (Group V PH), and autoimmune thyroid disease and aPL antibodies, no studies have yet linked these three phenomenon together. Studies with association of aPL antibodies and various WHO groups of PH are listed in Table 2.
Table 2. Studies on Association of antiphospholipid antibody with pulmonary hypertension.
| Author/Year | Type of Study | Number of subjects | Results |
|---|---|---|---|
| Karmochkine et al. (55) 1996 | Prospective | n = 38 PH (9PPH+29 SPH) aPL | (11/30) +aPL in pre capillary PAH (4/9) +aPL in PPH (44%) (7/29) +aPL in SPH (24%) |
| Wolf et al. (45) 2000 | Prospective |
n = 116: CTEPH n = 83: PPH |
(25/116) CTEPH + APA (8/83) PPH +APA Among 33 CTEPH or PPH patients: (22) + IgG (7) + IgG+IgM (4) + IgM, High titers of antibody 20% in CTEPH & 10% in PPH |
| Tanaseanu et al. (56) 2007 | Prospective | 30 SLE (15PH+/15PH-) 30 COPD (15 PH+/15PH-) |
Frequency of aPL antibody: SLE: (15/15(100%) in SLE/+PH) & (33% in SLE/-PH) COPD: (8/15(53%) in COPD/+PH) & (13% IN COPD/-PH) |
| Fois et al. (19) 2010 | Retrospective | 12 patients: SLE/+PH 9 SLE/+ isolated PH (Group1); 1 SLE/+ PH due to left heart disease(group2); 2 SLE/+PH related to lung disease (group3) |
(9/12) SLE/+PH had a + aCL antibody (frequency 75%) 2 SLE/+PH related due to lung disease: +aCL antibody 2/2 in this group |
| Kamel et al. (57) 2011 | Cross-sectional | Total SLE: 74 SLE/+PH: 8 |
7/8 (88%): SLE with PH/ + (aPL IgG), p = 0.08 Linear regression analyisis: + aCL were significantly associated with PAH occurrence in SLE with p value of 0.006 |
| Cefle et al. (18) 2011 | Retrospective | 10: SLE/+PH 97: SLE/-PH |
SLE/+PH: Total 10 2: + aCL IgG 4: + both aCL IgG & + aCL IgM 1: + aCL IgG, + aCLIgM, + LAC 1: + aCL IgM, + LAC aPL positivity: SLE/+PH (80%) vs. SLE/-PH (36%), p < 0.005. |
| Ware et al. (58) 2015 | Cross-sectional, observational |
n = 50 SLE 23/50: aPL + 11/23: aPL+/+PH |
(11/23) aPL+/+PH (14/ 23) + aCL (3 /23) + LAC (16/23) + Anti-beta-2-glycoprotein I antibodies (11) + 2 or more antibodies (PHT) 11/23 APA+ & 2/ 27 APA − Moderate to severe PH (7/ 11) APA + |
| Zuily S et al. (59) 2017 | Meta analysis | 4,480 SLE | Prevalence of PH: + aPL(12.3%) vs. - aPL (7.3%) The highest risk for PH: +LAC & + IgG aCL Low risk: IgM aCL & anti-β-2-glycoprotein I antibodies |
aCL: anticardiolipin antibodies; aPL: antiphospholipid antibodies; COPD: chronic obstructive pulmonary disease; CTEPH: Chronic thromboembolic pulmonary hypertension; LAC: lupus anticoagulant; PAH: pulmonary arterial hypertension; PH: Pulmonary hypertension; PPH: primary pulmonary hypertension; SLE: systemic lupus erythematosus; SPH: secondary pulmonary hypertension.
4. Group I PH — pulmonary arterial hypertension
4.1. Idiopathic pulmonary arterial hypertension
Idiopathic pulmonary arterial hypertension (IPAH) is defined as the presence of pulmonary arterial hypertension in the absence of another underlying disease. The presence of aPL antibodies has been reported in patients with IPAH (14). While the causative role of aPL antibodies in these patients is unclear, possible mechanisms include both platelet and endothelin-1 activation. Endothelin-1 (ET-1) is a potent vasoconstrictor and its role in PAH is well established (15). Platelet and endothelin activation can also lead to pulmonary vascular remodeling eventually leading to elevated pulmonary arterial pressures. This hypothesis is supported by the fact that increased levels of circulating ET-1 have been observed in patients with aPL antibodies present (16).
