ABSTRACT
We investigated changes in heart rate (HR) and HR variability as a function of age in newborn foals to old Thoroughbred horses. Experiments were performed on a total of 83 healthy and clinically normal Thoroughbred horses. Resting HR decreased with age from birth. The relationship between age and HR fit the equation Y=48.2X–0.129(R2=0.705); the relationship between age and HR for horses 0–7 years old fit the equation Y=44.1X–0.179(R2=0.882). Seven-day-old horses had the highest HR values (106 ± 10.3 beat/min). The low frequency (LF) and high frequency (HF) powers increased with age in newborn to old horses. These changes in HR and HR variability appear to result from the effects of ageing. Three- to seven-year-old race horses had the lowest HR values (32.9 ± 3.5 beat/min) and the highest LF and HF powers except for the HF powers in the oldest horses. Race training may have contributed to these changes. Horses of ages greater than 25 years old had the highest HF powers and the lowest LF/HF ratios. In individual horses, 8 of the 15 horses over 25 years old had LF/HF ratios of less than 1.0; their HR variability appears to be unique, and they may have a different autonomic balance than horses of younger age.
Keywords: autonomic nervous activity, foal, old Thoroughbred
Heart rate (HR) variability has been suggested to be a noninvasive index of autonomic nervous activity [1, 21, 22]. The high frequency (HF) power of the power spectrum of HR variability is thought to reflect primarily parasympathetic nervous activity. Both the sympathetic and the parasympathetic nervous systems have been shown to contribute to the low frequency (LF) power component of HR variability. Therefore, the LF/HF ratio has been utilized as an index of cardiac sympathovagal balance.
Heart rate variability has been applied as a method of assessing autonomic nervous activity in Thoroughbred horses as well as in humans and other animal species [6, 7, 9, 10, 15,16,17,18,19,20, 23, 25]. Although it has been reported that the response of HR variability to sympathetic beta-adrenergic blockade in Thoroughbred horses is different from that in other mammals, it has been shown that the magnitudes of LF and HF power reflect autonomic activity in Thoroughbred horses [9, 19]. Many studies have investigated effects on HR variability of stress, effects of medications, responses of horses being transported, horses in pain, horses during training, etc [6, 7, 9, 10, 15,16,17,18,19,20, 23, 25]. It has also been reported that HR variability may reflect the degree of relaxation in horses immersed in a hot spring bath [7]. However, most reports of HR variability have been based on measurements made on mature Thoroughbreds. There have been no reports about HR variability measurements in foals (0-year-old) and geriatric Thoroughbreds. The aim of this study was to investigate age-related changes in HR and HR variability in new born foals to geriatric Thoroughbred horses.
Materials and Methods
A protocol for the study was reviewed and approved by the Animal Use and Care Committee and the Animal Welfare and Ethics Committee of the Japan Racing Association’s Equine Research Institute.
Horses and ECG recording
Measurements were made on a total of 83 healthy and clinically normal Thoroughbred horses, 60 males and 23 females. Electrocardiograms (ECGs) were recorded longitudinally from 7 foals within 24 hr of birth and at 7, 30, 90 and 200 days old. Another 6 horses were recorded longitudinally at 1.5, 2.0, and 2.5 years of age; these 6 horses did not have any training under saddle. Another sixteen 1-year-old horses and 27 older horses more than 15 years old were also measured. Older horses were divided into two groups by age; one group ranged in age from 15–25 years old, and the other group comprised those more than 25 years old. Twenty-seven Thoroughbred race horses that ranged in age from 3–7 years also had ECGs recorded; these horses had been trained for racing and had raced.
Electrocardiograms were recorded using base-apex leads for over 30 min with horses in their own stalls so as to avoid potential biasing of measurements by conducting them in an unfamiliar environment. A Holter-type ECG (SM-60, Fukuda Denshi Co., Ltd., Tokyo, Japan) was used to obtain HR. Three ECG electrodes were affixed to the thorax with foam tape to provide the lead, and the Holter-type ECG monitor was then placed in a pocket on the blanket that the horse wore. All horses were free in their stalls without any restriction from 13:00–15:00 during the measurements so that diurnal variation would not bias the measurements.
