Abstract
Background
Previous clinical trials of α7-nicotinic acetylcholine receptor agonists (α7-nAChR agonists) showed mixed results in treating the cognitive and negative symptoms of schizophrenia.
Aims
To assess the efficacy and safety of α7-nAChR agonists in treating the cognitive and negative symptoms in schizophrenia.
Methods
A literature search was conducted to identify randomized double-blind placebo-controlled trials for schizophrenia published before May 26, 2017, by searching PubMed, Embase, ClinicalTrials.gov, the Cochrane Library and the Chinese language databases CNKI, Wanfang, and VIP Data. The effects of α7-nAChR agonists were evaluated for overall cognitive function and negative symptoms by calculating standard mean difference (SMDs) between active drugs and placebo added to antipsychotics.
Results
8 studies with low bias were included. We found no statistically significant effects of α7 nAChR agonists on the overall cognitive function (SMD=-0.10[-0.46, 0.25], I2 =88%) and negative symptoms (SMD=0.13 [-0.04, 0.30], I2 =64%) in patients with schizophrenia. Sensitivity analysis showed these results to be firm. And this drug is generally safe and well tolerated with no significant difference from placebo based on adverse events (RR=1.02, [0.85, 1.23]) and dropouts (RR=1.04, [0.61, 1.78]) data. Evidence based on outcomes from the meta-analysis was rated as ‘moderate’ as per the GRADE guidelines.
Conclusion
α7-nAChR agonists may not be effective in reversing overall cognitive impairments and negative symptoms in patients with schizophrenia as adjunctive therapies.
Key words: schizophrenia, cognitive dysfunction, nicotinic agonists, meta-analysis
Abstract
背景
现有α7- 烟碱型乙酰胆碱受体激动剂(α7-nAChR 受体激动剂) 对精神分裂症的认知障碍和阴性症状治 疗的临床研究结果不尽一致。
目的
评估α7- 烟碱型乙酰胆碱受体激动剂在治疗精 神分裂症认知缺损和阴性症状的临床疗效和安全性。
方法
PubMed、Embase、ClinicalTrials.gov、Cochrane Library 和中国知网、万方、VIP 数据库进行文献检索, 检索时间截止于 2017 年 5 月 26 日。Meta 分析双盲随 机对照试验中α7-nAChR 受体激动剂的作用,评估α7-nAChR 受体激动剂对精神分裂症的总体认知功能和阴 性症状的临床疗效。通过计算药物和安慰剂之间的平 均差(SMDs),评估α7-nAChR 受体激动剂能否作为有 效的抗精神病药物。
结果
8 个低偏倚研究纳入了meta 分析。我们没有 发现α7 乙酰受体激动剂对精神分裂症患者的认知障 碍 (SMD=-0.10(-0.46,0.25), I2 = 88%) 和阴性症状(SMD =0.13(-0.04,0.30), I2 = 64%) 有明显疗效。敏感性分析也 印证了此结果。药物总体安全且耐受性良好,在不良 事件(RR = 1.02, [0.85, 1.23]) 和脱落率 (RR = 1.04, [0.61, 1.78]) 与安慰剂对照无显著差异。根据GRADE 评级, 该meta 分析结果的证据强度为“ 中”。
结论
α7-nAChR 受体激动剂可能不是有效地改善精神 分裂症患者总体认知障碍和的阴性症状的药物。
1. Introduction
The diagnosis of schizophrenia is generally believed to be based on positive and negative symptoms. Positive symptoms such as delusions and hallucinations are often the target of attention and treatment. However, negative symptoms, such as blunted affect and social avoidance; and cognitive symptoms, such as poor attention and disorientation are also distinct symptom domains. One schizophrenia subtype known as deficit schizophrenia (DS), represents a stable clinical subtype of schizophrenia that, in comparison with non-deficit schizophrenia, is associated with a greater impairment of neurocognitive abilities and social cognition, poorer response to treatment and worse outcome. This homogeneous subtype of the schizophrenic syndrome was introduced by Carpenter et al to distinguish the primary, enduring negative symptoms of schizophrenia (termed “deficit symptoms”) from the more transient negative symptoms secondary to other factors.[1]
In the long term, positive symptoms vary, whereas negative symptoms remain relatively constant, possibly related to medication intake. Currently approved treatments such as first- and second-generation antipsychotics are primarily efficacious[2] at treating positive symptoms but do not adequately improve cognition or negative symptoms in patients.[3,4] Thus, there is an urgent need to develop new therapeutic targets for the treatment of the full constellation of symptoms.