4.2. Pulmonary hypertension associated with connective tissue disease
PH associated with connective tissue disorders (CTD) is classified as Group I PH. aPL antibodies have been identified in patients with CTD's such as SLE and scleroderma. A study compared the presence of aPL antibodies in patients with CTD and found the prevalence of aPL antibodies in SLE patients to be roughly six times more than those with scleroderma (17). Tanaseanu et al. studied the prevalence of aPL antibodies and its relationship with PAH in 30 patients with SLE. Presence of aPL antibodies was noted in all patients with SLE and PAH (15/15) as compared to only 5/15 in patients with SLE without PAH. Mutiple studies have noted the presence of positive anticardiolipin antibodies (aCL) has a significant association with PAH and SLE patients (18–21). Similarly Houman et al. found out that the frequency of PAH in SLE was higher in patients with antibodies against B2Glycoprotein I (B2GPI) than those without these antibodies (22).
Most patients with aPL antibodies do not have APS, but have symptoms similar to IPAH thereby suggesting a role of aPL antibodies in pulmonary vasculopathy. The role of aPL antibodies in CTD-PAH development is unknown. Zuily et al. did a meta-analysis of 31 studies with about 4,480 patients with SLE and showed that people with positive aPL antibodies had a higher prevalence of PH (12.3%) as compared with aPL antibody negative SLE patients (7.3%). The overall OR for PH in aPL-positive versus aPL-negative SLE patients was 2.28 (95% CI, 1.65 to 3.15; p < 0.0001).
The treatment of these patients is similar to other patients with pulmonary arterial hypertension with special focus on anticoagulation due to higher risk of VTE in patients with aPL antibodies (1). Current guidelines suggest the use of oral anticoagulants in patients with idiopathic PAH and PH associated with CTD on an individual basis and based upon the presence of thrombophilic predisposition (Grade II b, Level C) (23). The COMPERA study conformed the benefit of anticoagulation in IPAH. The number of SLE or other CTD patients was too small to draw any inferences (24). Thus it is unknown whether anticoagulation is of benefit in aPL antibody positive SLE patients with PH without thrombosis. In thrombotic APS patients with PH, anticoagulation is recommended (25). The presence of PH in SLE patients markedly worsens prognosis (26,27). It is unclear if aPL antibody positivity is a prognostic marker for survival or lung transplantation in these patients (10,27).
Pulmonary veno-occlusive disease (PVOD) is another subset of group I PH that is seen in patients with CTD, HIV infection, bone marrow transplantation, sarcoidosis and pulmonary langerhans cell granulomatosis (28). The authors are aware of only 1 case reporting the possible association of aPL antibodies to PVOD (29).
5. Group 2 PH — Pulmonary venous hypertension associated with heart valve disease
Cardiac valvular disease is the most frequent cardiac manifestation in patients with APS, with a prevalence of 30%. Heart valve lesions (vegetation, thickening and dysfunction) are usually reported in patients with APS with and without SLE, and in those with aPL antibodies alone. Several autoantibodies can directly affect the heart tissue. For example, aPL antibodies can affect the heart by enhancing atherosclerosis, causing thrombosis of coronary arteries or via means of an immune-complexes-mediated reaction. They lead to immune complex formation and deposition which acts as the initial triggers for valvular endothelial activation. This leads to the thickening of the valves or formation of sterile vegetations on these valves (mainly mitral and aortic) (30). This is known as Libman-Sacks endocarditis i.e. verrucous endocarditis of valve leaflets, papillary muscles and the mural endocardium. This endocarditis is present in about 20% to 30% of patients with SLE and about a third of patients with primary APS (31–33). These lead to a significant amount of valvular regurgitation and can lead to left heart failure and associated pulmonary venous hypertension (PVH) (34). The risk of valvular heart disease is highest for patients with lupus anticoagulant and anti-cardiolipin antibodies (IgG) (35).
The presence of aPL antibodies in SLE patients is associated with a threefold greater risk of cardiac valvular disease than those without aPL antibodies, leading to thrombotic manifestations on valves because of hypercoagulability. APS patients undergoing valve-replacement surgery are at high risk of thrombotic and bleeding complications. Thus aPL antibody-related cardiac valvular disease affects APS management.