Analyzing HR variability
The recorded ECGs were analyzed with an ECG processor analyzing system (Softron Co., Ltd., Tokyo, Japan) that has been previously described [9]. The program first detected R waves and calculated the R-R interval tachogram as the raw HR variability in sequence order. Horses that had the frequency of second-degree atrioventricular block >10% of the total frequency of the HR were excluded. Noise that the software identified as R waves was eliminated manually by use of visual inspection and examination of values that were outside the interval of 75 to 125% of the mean value. From this tachogram, data sets of 512 points were resampled at 200 ms. We applied each set of data to a Hamming window and a Fast-Fourier Transform to obtain the power spectrum of the fluctuation. We set the LF power at 0.01–0.07 Hz and the HF power at 0.07–0.6 Hz; heart rate, LF power, HF power, and the LF/HF ratio were obtained from each recording.
Data analysis
Data are shown as mean ± SD. Data were fit using a nonlinear regression to analyze the relationship between HR and age. A P-value ≤0.05 was considered significant.
Results
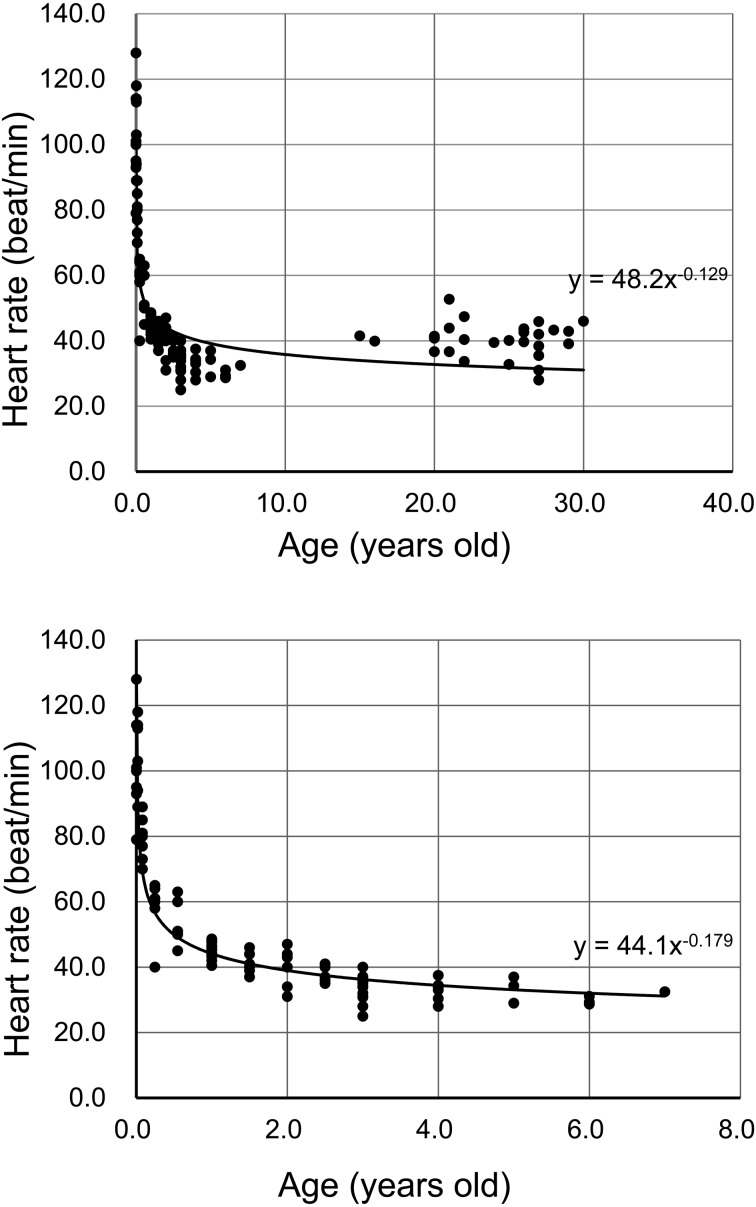
Table 1 shows detailed information about the horses and the results for HR and HR variability. Heart rate decreased with age from birth. Seven-day-old horses had the highest HR values, 106.2 ± 10.3 beat/min. The 3- to 7-year-old horses had the lowest HR values (32.9 ± 3.5 beat/min). The relationship between age and HR fit the equation Y=48.2X–0.129(R2=0.705, P<0.05, Fig. 1, top). The relationship between age and HR in 0- to 7-year-old horses fit the equation Y=44.1X–0.179(R2=0.882, P<0.05, Fig. 1, bottom). The LF power increased with age from birth; the highest values of LF power measured were in horses 3–7 years old. The HF power increased with age from birth; the highest values of HF power were in the oldest horses. Horses aged more than 25 years old had low values of the LF/HF ratio. Table 2 shows individual values of the 25-year-old horses; eight of the 15 older horses had values of less than 1.0 for the LF/HF ratio.