Some authors have reported that nAChRs stimulation of the brain dopamine receptor system normalizes a number of sensory processing deficits associated with schizophrenia, including sensory, PPI and eye- tracking deficits, the hypothesis of nAChR “hypofunction” in schizophrenia has stimulated the development of selective nicotinic receptor agonists as putative treatments for negative symptoms and cognitive dysfunction.[5]
One therapeutic target that has recently been found is the α7-nicotinic acetylcholine receptor (α7 receptor), which was first identified in animal models of a sensory gating deficit associated with schizophrenia.[6] α7 receptors are localized on the presynaptic and postsynaptic elements in the hippocampus and cerebral cortex, regions critical to the synaptic plasticity underlying learning and memory. In schizophrenia, α7 receptors protein expression is decreased with altered transcription seen in post-mortem brain samples. Additionally, α7 receptors are present on the pre- and postsynapses of neurons containing other neurotransmitters(g-aminobutyric acid, acetylcholine, glutamate) that are important for cognition.[7] Activation of the α7 receptors increases cholinergic neurotransmission and the release of glutamate (Glu) and dopamine (DA) exerts procognitive effects in rats.[8]
Notably, in the past several years, several compounds have been identified as α7-nicotinic acetylcholine receptor agonists. These compounds include encenicline, RG3487, TC-5619, ABT-126, DMXB-A and so on. Some phase 2 clinical trials have been conducted to investigate the procognitive effects of these new drugs adjunct to some second-generation antipsychotics. However, the outcomes published seem diverse. Here, we conduct a comprehensive meta-analysis to provide robust evidence on the effects of α7-nicotinic acetylcholine receptor agonists on cognitive functions and negative symptoms in patients with schizophrenia.
2. Methods
2.1 Criteria for considering studies
Study types considered for meta-analysis included all relevant randomized double-blind controlled trials with individuals who had a diagnosis of schizophrenia according the DSM-IV.[26] Types of interventions included were α7 nAChR agonists including encenicline, varenicline and tropisetron(4) or α7 nAChR positive allosteric modulators in combination with choline that were given orally, and adjunctively to ongoing stable antipsychotic treatment. Placebo was given as a control group.
Types of outcome measures included: (i) Primary outcome-Cognitive function: Mean change of scale overall scores from baseline to treatment endpoint in which cognitive function is measured. This was done mainly using the Measurement And Treatment Research to Improve Cognition in Schizophrenia(MATRICS) Consensus Cognitive Battery (MCCB). MCCB packaging 10 tests, is a method developed by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) group to evaluate the efficacy of treatments targeting cognitive impairments in schizophrenia. It measures 7 cognitive domains: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. This was a continuous outcome measure.[9] Studies which reported the scores change of the 7 domains should also be reported.
(ii) Secondary outcomes--Negative symptoms: Mean change of the positive and negative syndrome scale(PANSS) negative symptoms subscale score or Scale for the Assessment of Negative Symptoms(SANS) score from baseline to endpoint. PANSS is a scale used for measuring symptom severity for patients with schizophrenia. SANS is used specifically for the assessment of negative symptoms in schizophrenia. Safety and tolerability should also be reported as adverse event rates and dropout rates.
2.2 Search methods for identification of studies
Relevant randomized controlled trials were identified by searching several electronic databases (PubMed, Embase, ClinicalTrials.gov, the Cochrane Library and CNKI, WanFang Data, VIP Data) for papers published before May 26, 2017. Keywords included synonyms of “schizophrenia”, “α7-nicotinic acetylcholine receptor agonist”, and “cognitive dysfunction” (and the Chinese equivalents). A set of search terms were [(alpha7 OR alpha-7) AND agonist OR positive allosteric modulator OR encenicline(3) OR varenicline OR tropisetron(4)) and (Schizophrenia OR Schizoaffective Disorder OR Schizophreniform Disorder OR Dementia Praecox) AND (random* OR RCT OR control* OR compare* OR placebo)] were also utilized. Various combinations of these keywords were used to search for articles. Limits were set for “clinical trials” and “humans” where applicable. Reference lists of retrieved studies and review articles were manually searched for additional studies relevant for meta-analysis.