6. Group 3 PH — Pulmonary hypertension associated with interstitial lung disease
Patients with interstitial lung disease (ILD) present with onset of chronic cough, dyspnea, and decreased exercise tolerance. PH-ILD is secondary to parenchymal and vascular remodeling in the lungs. Its prevalence in ILD is estimated at 30–40% and causes severe exercise limitation and dismal prognosis for ILD patients (36). Mejia M et al. (37) in 2017 have found several auto-antibodies associated with ILD. Many studies have shown association of ILD with SLE, but there is a paucity of literature showing a specific relationship of aPL antibodies with PH in this group (38). Similarly the association of other CTD's with ILD is well established (39). As discussed earlier, there is an increased incidence of PH in patients with CTD and the presence of aPL antibodies. Thus by association, it is likely that aPL antibodies are present in patients with both ILD and PH, especially in those with underlying CTD.
Morrisroe et al. studied 940 patients with systemic sclerosis and found the presence of aPL antibodies in 226 patients. Elevated titres of both anticardiolipin antibody IgM (OR 2.04, 95% CI: 1.4–3.0, p < 0.0001) and IgG (OR 1.84, 95% CI: 1.2–2.8, p = 0.005) were associated with an increased likelihood of ILD in these patients. Positive anticardiolipin IgG was also noted to be a marker of co-existent ILD and PH (OR 2.10, 95% CI: 1.1–4.2, p = 0.036) (40).
7. Group 4 PH — Chronic thromboembolic pulmonary hypertension
Chronic thromboembolic pulmonary hypertension (CTEPH) is the form of pulmonary hypertension defined as pre-capillary hypertension with at least one segmental perfusion defect at scintigraphy and typical findings at conventional or computed tomographic pulmonary angiography, after at least 3 months of anticoagulation (41). It occurs usually as a consequence of incomplete resolution of acute pulmonary emboli, progressively organized into fibrotic material obstructing large pulmonary arteries, which leads to elevated pulmonary artery pressures (42). There has been an increased awareness regarding the pathogenesis of CTEPH in the last two decades with several reports of association of aPL antibodies and CTEPH. Colorio et al. noted a high proportion of positive aPL antibodies (50%) in 24 patients with CTEPH (43). In a retrospective study, D'Armini et al. detected aPL antibodies in 15% of patients (28/184) who underwent pulmonary endarterectomy for CTEPH (44). Wolf et al. studied the presence of thrombotic risk factors in patients with CTEPH and PPH. The study showed 20% positive aPL antibodies in patients with CTEPH and 10% positive aPL antibodies in patients with PPH (45). Similarly Martinuzzo et al. found a higher prevalence of aPL antibodies in patients with CTEPH as compared to patients with primary or secondary PH (46).
Patients with positive aPL antibodies especially in APS are prone to venous thrombosis and may have a recurrent pulmonary embolism leading to CTEPH. APS is the most common acquired cause of venous thromboembolism (VTE). The curative treatment for CTEPH is pulmonary thromboendarterectomy (PTE), a surgical procedure in which the blood vessels of the lungs are cleared of clot and scar material. But about 20–40% CTEPH patients are not operable either due to distal and inaccessible nature of the lesions or comorbid conditions, and in up to 35% of patients, the disease persists or reoccurs after PTE (47). Although several vasodilators are in the market for the treatment of PAH, none have been shown to be invariably effective in the treatment of CTEPH. Patients that are ineligible for surgery have been treated with Riociguat, a soluble guanylate cyclase stimulator (48). Lifelong anticoagulation is recommended for all patients with CTEPH in order to prevent recurrent VTE and progressive PH. Vitamin K antagonists (e. g. warfarin) are used for anticoagulation. Since the underlying mechanism of aPL antibodies causing CTEPH is immunological, the addition of immunosuppressive agents to the therapeutic regimen may be of benefit. It is therefore important to consider CTEPH in all patients with CTDs and PH as the management of CTEPH is significantly different from other forms of PH.