Table 1. Horse group information and the heart rate (HR) and variables of HR variability results.
| Number | Male | Female | Age | Body weight (kg) |
Heart rate (beat/min) |
LF power (msec2) |
HF power (msec2) |
LF/HF ratio |
|---|---|---|---|---|---|---|---|---|
| 7 | 4 | 3 | 0 days old | 56.4 ± 5.1 | 101.4 ± 14.5 | 158 ± 87 | 58 ± 35 | 2.9 ± 0.7 |
| (51–64) | (79–100) | (71–299) | (23–128) | (1.6–3.7) | ||||
| 7 days old | 72 ± 6.4 | 106.2 ± 10.3 | 171 ± 95 | 48 ± 26 | 3.8 ± 1.1 | |||
| (62–84) | (89–118) | (73–381) | (22–103) | (2.4–5.6) | ||||
| 30 days old | 115 ± 10 | 79.3 ± 6.1 | 364 ± 120 | 111 ± 83 | 4.0 ± 1.6 | |||
| (96–128) | (73–89) | (170–538) | (53–307) | (1.8–7.1) | ||||
| 90 days old | 183 ± 24 | 60.1 ± 4.0 | 574 ± 280 | 155 ± 101 | 4.2 ± 1.5 | |||
| (152–222) | (52–65) | (238–1,121) | (48–383) | (1.7–6.6) | ||||
| 200 days old | 267 ± 28 | 53.3 ± 6.2 | 1,157 ± 664 | 325 ± 146 | 3.9 ± 1.7 | |||
| (211–291) | (45–63) | (539–2,493) | (126–560) | (1.4–6.6) | ||||
| 16 | 6 | 10 | 1 year old | 45.4 ± 2.4 | 1,184 ± 535 | 289 ± 196 | 4.9 ± 2.3 | |
| (40.5–48.6) | (373–2,189) | (133–878) | (1.1–10.3) | |||||
| 6 | 4 | 2 | 1.5 years old | 433 ± 11 | 41.2 ± 3.0 | 1,926 ± 1,597 | 306 ± 157 | 6.0 ± 2.7 |
| (417–450) | (37–46) | (444–5,356) | (137–597) | (2.8–9.0) | ||||
| 2 years old | 480 ± 31 | 39.8 ± 5.6 | 1,744 ± 809 | 336 ± 113 | 5.7 ± 2.7 | |||
| (426–515) | (31–47) | (805–3,338) | (173–486) | (2.4–10.4) | ||||
| 2.5 years old | 505 ± 34 | 37.7 ± 2.1 | 1,853 ± 738 | 363 ± 96 | 5.4 ± 2.0 | |||
| (457–541) | (35–40) | (1,050–3,062) | (187–459) | (2.3–8.1) | ||||
| 27 | 24 | 3 | 3.9 ± 1.2 years old | 32.9 ± 3.5 | 3,138 ± 1,045 | 844 ± 597 | 5.3 ± 2.9 | |
| (3–7) | (25.0–40.0) | (1,575–5,488) | (213–2,545) | (1.1–13.2) | ||||
| 12 | 11 | 1 | 20.3 ± 2.5 years old | 41.2 ± 5.0 | 1,371 ± 628 | 452 ± 185 | 3.1 ± 0.9 | |
| (15–24) | (33.7–52.7) | (623–2,391) | (206–796) | (1.7–5.1) | ||||
| 15 | 11 | 4 | 27.1 ± 1.4 years old | 39.4 ± 5.4 | 1,665 ± 1,425 | 2,679 ± 4,281 | 1.5 ± 1.3 | |
| (25–30) | (28.0–46.0) | (168–4,529) | (183–13,050) | (0.3–4.4) | ||||
Values are means ± SD. LF power represents the low frequency power spectrum, and HF power represents the high frequency power spectrum of HR variability.