2.3 Data collection and analysis
Selection of trials
Two authors (JY and WQ) independently inspected the abstract of each reference identified by the search to see if the study was likely to be relevant. When it was unclear from an abstract whether a study was a randomised trial or if there was disagreement between the two authors, the full article was obtained. The article was then inspected independently by the two authors to assess its relevance to the analysis.
Data collection
Both authors independently extracted the data from the included trials. Again, any disagreement was discussed, the decisions documented. The remaining problems were arbitrated by the third reviewer (LXB).
Data synthesis
Meta-analysis: The meta-analysis was performed using Review Manager Version 5.3. Because the outcomes are continuous, standard mean differences were calculated using the inverse variance statistical method and random effects model to adjust for study heterogeneity. Unreported SD values were calculated from other available data in the articles (SD=SE×√N) according to the Cochrane Handbook for Systematic Reviews of Interventions(Chapters 7.7.3.2). Count data were shown by using relative risk (RR). The results of the combined calculation were shown in the forest plot. Two-sided 95% CIs were used to assess significance, according to whether the CIs included the null value.
Heterogeneity: Study heterogeneity was quantified for the outcome analysis using the I2 statistic alongside the Chi2 ‘P’ value with I2 ≥ 50% indicating significant heterogeneity.[29] When heterogeneity is present, sensitivity analyses should be conducted to assess potential influences of any one single study on the pooled MD and associated P-values, and if necessary, subgroup analyses should be conducted to explore the source of heterogeneity.[29]
Assessment of risk of bias: Included trials were assessed with the Cochrane Risk of Bias Tool for methodological quality of sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.[10]
The quality assessment of meta-analytic outcomes: The grading of recommendations assessment, and evaluation (GRADE) system was used to assess the quality of evidence for main outcomes.[11] The overall level of evidence was rated as ‘high’, ‘moderate’, ‘low’, or ‘very low’.
Assessment of publication biases: Publication biases arise when the dissemination of research findings is influenced by the nature and direction of results. We are aware that funnel plots may be useful in investigating publication biases but are of limited power to detect small-study effects. We intended not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. [31]
3. Results
3.1 Characteristics of included studies
Eight double-blind randomized placebo-controlled trials were included. (total number of subjects, N= 1438)[7,8,12-17] The PRISMA flow diagram is displayed in Figure 1. Study characteristics are summarized in Table 1.
Figure 1.
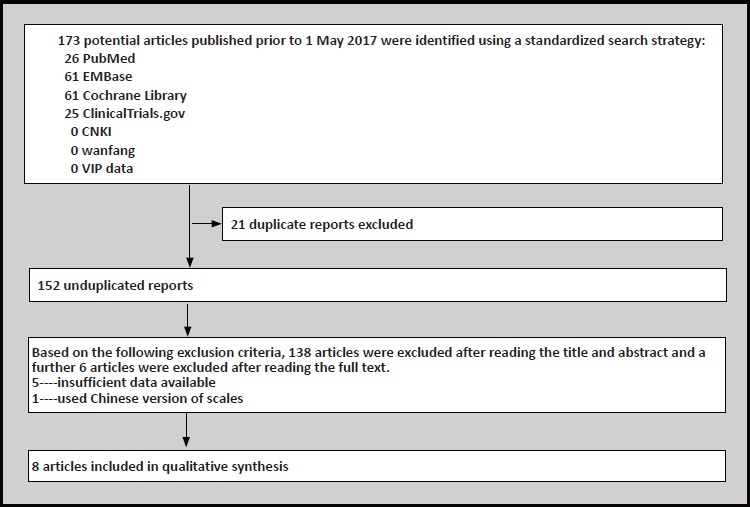
Flowchart of literature search and exclusion process
Table 1.