8. Group 5 PH
8.1. PH and autoimmune thyroid disease
WHO group 5 PH includes patients with thyroid disorders, especially hyperthyroidism. It is unclear whether the association between pulmonary hypertension (PH) and hyperthyroidism is an incidental one or if there is an unexplained pathological mechanism (49). The autoimmune hypothesis can possibly help answer this question. Sugiura et al. found a direct linear correlation between pulmonary artery pressure's and thyroid stimulating hormone receptor antibodies (TRAb) (r = 0.74, p < 0.001) (50). This hypothesis is based upon a possible indirect influence of TRAb in inducing immune-mediated damage of the endothelium that could be able to promote endothelial dysfunction leading to PH. Pagi et al. and Marongiu et al. noted increased incidence of aPL antibodies in patients with autoimmune thyroid disease, especially Grave's disease. Nabriski et al. similarly demonstrated the prevalence of aPL antibodies is increased in patients with autoimmune thyroid disorders. They suggested that non-specific autoantibody production may accompany the synthesis of tissue-specific immunoglobulin in autoimmune disorders (51,52). Thus while there might be an association between autoimmune thyroid disease, aPL antibodies and PH, this has not yet been studied and requires further research.
8.2. PH and Sarcoidosis
Ina et al. studied the correlation between aPL antibodies and sarcoidosis. While no correlation was observed between the occurrence of aPL antibodies and disease activity of sarcoidosis, the presence of aPL antibodies in sarcoidosis was found to be a useful index to judge the prolongation of the disease (53). Recently Pathak et al. performed a systematic review to study the association of APS and sarcoidosis and identified 4 cases of sarcoidosis in patients with APS. All patients were noted to have a one of the thrombotic manifestations of APS. None of the studies recorded the incidence of association with PH (54). Thus similar to autoimmune thyroid disease the possible association of PH with sarcoidosis and aPL antibodies needs future studies.
9. Conclusion
The association of aPL antibodies with VTE and PE is well established. Now we know that the effects of aPL antibodies on the pulmonary vasculature extend beyond CTEPH with manifestations that are similar to pulmonary arterial hypertension. There is emerging literature studying the association of aPL antibodies with cardiac valvular disease which can eventually lead to pulmonary venous hypertension. Thus the presence of aPL antibodies will make clinicians think about PH across all WHO groups. Our hypothesis is that aPL antibodies are a harbinger of pulmonary vasculopathy and thus the presence of aPL antibodies can be possibly used as a screening tool for PH patients. Further research is needed to clarify the pathogenesis of aPL antibodies and PH, and to lay guidelines regarding the routine screening of these patients.
References
- 1. Misita CP, Moll S. Antiphospholipid antibodies. Circulation. 2005; 112:e39-e44. [DOI] [PubMed] [Google Scholar]
- 2. Asherson RA, Cervera R, Merrill JT, Erkan D. Antiphospholipid antibodies and the antiphospholipid syndrome: Clinical significance and treatment. Semin Thromb Hemost. 2008; 34:256-266. [DOI] [PubMed] [Google Scholar]
- 3. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006; 4:295-306. [DOI] [PubMed] [Google Scholar]
- 4. Ford HJ, Roubey RA. Pulmonary manifestations of the antiphospholipid antibody syndrome. Clin Chest Med. 2010; 31:537-545. [DOI] [PubMed] [Google Scholar]
- 5. Stojanovich L. Pulmonary manifestations in antiphospholipid syndrome. Autoimmun Rev. 2006; 5:344-348. [DOI] [PubMed] [Google Scholar]