Fig. 1.
(Top) The relationship between age and HR fit the equation Y=48.2X–0.129 (R2=0.705). (Bottom) The relationship between age and HR from 0–7 years of age fit the equation Y=44.1X–0.179 (R2=0.882).
Table 2. Individual data of HR and variables of HR variability for horses over 25 years old.
| Horse | Gender | Age (years) |
Heart rate (beat/min) |
LF power (msec2) |
HF power (msec2) |
LF/HF ratio |
|---|---|---|---|---|---|---|
| 1 | Male | 26 | 39.7 | 3,234 | 12,900 | 0.25 |
| 2 | Male | 26 | 43.7 | 4,308 | 13,050 | 0.33 |
| 3 | Female | 28 | 43.3 | 1,061 | 2,694 | 0.39 |
| 4 | Male | 25 | 40.1 | 838 | 1,905 | 0.44 |
| 5 | Male | 27 | 31 | 689 | 1,398 | 0.49 |
| 6 | Female | 30 | 46 | 168 | 254 | 0.66 |
| 7 | Male | 27 | 38.4 | 608 | 629 | 0.97 |
| 8 | Male | 29 | 42.9 | 533 | 536 | 0.99 |
| 9 | Male | 27 | 28 | 4,529 | 3,302 | 1.37 |
| 10 | Female | 29 | 39.1 | 266 | 183 | 1.45 |
| 11 | Male | 27 | 45.9 | 2,422 | 1,381 | 1.75 |
| 12 | Male | 27 | 42 | 1,102 | 509 | 2.16 |
| 13 | Male | 25 | 32.8 | 1,243 | 440 | 2.82 |
| 14 | Male | 27 | 35.5 | 2,665 | 703 | 3.79 |
| 15 | Female | 26 | 42.6 | 1,309 | 300 | 4.37 |
Discussion
We investigated changes in HR and HR variability as a function of age in newborn foals to geriatric Thoroughbred horses. The mean HR decreased with age in 7-day-old to geriatric horses. The LF and HF powers tended to increase with age in newborn foals to geriatric horses. These changes in HR and HR variability appear to result from the effects of ageing. The 3- to 7-year-old horses had the lowest HR values and the highest LF and HF powers except for the HF powers of the oldest horses, presumably because of the effects of training. Horses more than 25 years old had the highest HF powers and the lowest LF/HF ratios. Their HR variability is unique and may indicate that they have a different autonomic balance from that of younger horses.
It was reported that resting HR decreased and the LF and HF powers increased with training in young Thoroughbred as age increased from 1 (yearling) to 2 years old [16]. The authors concluded that parasympathetic nervous activity increased and resting HR decreased in young Thoroughbreds with 7 months of training [16]. Therefore, it speculated that the decrease of resting HR may be dependent on the training-induced increase of parasympathetic nervous activity in Thoroughbred horses. In this study, 3- to 7-year-old horses had the lowest HR values and the highest LF and HF powers except for the HF powers of the oldest horses. All of the 3- to 7-year-old horses were racehorses and had been trained 6 days a week. The other young horses and older horses were not being trained when ECGs were recorded. Therefore, these changes in HR and HR variability in the 3- to 7-year-old horses may have resulted from the effects of training for racing.
Positive correlation between HR and energy expenditure has been documented for a number of mammalian species [2,3,4,5, 8, 11,12,13,14]. It was reported that changes in HR and HR variability, as indices of autonomic nervous activity, could be associated with metabolic rate in Thoroughbred horses [15]. In this study, HR decreased with age in newborn foals up to the oldest horses. The relationship between age and HR fit well the hyperbolic curve determined by nonlinear regression. Generally speaking, younger animals and smaller animals have higher metabolic rates than do older and larger animals, as dictated by allometric relationships [24]. We speculate that foals may need to have higher HR values to support a relatively high cardiac output in order to support their higher energy expenditure. However, further studies will be needed to clarify the association between HR and age on energy expenditure in Thoroughbreds.