Characteristics of included studies
| Study | alpha7 agonist | Dose(s) (mg/day) | antipsychotics | N | age (year) | study duration (weeks) | smokers | duration of illness (year) | PANSS total score | outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|
| Shiina 2010 | tropisetron | 10 | risperidone | 40 | 18-55 | 8 | 27.5% | 10.9(7.6) | 64.6 (15.2) | PANSS, QLS |
| Noroozian 2013 | tropisetron | 10 | risperidone | 40 | 45.1 (9.9) | 8 | unclear | > 2 | 46.6 (2.8) | PANSS, ANOVA |
| Umbricht 2014 | RG3487 | 5, 15, 50 | aripiprazole, risperidone paliperidone | 215 | 21-48 | 8 | 76.7% | unclear | ≤ 70 | MCCB, SANS |
| Keefe 2015 | encenicline | 0.27, 0.90 | unclear | 317 | 18-55 | 12 | 56.7% | unclear | unclear | PANSS, MCCB |
| Walling 2015 | TC-5619 | 5, 50 | unclear | 477 | 18-65 | 24 | 55% | unclear | unclear | PANSS, CogState OCI, SANS, CSB |
| Jeon 2016 | varenicline | 2 | unclear | 59 | 20-55 | 8 | 100% | > 2 | ≤75 | PANSS, SANS, HAM-D |
| Smith 2016 | varenicline | 2 | unclear | 87 | 33.8 (6.4) | 8 | 100% | unclear | 57.46 (16.79) | PANSS |
| Haig 2016 | ABT-126 | 10, 25 | unclear | 203 | 18-60 | 12 | 74.9% | unclear | unclear | MCCB, PANSS |
QLS: Quality of life scale
ANOVA: analysis of varivance
CogState OCI: CogState overall cognition index
HAM-D: Hamilton depression scale
All the included studies involved participants who had been diagnosed using operationalised criteria (DSM-IV). Six different kinds of α7-nicotinic agonist were used as the intervention, varenicline,[12,13] tropisetron,[14,15] ABT-126,[7] encenicline,[8] TC-5619,[16] and RG3487.[17]
The mean(sd) duration of the studies was 14.7(6.7) weeks (range: 8–24 weeks). 5 studies (Umbricht 2014, Haig 2016, Keefe 2015, Smith 2016 and Walling 2016) were included in the analysis of cognitive function, which used MCCB and CSB(Cogstate Schizophrenia Batery),[18] and 8 studies(Haig 2016, Walling 2015, Keefe 2015, Jeon 2016, Noroozian 2013, Shiina 2010, Walling 2016 and Smith 2016) were included in the analysis of negative symptoms, which used PANSS and SANS.
The controversial problem during data extraction process was that one study (Keefe, 2015) used LOCF [19] (Last observation carried forward) analysis to achieve ITT(Intention-to Treat)[20] analysis. However, this method would lower the reliability of study outcomes. The decision was made by the third author (LXB) to include this LOCF outcome.
3.2 Risk of bias
The risks of bias of included studies are summarized in Table 2. Although all studies were randomized trials, the methodology of allocation concealment was often unreported, leading to ‘unclear risk’ for selection bias in 6 studies (75%). One study (12.5%) was judged to have ‘unclear risk’ for detection bias since the blinding of outcome assessment was unspecified. One study (12.5%) has attrition bias and 4 studies (50%) did not report other bias.
Table 2.
Risk of bias summary
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | other bias |
|---|---|---|---|---|---|---|---|
| Shiina 2010 | low | Unclear | Low | Low | Low | Low | Unclear |
| Noroozian 2013 | low | unclear | Low | Low | Low | Low | Unclear |
| Umbricht 2014 | low | Low | Low | low | Low | Low | Unclear |
| Keefe 2015 | low | unclear | low | unclear | low | low | Unclear |
| Haig 2016 | low | low | low | low | unclear | low | Low |
| Jeon 2016 | low | Unclear | Low | Low | Low | Low | Low |
| Smith 2016 | low | Unclear | Low | Low | Low | Low | Unclear |
| Walliing 2016 | low | unclear | low | low | low | low | unclear |
3.3 Meta-analyses
Primary outcome--Effects of α7-nicotinic agonist on cognitive function
There was no statistically significant difference favouring α7-nicotinic agonists in terms of overall cognitive function (SMD=-0.10[-0.46, 0.25], I2=88%) (Figure 2) in patients with schizophrenia according to MCCB overall scores. However, one study (Freedman 2008) [6] reported two domains (attention/vigilance and working memory) significantly improved over baseline with DMXB-A treatment, but it was only a secondary analysis using the results from one arm of the study with limited power.
