Abstract
Background
Metastases to the thyroid are rare. The most common primary cancer to metastasize to the thyroid is renal cell carcinoma, followed by malignancies of the gastrointestinal tract, lungs, and skin, with breast cancer metastases to the thyroid being rare. Overall, the outcomes in malignancies that have metastasized to the thyroid are poor. There are no prospective studies addressing the role of surgery in metastatic disease of the thyroid. Isolated thyroidectomy has been proposed as a local disease control option to palliate and prevent the potential morbidity of tumor extension related to the airway. Here, we present a case of a patient with breast cancer metastases to the thyroid gland and discuss the role of thyroidectomy in the context of the current literature.
Case presentation
A 62-year-old Afro-Caribbean woman was diagnosed as having bilateral breast carcinoma in 2004, for which she underwent bilateral mastectomy. The pathology revealed multifocal disease on the right, T2N0(0/20)M0 grade 1 and 2 invasive ductal carcinoma, and on the left side, T3N1(2/18)M0 grade 1 invasive ductal carcinoma. Surgery was followed by adjuvant chemotherapy and regional radiotherapy. The disease was under control on hormonal therapy until 2016, when she developed cervical lymphadenopathy. The fine-needle aspiration cytology of the thyroid was reported as papillary thyroid cancer; and the fine-needle biopsy of the left lateral nodal disease was more suggestive of breast malignancy. She underwent a total thyroidectomy and a clearance of the central compartment lymph nodes and a biopsy of the lateral nodal disease. The histopathological analysis was consistent with metastatic breast cancer in the thyroid and lymph nodes with no evidence of a primary thyroid malignancy.
Conclusions
A past history of a malignancy elsewhere should raise the index of suspicion of metastatic disease in patients presenting with thyroid lumps with or without cervical lymphadenopathy. Detection of metastases to the thyroid generally indicates poor prognosis, obviating the need of surgery in an already compromised patient. An empirical thyroidectomy should be considered in select patients for local disease control.
Keywords: Thyroid disorders, Breast cancer, Clinical oncology, Endocrine surgery
Background
Breast cancer is the most commonly diagnosed cancer among women [1]. The common sites for metastatic spread are bone, lungs, and liver [2]. Metastases to the thyroid gland from a non-thyroid primary are uncommon and are mostly from the kidney, followed by gastrointestinal tract, lungs, skin, and rarely breast [3–7]. It is usually associated with a poor prognosis. There are no prospective studies addressing the role of surgery in metastatic disease of the thyroid. Isolated thyroidectomy has been proposed as a local disease control option to palliate and prevent the potential morbidity of tumor extension related to the airway. Here, we present a rare case of a patient with breast cancer metastases to the thyroid gland, and review the evidence for the role of thyroidectomy in the context of the current literature.
Case presentation
A 62-year-old Afro-Caribbean woman was diagnosed as having bilateral carcinoma of the breast in 2004. Her past medical history included hypertension, controlled by amlodipine and losartan, in addition to diabetes on treatment with metformin. She underwent bilateral mastectomy and axillary node clearance with immediate implant-based reconstruction. The pathology revealed multifocal disease on the right, T2N0(0/20)M0 grade 1 and 2 invasive ductal carcinoma (IDC), and on the left side, T3N1(2/18)M0 grade 1 IDC. The disease was estrogen receptor (ER)-positive, weak progesterone receptor (PR)-positive, and human epidermal growth factor receptor 2 (HER2)-negative. Surgery was followed by adjuvant chemotherapy, consisting of the 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) regimen and