- 6. Espinosa G, Cervera R, Font J, Asherson RA. The lung in the antiphospholipid syndrome. Ann Rheum Dis. 2002; 61:195-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sikachi RR, Sahni S, Mehta D, Agarwal A, Agrawal A. Nationwide trends in inpatient admissions of pulmonary hypertension in the United States from 2000 to 2013. Adv Respir Med. 2017; 85:77-86. [DOI] [PubMed] [Google Scholar]
- 8. Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013; 62:D42-D50. [DOI] [PubMed] [Google Scholar]
- 9. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013; 62:D34-D41. [DOI] [PubMed] [Google Scholar]
- 10. Zuily S, Wahl D. Pulmonary hypertension in antiphospholipid syndrome. Curr Rheumatol Rep. 2015; 17:478. [DOI] [PubMed] [Google Scholar]
- 11. Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012; 185:311-321. [DOI] [PubMed] [Google Scholar]
- 12. Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012; 186:261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asherson RA, Morgan SH, Harris N, Gharavi AE, Hughes GR, Millar AB. Pulmonary hypertension and chronic cutaneous lupus erythematosus: Association with the lupus anticoagulant. Arthritis Rheum. 1985; 28:118. [DOI] [PubMed] [Google Scholar]
- 14. Asherson RA, Higenbottam TW, Dinh Xuan AT, Khamashta MA, Hughes GR. Pulmonary hypertension in a lupus clinic: Experience with twenty-four patients. J Rheumatol. 1990; 17:1292-1298. [PubMed] [Google Scholar]
- 15. Palkar AV, Agrawal A, Verma S, Iftikhar A, Miller EJ, Talwar A. Post splenectomy related pulmonary hypertension. World J Respirol. 2015; 5:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atsumi T, Khamashta MA, Haworth RS, Brooks G, Amengual O, Ichikawa K, Koike T, Hughes GR. Arterial disease and thrombosis in the antiphospholipid syndrome: a pathogenic role for endothelin 1. Arthritis Rheum. 1998; 41:800-807. [DOI] [PubMed] [Google Scholar]
- 17. Merkel PA, Chang Y, Pierangeli SS, Convery K, Harris EN, Polisson RP. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996; 101:576-583. [DOI] [PubMed] [Google Scholar]
- 18. Cefle A, Inanc M, Sayarlioglu M, Kamali S, Gul A, Ocal L, Aral O, Konice M. Pulmonary hypertension in systemic lupus erythematosus: Relationship with antiphospholipid antibodies and severe disease outcome. Rheumatol Int. 2011; 31:183-189. [DOI] [PubMed] [Google Scholar]
- 19. Fois E, Le Guern V, Dupuy A, Humbert M, Mouthon L, Guillevin L. Noninvasive assessment of systolic pulmonary ar tery pressure in systemic lupus erythematosus: retrospective analysis of 93 patients. Clin Exp Rheumatol. 2010; 28:836-841. [PubMed] [Google Scholar]
- 20. Lian F, Chen D, Wang Y, Ye Y, Wang X, Zhan Z, Xu H, Liang L, Yang X. Clinical features and independent predictors of pulmonary arterial hypertension in systemic lupus erythematosus. Rheumatol Int. 2012; 32:1727-1731. [DOI] [PubMed] [Google Scholar]
- 21. Lee JH, Im Cho K. Arterial stiffness, antiphospholipid antibodies, and pulmonary arterial hypertension in systemic lupus erythematosus. J Cardiol. 2014; 64:450-455. [DOI] [PubMed] [Google Scholar]
- 22. Houman MH, Smiti-Khanfir M, Ben Ghorbell I, Miled M. Systemic lupus erythematosus in Tunisia: demographic and clinical analysis of 100 patients. Lupus. 2004; 13:204-211. [DOI] [PubMed] [Google Scholar]
- 23. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67-119. [DOI] [PubMed] [Google Scholar]
- 24. Olsson KM, Delcroix M, Ghofrani HA, et al. Response to letters regarding article, “Anticoagulation and survival in pulmonary arterial hypertension: Results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA)”. Circulation. 2014; 130:e110-e112. [DOI] [PubMed] [Google Scholar]
- 25. Lockshin M, Tenedios F, Petri M, McCarty G, Forastiero R, Krilis S, Tincani A, Erkan D, Khamashta MA, Shoenfeld Y. Cardiac disease in the antiphospholipid syndrome: Recommendations for treatment. Committee consensus report. Lupus. 2003; 12:518-523. [DOI] [PubMed] [Google Scholar]