Figure 2.
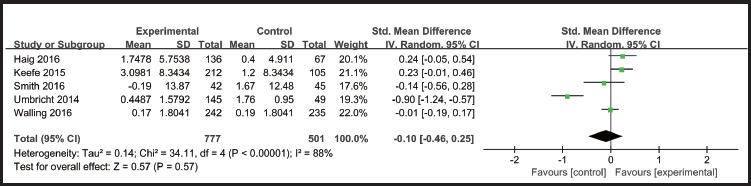
Effect of α7-nicotinic agonist on cognitive function
Secondary outcome--Effects of α7-nicotinic agonist on negative symptoms
There was no statistically significant difference between α7-nicotinic agonists and placebo in terms of negative symptoms (SMD=0.13 [-0.04, 0.30], I2=64%) in patients with schizophrenia. (Figure 3)
Figure 3.
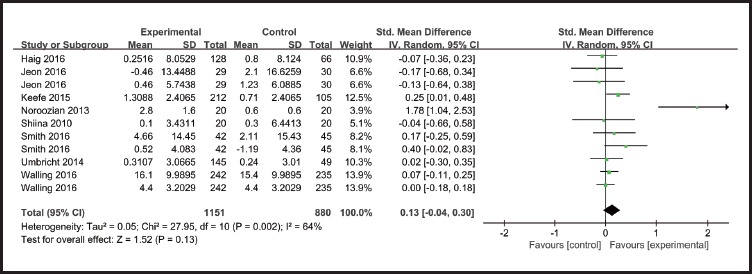
Effect of α7-nicotinic agonist on negative symptoms
Secondary outcome-- Tolerability and safety
Most adverse events reported in the articles were considered by study investigators to be mild or moderate in severity. Common adverse events include dizziness, headache, nausea, fatigue, nasopharyngitis and so on. We got data of adverse events and dropouts rates ofα7-nicotinic agonists as well as placebo in four studies (Umbricht 2014, Keefe 2015, Walling 2015 and Haig 2016). For the other four studies (Shiina 2010, Noroozian 2013, Smith 2016 and Jeon 2016), the overall adverse rates and dropouts rates were unclear. There were no statistically significant differences between α7-nicotinic agonist group and the placebo group in the incidence of adverse events (RR=1.02, p=0.84)(Figure.4) and dropouts(RR=1.04, p=0.88)(Figure.5).
Figure 4.
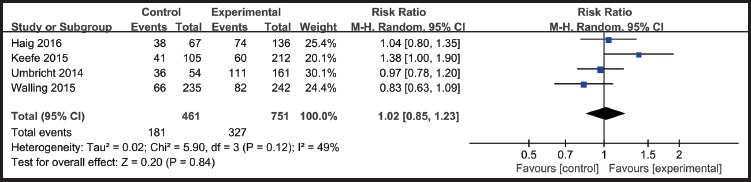
Adverse events rates: α7-nicotinic agonists VS placebo
Figure 5.
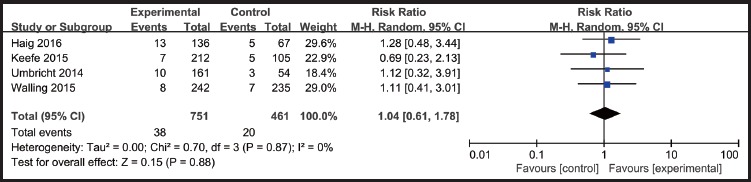
Dropouts rates: α7-nicotinic agonists VS placebo
Sensitivity analysis
In order to explain the heterogeneity of cognition outcome(I2 =88%), sensitivity analysis was conducted, and after one outlier(SMD=-0.90 [-1.24, -0.57]) study(Umbricht 2014) was removed, the results remained not statistically significant(SMD= 0.10 [-0.07, 0.26], I2 =37%). With regards to the negative symptoms outcome (I2 =64%), sensitivity analysis also found the results remained not statistically significant (SMD=0.07 [-0.01, 0.16], I2 =0%) after removing one outlier study (SMD=1.78 [1.04, 2.53]), (Noroozian 2013).