regional radiotherapy. Hormonal therapy initially consisted of 20 mg daily of tamoxifen. After 3 years this was switched to an aromatase inhibitor (anastrozole 1 mg daily) until 2009 when she completed 5 years of adjuvant endocrine therapy. She then subsequently relapsed with metastatic disease with lung nodules in 2008 and bone metastases were noted on a bone scan 4 years later. She was commenced on 25 mg once a day of exemestane and 4 mg intravenously administered monthly injections of zoledronic acid in early 2014. Due to disease progression, capecitabine 1250 mg/m2 (based on total body surface area) twice daily was commenced until after six cycles when it was discontinued due to capecitabine-related toxicity and she was started on 2.5 mg once a day of letrozole and 150 mg once a day of ibandronic acid. In February 2016 she presented with neck swelling with intermittent neck discomfort without airway pressure symptoms. On clinical examination she was found to have cervical lymphadenopathy. Laboratory findings revealed a white cell count of 5.2 × 109/L, hemoglobin of 115 g/L, and normal liver and renal function with an estimated glomerular filtration rate of 67 ml/minute/1.73 m2. The neck swelling was investigated with an ultrasound and confirmed both lateral cervical nodal disease in levels II to IV and a goiter with left-sided dominance. The fine-needle aspiration cytology (FNAC) of her thyroid was reported as in keeping with a papillary thyroid cancer; however, the cytology of the left lateral nodal disease was described as more suggestive of a breast malignancy. She had no personal or familial risk factors for thyroid malignancy. Staging investigations including magnetic resonance imaging (MRI) of her spine demonstrated stable deposits involving C2, C5, T4, and L1 without neural compromise (Fig. 1) and computed tomography (CT) of her thorax demonstrated no change in the lung nodules (Fig. 2). Since the diagnosis was not clear, following a multidisciplinary team discussion the decision was made to proceed with a total thyroidectomy and a clearance of the central compartment lymph nodes coupled with an excision biopsy of the laterocervical lymph nodes. Histopathological analysis of the specimen demonstrated an ill-circumscribed white tumor at the posterior margin of the left lobe measuring 1.2 × 0.9 × 1.5 cm. On immunocytochemistry the tumor cells were positive for carcinoembryonic antigen (CEA), synaptophysin, GATA3, and ER (5/8), focally positive for cytokeratin (CK) 7 and gross cystic disease fluid protein 15 (GCDFP-15), and negative for thyroid transcription factor 1 (TTF-1), calcitonin, thyroglobulin, CK20, PR, and HER2. The overall appearances were consistent with metastatic breast cancer (Figs. 3 and 4) with no evidence of a primary thyroid malignancy. The level IV and level VI lymph nodes contained metastatic breast cancer. She was discharged on daily 125 mcg of levothyroxine. The chemotherapy was switched to 500 mg intramuscular monthly injections of fulvestrant and she continues to take the ibandronic acid. Currently, 14 months following the thyroidectomy, she remains clinically stable. She developed local recurrences in the level II to IV lymph nodes in her neck and a recent MRI of her spine showed stable spinal metastatic disease.
Fig. 1.
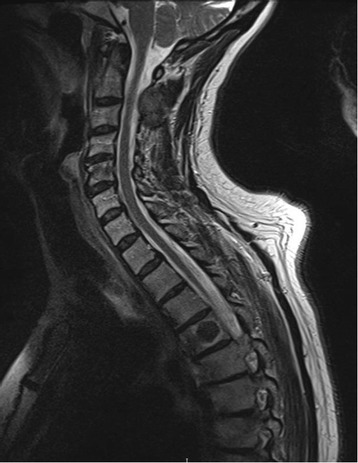
T2-weighted sagittal magnetic resonance image demonstrating the deposits in C5 and T4. They appeared confined to the vertebral body with no evidence of vertebral body collapse
Fig. 2.
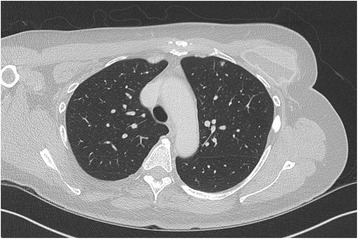
Computed tomography of the thorax demonstrating a small (5 mm in diameter) subpleural nodule within the anterior left upper lobe, which remained unchanged since the previous scan
Fig. 3.
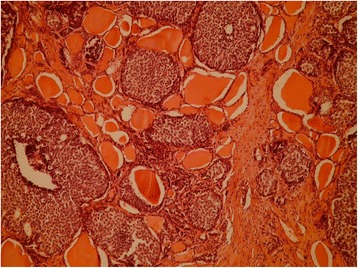
Hematoxylin and eosin stain at × 100 magnification demonstrating solid nests of atypical epithelial cells among normal colloid-filled thyroid follicles
Fig. 4.