- 26. Chen Y, Chen GL, Zhu CQ, Lu X, Ye S, Yang CD. Severe systemic lupus erythematosus in emergency department: A retrospective single-center study from China. Clin Rheumatol. 2011; 30:1463-1469. [DOI] [PubMed] [Google Scholar]
- 27. Chow SL, Chandran V, Fazelzad R, Johnson SR. Prognostic factors for survival in systemic lupus erythematosus associated pulmonary hypertension. Lupus. 2012; 21:353-364. [DOI] [PubMed] [Google Scholar]
- 28. Montani D, Price LC, Dorfmuller P, Achouh L, Jais X, Yaici A, Sitbon O, Musset D, Simonneau G, Humbert M. Pulmonary veno-occlusive disease. Eur Respir J. 2009; 33:189-200. [DOI] [PubMed] [Google Scholar]
- 29. Hussein A, Trowitzsch E, Brockmann M. Pulmonary veno-occlusive disease, antiphospholipid antibody and pulmonary hypertension in an adolescent. Klin Padiatr. 1999; 211:92-95. [DOI] [PubMed] [Google Scholar]
- 30. Blank M, Aron-Maor A, Shoenfeld Y. From rheumatic fever to Libman-Sacks endocarditis: Is there any possible pathogenetic link? Lupus. 2005; 14:697-701. [DOI] [PubMed] [Google Scholar]
- 31. Galve E, Ordi J, Barquinero J, Evangelista A, Vilardell M, Soler-Soler J. Valvular heart disease in the primary antiphospholipid syndrome. Ann Intern Med. 1992; 116:293-298. [DOI] [PubMed] [Google Scholar]
- 32. Khamashta MA, Cervera R, Asherson RA, et al. Association of antibodies against phospholipids with heart valve disease in systemic lupus erythematosus. Lancet. 1990; 335:1541-1544. [DOI] [PubMed] [Google Scholar]
- 33. Prete M, Fatone MC, Vacca A, Racanelli V, Perosa F. Severe pulmonary hypertension as the initial manifestation of systemic lupus erythematosus: A case report and review of the literature. Clin Exp Rheumatol. 2014; 32:267-274. [PubMed] [Google Scholar]
- 34. Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016; 37:942-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zuily S, Regnault V, Selton-Suty C, Eschwege V, Bruntz JF, Bode-Dotto E, De Maistre E, Dotto P, Perret-Guillaume C, Lecompte T, Wahl D. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: Meta-analysis of echocardiographic studies. Circulation. 2011; 124:215-224. [DOI] [PubMed] [Google Scholar]
- 36. Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008; 31:1357-1367. [DOI] [PubMed] [Google Scholar]
- 37. Mejia M, Herrera-Bringas D, Perez-Roman DI, Rivero H, Mateos-Toledo H, Castorena-Garcia P, Figueroa JE, Rojas-Serrano J. Interstitial lung disease and myositis-specific and associated autoantibodies: Clinical manifestations, survival and the performance of the new ATS/ERS criteria for interstitial pneumonia with autoimmune features (IPAF). Respir Med. 2017; 123:79-86. [DOI] [PubMed] [Google Scholar]
- 38. Huang C, Li M, Liu Y, Wang Q, Guo X, Zhao J, Lai J, Tian Z, Zhao Y, Zeng X. Baseline Characteristics and Risk Factors of Pulmonary Arterial Hypertension in Systemic Lupus Erythematosus Patients. Medicine (Baltimore). 2016; 95:e2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013; 143:814-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morrisroe KB, Stevens W, Nandurkar H, et al. The association of antiphospholipid antibodies with cardiopulmonary manifestations of systemic sclerosis. Clin Exp Rheumatol. 2014; 32:S-133-137. [PubMed] [Google Scholar]
- 41. Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation. 2011; 124:1973-1981. [DOI] [PubMed] [Google Scholar]
- 42. Blauwet LA, Edwards WD, Tazelaar HD, McGregor CG. Surgical pathology of pulmonary thromboendarterectomy: A study of 54 cases from 1990 to 2001. Hum Pathol. 2003; 34:1290-1298. [DOI] [PubMed] [Google Scholar]