Assessment of publication biases
There were only eight studies included in the meta-analysis, so it was not possible to assess publication bias using funnel plots (which requires at least ten studies).[30] However, we assume our results have publication biases in assessment of evidence quality using the GRADE approach.
Assessment of evidence quality
The evidence quality for 4 outcome measures as assessed by the GRADE approach were moderate. (Table 3)
Table 3.
Summary of meta-analysis and GRADE assessments of quality of data
| outcome measure | number of studies (pooled sample) |
Heterogeneity | Total effects | Measure compared | 95%CI | GRADE result | ||
|---|---|---|---|---|---|---|---|---|
| I2 | p | Z | p | |||||
| Cognition | 5(1278) | 88% | <0.00001 | 0.57 | 0.57 | SMD=-0.1 | [-0.46,0.25] | moderate |
| Negative symptoms | 8(1438) | 64% | 0.002 | 1.52 | 0.13 | SMD=0.13 | [-0.04,0.30] | Moderate |
| Adverse events | 4(1212) | 49% | 0.12 | 0.20 | 0.84 | OR=1.02 | [0.85, 1.23] | high |
| Dropouts rates | 4(1212) | 0% | 0.87 | 0.15 | 0.88 | OR=1.04 | [0.61, 1.78] | high |
4. Discussion
4.1 Main findings
To our knowledge, this is the first comprehensive meta-analysis to examine the effects of α7-nicotinic acetylcholine receptor agonists on both cognitive deficits and negative symptoms in patients with schizophrenia. As a whole, α7-nicotinic agonists were not found to be superior to placebo as an adjunctive therapy to antipsychotics in cognitive function (no statistically significant difference favoring α7 agonists) even after sensitivity analysis removing one outlier study (Umbricht 2014). Outcomes for negative symptoms [8,14] also showed no significant difference between this drug and placebo and sensitivity analysis indicated that this result was firm.
This result is consistent with a recent meta-analysis by Lewis and colleagues.[21] However, the present study has the additional advantage of analyzing the intervention’s effect on the negative symptoms of schizophrenia (besides cognition).
4.2 Limitations
The present report must be considered in light of various limitations. First, the number of included individual studies was small. Second, we did not examine the long-term effects of α7-nicotinic agonists since the duration of individual studies did not exceed 24 weeks. Third, influences of concomitant antipsychotics are not clear. Fourth, we cannot analyze the relationship between tobacco use and the effects of α7-nicotinic agonists because the articles we found provided insufficient data on nonsmokers. Fifth, we excluded 6 RCT studies, Deutsch 2008, [22] Shim 2012,[23] Preskorn 2014,[24] Lieberman 2013,[25] Freedman 2008,[6] Zhang 2012,[27] because the former 4 studies did not present enough data needed for meta-analysis and we were unable to successfully contact the authors. The fifth study was a crossover trial because we cannot ignore the long-term effect of this kind of agent [28]; and the last study used a Chinese version of the scales. Sixth, the scales we chose for our meta-analysis measuring cognition and negative symptoms were limited to MCCB, CSB, SANS and PANSS negative subscale, which did not make full use of the data given from many other scales. Seventh, the individual pharmacological profiles of these agents are likely to play a role in the pharmacodynamic heterogeneity; for example, TC-5619 is a full agonist at the a7nAChR, and EVP-6124 and RG3487 are partial agonists at this receptor, with differences in 5-HT3 receptor antagonism.[17] Eighth, the relationship between dose and effects of this drug cannot be ignored since some previous clinical studies divided subjects into different dose groups. Ninth, the study data we used for our meta-analysis was change scores from baseline which we were unclear if this data was skewed or not. Finally, a possibility of publication bias should not be dismissed.