Horses over 25 years old had the highest HF powers and the lowest LF/HF ratios. Eight of the 15 older horses had values of less than 1.0 for the LF/HF ratio. It has been reported that the responses of the LF/HF ratio to the sympathetic beta-adrenergic blocker propranolol in Thoroughbreds did not change [9, 19]. It has also been reported that parasympathetic nervous activity might play a role in mediating both LF power and HF power, and similar changes in both LF power and HF power may result in the LF/HF ratio maintaining a relatively constant value in the horses [19]. Therefore, it was thought that changes in parasympathetic activity are not reflected in the LF/HF ratio. Moreover, a relaxing condition, e.g., immersion in hot spring water, resulted in no change in the LF/HF ratio either, although both the LF and HF powers increased [7]. We never observed higher HF power in combination with a lower LF/HF ratio in the horses in this study. We speculated that horses over 25 years old had increased cardiac vagal tone. However, it was difficult to explain that only increased cardiac vagal tone was the reason why they had higher HF power in combination with a lower LF/HF ratio. Their HR variability may be unique, and they may have a different autonomic balance than younger horses.
In conclusion, we observed the effect of ageing by changes in HR and HR variability in newborn foals to geriatric Thoroughbred horses. Resting HR decreased with age from birth, and the relationship between age and HR fit well a hyperbolic equation. Horses aged over 25 years old may have a different autonomic balance than younger horses, as they had the highest HF powers and the lowest LF/HF ratios.
References
- 1.Akselrod S., Gordon D., Ubel F.A., Shannon D.C., Berger A.C., Cohen R.J. 1981. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn M.W., Calloway D.H. 1985. Heart rate and energy expenditure of pregnant and lactating women. Am. J. Clin. Nutr. 42: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 3.Brosh A., Aharoni Y., Degen A.A., Wright D., Young B. 1998. Estimation of energy expenditure from heart rate measurements in cattle maintained under different conditions. J. Anim. Sci. 76: 3054–3064. [DOI] [PubMed] [Google Scholar]
- 4.Ceesay S.M., Prentice A.M., Day K.C., Murgatroyd P.R., Goldberg G.R., Scott W., Spurr G.B. 1989. The use of heart rate monitoring in the estimation of energy expenditure: a validation study using indirect whole-body calorimetry. Br. J. Nutr. 61: 175–186. [DOI] [PubMed] [Google Scholar]
- 5.Hebestreit H., Bar-Or O., McKinty C., Riddell M., Zehr P. 1995. Climate-related corrections for improved estimation of energy expenditure from heart rate in children. J. Appl. Physiol. 79: 47–54. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya K., Ohmura H., Eto D., Mukai K., Ushiya S., Hiraga A., Yokota S. 2003. Heart size and heart rate variability of the top earning racehorse in Japan, T. M. Opera O. J. Equine Sci. 14: 97–100. [Google Scholar]
- 7.Kato T., Ohmura H., Hiraga A., Wada S., Kuwahara M., Tsubone H. 2003. Changes in heart rate variability in horses during immersion in warm springwater. Am. J. Vet. Res. 64: 1482–1485. [DOI] [PubMed] [Google Scholar]
- 8.Kriemler S., Hebestreit H., Bar-Or O. 2002. Temperature-related overestimation of energy expenditure, based on heart-rate monitoring in obese boys. Eur. J. Appl. Physiol. 87: 245–250. [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara M., Hashimoto S., Ishii K., Yagi Y., Hada T., Hiraga A., Kai M., Kubo K., Oki H., Tsubone H., Sugano S. 1996. Assessment of autonomic nervous function by power spectral analysis of heart rate variability in the horse. J. Auton. Nerv. Syst. 60: 43–48. [DOI] [PubMed] [Google Scholar]
- 10.le Jeune S.S., Williams C.A., Pypendop B.H., Ohmura H., Jones J.H. 2014. Does acupuncture acutely affect heart rate variability in horses? J. Equine Vet. Sci. 34: 1084–1090. [Google Scholar]