- 43. Colorio CC, Martinuzzo ME, Forastiero RR, Pombo G, Adamczuk Y, Carreras LO. Thrombophilic factors in chronic thromboembolic pulmonary hypertension. Blood Coagul Fibrinolysis. 2001; 12:427-432. [DOI] [PubMed] [Google Scholar]
- 44. D'Armini AM, Totaro P, Nicolardi S, Morsolini M, Silvaggio G, Toscano F, Toscano M, Vigano M. Impact of high titre of antiphospholipid antibodies on postoperative outcome following pulmonary endarterectomy. Interact Cardiovasc Thorac Surg. 2010; 10:418-422. [DOI] [PubMed] [Google Scholar]
- 45. Wolf M, Boyer-Neumann C, Parent F, Eschwege V, Jaillet H, Meyer D, Simonneau G. Thrombotic risk factors in pulmonary hypertension. Eur Respir J. 2000; 15:395-399. [DOI] [PubMed] [Google Scholar]
- 46. Martinuzzo ME, Pombo G, Forastiero RR, Cerrato GS, Colorio CC, Carreras LO. Lupus anticoagulant, high levels of anticardiolipin, and anti-beta2-glycoprotein I antibodies are associated with chronic thromboembolic pulmonary hypertension. J Rheumatol. 1998; 25:1313-1319. [PubMed] [Google Scholar]
- 47. Smith ZR, Makowski CT, Awdish RL. Treatment of patients with chronic thrombo embolic pulmonary hypertension: Focus on riociguat. Ther Clin Risk Manag. 2016; 12:957-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C, Group C-S. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013; 369:319-329. [DOI] [PubMed] [Google Scholar]
- 49. Scicchitano P, Dentamaro I, Tunzi F, Ricci G, Carbonara S, Devito F, Zito A, Ciampolillo A, Ciccone MM. Pulmonary hypertension in thyroid diseases. Endocrine. 2016; 54:578-587. [DOI] [PubMed] [Google Scholar]
- 50. Sugiura T, Yamanaka S, Takeuchi H, Morimoto N, Kamioka M, Matsumura Y. Autoimmunity and pulmonary hypertension in patients with Graves' disease. Heart Vessels. 2015; 30:642-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991; 49:193-280. [DOI] [PubMed] [Google Scholar]
- 52. Nabriski D, Ellis M, Ness-Abramof R, Shapiro M, Shenkman L. Autoimmune thyroid disease and antiphospholipid antibodies. Am J Hematol. 2000; 64:73-75. [DOI] [PubMed] [Google Scholar]
- 53. Ina Y, Takada K, Yamamoto M, Sato T, Ito S, Sato S. Antiphospholipid antibodies. A prognostic factor in sarcoidosis? Chest. 1994; 105:1179-1183. [DOI] [PubMed] [Google Scholar]
- 54. Pathak R, Khanal R, Aryal MR, Giri S, Karmacharya P, Pathak B, Acharya U, Bhatt VR. Sarcoidosis and antiphospholipid syndrome: A systematic review of cases. N Am J Med Sci. 2015; 7:379-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Karmochkine M, Mazoyer E, Marcelli A, Boffa MC, Piette JC. High prevalence of antiphospholipid antibodies in disseminated intravascular coagulation. Thromb Haemost. 1996; 75:971. [PubMed] [Google Scholar]
- 56. Tanaseanu C, Tudor S, Tamsulea I, Marta D, Manea G, Moldoveanu E. Vascular endothelial growth factor, lipoporotein-associated phospholipase A2, sP-selectin and antiphospholipid antibodies, biological markers with prognostic value in pulmonary hypertension associated with chronic obstructive pulmonary disease and systemic lupus erithematosus. Eur J Med Res. 2007; 12:145-151. [PubMed] [Google Scholar]
- 57. Kamel SR, Omar GM, Darwish AF, Asklany HT, Ellabban AS. Asymptomatic pulmonary hypertension in systemic lupus erythematosus. Clin Med Insights Arthritis Musculoskelet Disord. 2011; 4:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ware D, Sharma V, Kalekar L, Kamble A, Mahajan A, Gokhale Y. Higher incidence of pulmonary hypertension in antiphospholipid antibody positive lupus. J Assoc Physicians India. 2015; 63:17-20. [PubMed] [Google Scholar]
- 59. Zuily S, Domingues V, Suty-Selton C, Eschwege V, Bertoletti L, Chaouat A, Chabot F, Regnault V, Horn EM, Erkan D, Wahl D. Antiphospholipid antibodies can identify lupus patients at risk of pulmonary hypertension: A systematic review and meta-analysis. Autoimmun Rev. 2017; 16:576-586. [DOI] [PubMed] [Google Scholar]