4.3 Implications
In this meta-analysis, no statistical significant difference was found between α7-nicotinic acetylcholine receptor agonists and placebo for overall cognitive deficits and negative symptoms in patients with schizophrenia. However, some previous preclinical models and pharmaceutical phase II proof-of-concept trials have demonstrated a7nAChR agonist efficacy, and there are signals of clinical efficacy on several specific cognitive domains, such as attention. Previous studies [21] indicated that choice of dose and dosing frequency are critical to the demonstration of therapeutic effects in clinical trials. Further research, those for example, with a large sample and low heterogeneity are required to elucidate the role of α7-nicotinic agonists in schizophrenia and explain its mechanism.
Acknowledgment
We thank the reviewers of this analysis for their useful comments.
Biography

Ye Jin obtained a bachelor degree from China Medical University in 2016, and is now working on her Master’s at China Medical University, Shenyang, China. She has been working at the department of psychiatry of the First Affiliated Hospital of China Medical University in Shenyang, China since 2016. Her research interests include psychopharmacology and biological psychiatry.
Footnotes
Funding statement
This work was supported by the Natural Science Foundation of Liaoning Province (Project No. 2015020481).
Conflict of interest statement
The authors claim no financial conflict of interests related to this manuscript.
Authors’ contributions
Ye Jin contributed to the conception of the study.
Xiaobai Li contributed significantly to analysis and manuscript preparation.
Qi Wang performed the data analyses and wrote the manuscript;
Yan Wang, Mengxi Liu, Anji Sun, Yiwei lin and Zhongli Geng helped perform the analysis.
References
- 1.Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988; 145(5): 578–583. doi: http://dx.doi.org/10.1176/ajp.145.5.578 [DOI] [PubMed] [Google Scholar]
- 2.Galderisi S, Merlotti E, Mucci A. Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2015; 265(7): 543-558. doi: http://dx.doi.org/10.1007/s00406-015-0590-4 [DOI] [PubMed] [Google Scholar]
- 3.Citrome L. Unmet needs in the treatment of schizophrenia: new targets to help different symptom domains. J Clin Psychiatry. 2014; 75 (Suppl 1): 21-26. doi: http://dx.doi.org/10.4088/JCP.13049su1c.04 [DOI] [PubMed] [Google Scholar]
- 4.Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013; 86(8): 1122-1132. doi: http://dx.doi.org/10.1016/j.bcp.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbronner U, Samara M, Leucht S, Falkai P, Schulze TG. The longitudinal course of schizophrenia across the lifespan: Clinical, cognitive, and neurobiological aspects. Harv Rev Psychiatry. 2016; 24(2): 118-128. doi: http://dx.doi.org/10.1097/HRP.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008; 165(8): 1040-1047. doi: http://dx.doi.org/10.1176/appi.ajp.2008.07071135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haig GM, Bain EE, Robieson WZ, Baker JD, Othman AA. A randomized trial to assess the efficacy and safety of abt-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016; 173(8): 827-835. doi: http://dx.doi.org/10.1176/appi.ajp.2015.15010093 [DOI] [PubMed] [Google Scholar]
- 8.Keefe RS, Meltzer HA, Dgetluck N, Gawryl M, Koenig G, Moebius HJ, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015; 40(13): 3053-3060. doi: http://dx.doi.org/10.1038/npp.2015.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lystad JU, Falkum E, Mohn C, Haaland VO, Bull H, Evensen S, et al. The MATRICS Consensus Cognitive Battery (MCCB): performance and functional correlates. Psychiatry Res. 2014; 220(3): 1094-1101. doi: http://dx.doi.org/10.1016/j.psychres.2014.08.060 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org [Google Scholar]
- 11.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64(4): 401 doi: http://dx.doi.org/10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 12.Jeon DW, Shim JC, Kong BG, Moon JJ, Seo YS, Kim SJ, et al. Adjunctive varenicline treatment for smoking reduction in patients with schizophrenia: A randomized double-blind placebo-controlled trial. Schizophre Res. 2016; 176(2-3): 206-211. doi: http://dx.doi.org/10.1016/j.schres.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 13.Smith RC, Amiaz R, Si TM, Maayan L, Jin H, Boules S, et al. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia: A double-blind randomized trial. PloS One. 2016; 11(1): e0143490 doi: http://dx.doi.org/10.1371/journal.pone.0143490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, et al. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl). 2013; 228(4): 595-602. doi: http://dx.doi.org/10.1007/s00213-013-3064-2 [DOI] [PubMed] [Google Scholar]