- 11.Livingstone M.B., Coward W.A., Prentice A.M., Davies P.S., Strain J.J., McKenna P.G., Mahoney C.A., White J.A., Stewart C.M., Kerr M.J. 1992. Daily energy expenditure in free-living children: comparison of heart-rate monitoring with the doubly labeled water (2H2(18)O) method. Am. J. Clin. Nutr. 56: 343–352. [DOI] [PubMed] [Google Scholar]
- 12.Livingstone M.B., Prentice A.M., Coward W.A., Ceesay S.M., Strain J.J., McKenna P.G., Nevin G.B., Barker M.E., Hickey R.J. 1990. Simultaneous measurement of free-living energy expenditure by the doubly labeled water method and heart-rate monitoring. Am. J. Clin. Nutr. 52: 59–65. [DOI] [PubMed] [Google Scholar]
- 13.Livingstone M.B., Robson P.J., Totton M. 2000. Energy expenditure by heart rate in children: an evaluation of calibration techniques. Med. Sci. Sports Exerc. 32: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 14.Maffeis C., Pinelli L., Zaffanello M., Schena F., Iacumin P., Schutz Y. 1995. Daily energy expenditure in free-living conditions in obese and non-obese children: comparison of doubly labelled water (2H2(18)O) method and heart-rate monitoring. Int. J. Obes. Relat. Metab. Disord. 19: 671–677. [PubMed] [Google Scholar]
- 15.Ohmura H., Boscan P.L., Solano A.M., Stanley S.D., Jones J.H. 2012. Changes in heart rate, heart rate variability, and atrioventricular block during withholding of food in Thoroughbreds. Am. J. Vet. Res. 73: 508–514. [DOI] [PubMed] [Google Scholar]
- 16.Ohmura H., Hiraga A., Aida H., Kuwahara M., Tsubone H. 2002. Effects of initial handling and training on autonomic nervous function in young Thoroughbreds. Am. J. Vet. Res. 63: 1488–1491. [DOI] [PubMed] [Google Scholar]
- 17.Ohmura H., Hiraga A., Aida H., Kuwahara M., Tsubone H. 2003. Influence of quinidine and flecainide on autonomic nervous activity in thoroughbred horses. Vet. Rec. 152: 114–116. [DOI] [PubMed] [Google Scholar]
- 18.Ohmura H., Hiraga A., Aida H., Kuwahara M., Tsubone H., Jones J.H. 2006. Changes in heart rate and heart rate variability in Thoroughbreds during prolonged road transportation. Am. J. Vet. Res. 67: 455–462. [DOI] [PubMed] [Google Scholar]
- 19.Ohmura H., Hiraga A., Aida H., Kuwahara M., Tsubone H. 2001. Effects of repeated atropine injection on heart rate variability in Thoroughbred horses. J. Vet. Med. Sci. 63: 1359–1360. [DOI] [PubMed] [Google Scholar]
- 20.Ohmura H., Hobo S., Hiraga A., Jones J.H. 2012. Changes in heart rate and heart rate variability during transportation of horses by road and air. Am. J. Vet. Res. 73: 515–521. [DOI] [PubMed] [Google Scholar]
- 21.Pagani M., Lombardi F., Guzzetti S., Rimoldi O., Furlan R., Pizzinelli P., Sandrone G., Malfatto G., Dell’Orto S., Piccaluga E., et al. 1986. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59: 178–193. [DOI] [PubMed] [Google Scholar]
- 22.Pomeranz B., Macaulay R.J., Caudill M.A., Kutz I., Adam D., Gordon D., Kilborn K.M., Barger A.C., Shannon D.C., Cohen R.J., et al. 1985. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 248: H151–H153. [DOI] [PubMed] [Google Scholar]
- 23.Rietmann T.R., Stauffacher M., Bernasconi P., Auer J.A., Weishaupt M.A. 2004. The association between heart rate, heart rate variability, endocrine and behavioural pain measures in horses suffering from laminitis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 51: 218–225. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Nielsen K. 1984. Body temperature and temperature regulation. pp. 197–208. In: Scaling: Why is Animal Size So Important? Cambridge University Press, Cambridge. [Google Scholar]
- 25.Younes M., Robert C., Barrey E., Cottin F. 2016. Effects of age, exercise duration, and test conditions on heart rate variability in young endurance horses. Front. Physiol. 7: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]