- 15.Shiina A, Shirayama Y, Niitsu T, Hashimoto T, Yoshida T, Hasegawa T, et al. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry. 2010; 9: 27 doi: http://dx.doi.org/10.1186/1744-859X-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walling D, Marder SR, Kane J, Fleischhacker WW, Keefe RS, Hosford DA, et al. Phase 2 trial of an alpha-7 nicotinic receptor agonist (tc-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016; 42(2): 335-343. doi: http://dx.doi.org/10.1093/schbul/sbv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umbricht D, Keefe RS, Murray S, Lowe DA, Porter R, Garibaldi G, et al. A randomized, placebo-controlled study investigating the nicotinic alpha7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014; 39(7): 1568-1577. doi: http://dx.doi.org/10.1038/npp.2014.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009; 24(2): 165-178. doi: https://doi.org/10.1093/arclin/acp010 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Florez ID, Colunga Lozano LE, Bakar Aloweni FA, Kennedy SA, Li A, et al. Reporting and methods for handling missing participant data for continuous outcomes in randomized controlled trials: a systematic survey. J Clin Epidemiol. 2017; pii: S0895-4356(17)30573-5. doi: http://dx.doi.org/10.1016/j.jclinepi.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Dossing A, Tarp S, Furst DE, Gluud C, Wells GA, Beyene J, et al. Modified intention-to-treat analysis did not bias trial results. J Clin Epidemiol. 2016; 72: 66-74. doi: http://dx.doi.org/10.1016/j.jclinepi.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Lewis AS, van Schalkwyk GI, Bloch MH. Alpha-7 nicotinic agonists for cognitive deficits in neuropsychiatric disorders: A translational meta-analysis of rodent and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2017; 75: 45-53. doi: http://dx.doi.org/10.1016/j.pnpbp.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutsch SI, Schwartz BL, Schooler NR, Rosse RB, Mastropaolo J, Gaskins B. First administration of cytidine diphosphocholine and galantamine in schizophrenia: a sustained alpha7 nicotinic agonist strategy. Clinical Neuropharmacology. 2008; 31(1): 34-39. doi: http://dx.doi.org/10.1097/wnf.0b013e31806462ba [DOI] [PubMed] [Google Scholar]
- 23.Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012; 37(3): 660-668. doi: http://dx.doi.org/10.1038/npp.2011.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract. 2014; 20(1): 12-24. doi: http://dx.doi.org/10.1097/01.pra.0000442935.15833.c5 [DOI] [PubMed] [Google Scholar]
- 25.Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013; 38(6): 968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu RJ, Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Encyclopedia of the Neurological Sciences. 2003; 35: 4–8. doi: http://dx.doi.org/10.1016/B0-12-226870-9/01070-4 [Google Scholar]
- 27.Zhang X Y, Liu L, Liu S, Hong X, Chen DC, Xiu MH, et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012; 169(9): 974-981. doi: https://doi.org/10.1176/appi.ajp.2012.11081289 [DOI] [PubMed] [Google Scholar]
- 28.Gary Remington, Pierre Chue, Emmanuel Stip, Lili Kopala, Todd Girard, Bruce Christensen. The crossover approach to switching antipsychotics: What is the evidence? Schizophr Res. 2005; 76(2-3): 267-272. doi: http://dx.doi.org/10.1016/j.schres.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Deeks JJ, Higgins JPT, Altman DG. (editors). Chapter 9: Analysing data and undertaking Meta-analyses. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011 [Google Scholar]
- 30.Sterne JAC, Higgins JPT. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343: d4002 doi: http://dx.doi.org/10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 31.Sterne JAC, Egger M, Moher D. (editors). Chapter 10: Addressing reporting biases. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; 2008 [Google Scholar]