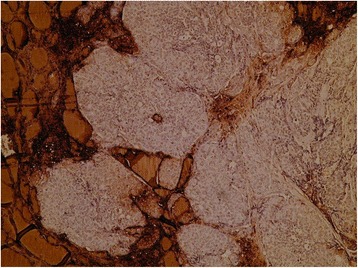
Immunoperoxidase for thyroglobulin showing the solid nests, which are negative while the follicles are positive, including a small trapped microfollicle within the larger nest of metastatic cells. Thyroid transcription factor 1 and calcitonin were equally negative; however, cytokeratin 7 was focally positive and synaptophysin was expressed by the majority of cells. This raises the possibility of a carcinoma with neuroendocrine features
Discussion
Metastatic deposits have a predilection for highly vascularized organs but despite one of the highest blood supplies per weight of tissue (4 to 6 mL/minute/g) [8] the thyroid is rarely the site for metastatic deposits. It is difficult to establish the true rate of metastases from breast cancer to the thyroid gland with a quoted range of prevalence from 3% of all thyroid metastases [4] to 34% (Table 1) [3, 4, 6, 7, 9–27]. Metastases to the thyroid gland represent an indication for surgery in under 1 in a 1000 thyroidectomies [24, 28] of which almost half are from a renal cell carcinoma primary [7, 29]. Other primary tumors that have been documented to metastasize to the thyroid include colorectal, lung, and malignant melanoma [3–7] and gastrointestinal tract tumors [10].
Table 1.
Clinical studies (case reports and case series) of breast metastases to the thyroid gland published so far
| Author | Study years | Number of patients | Percentage of thyroid metastases from breast |
|---|---|---|---|
| Harcourt-Webster [9] | – | 2 | 18% |
| Lam and Lo [10] | – | 7 | 9% |
| Mayo and Schlicke [11] | – | 2 | 11% |
| Elliott and Frantz [12] | 1947–1958 | 4 | 29% |
| Wychulis et al. [13] | 1907–1962 | 4 | 29% |
| Pillay et al. [14] | 1974–1976 | 1 | 10% |
| Lin et al. [15] | 1977–1995 | 1 | 7% |
| Chacho et al. [16] | 1978–1985 | 1 | 13% |
| Rosen et al. [17] | 1978–1993 | 1 | 9% |
| Hegerova et al. [7] | 1980–2010 | 11 | 11% |
| De Ridder et al. [18] | 1982–2002 | 1 | 17% |
| Russell et al. [19] | 1983–2013 | 2 | 12% |
| Cichon et al. [20] | 1984–2003 | 1 | 6% |
| Nakhjavani et al. [21] | 1985–1994 | 7 | 16% |
| Wood et al. [22] | 1985–2002 | 1 | 7% |
| HooKim et al. [6] | 1986–2013 | 3 | 11% |
| Saito et al. [23] | 1987–2008 | 3 | 34% |
| Papi et al. [24] | 1993–2003 | 5 | 14% |
| Moghaddam et al. [3] | 1993–2013 | 1 | 10% |
| Calzolari et al. [25] | 1995–2005 | 1 | 4% |
| Kim et al. [26] | 1997–2004 | 5 | 23% |
| Surov et al. [4] | 1997–2013 | 1 | 3% |
| Choi et al. [27] | 2001–2013 | 7 | 15% |
Breast cancer is the most common malignant tumor among women [1]; while being uncommon, thyroid cancers are the most common endocrine malignancies and the incidence is rising [30]. It has been suggested that possibly due to some common risk factors (genetic, lifestyle, diet habits, hormonal, menstrual, and reproductive factors), individuals with breast cancer are more likely to develop primary thyroid cancer [31, 32]. Therefore, an individual presenting with both thyroid and breast malignancy is more likely to have primary cancer of thyroid and breast, rather than breast metastases to the thyroid.
Up to 80% of thyroid metastases are metachronous [29] with mean intervals from as little as 2.3 years in head and neck cancer [7, 21] to as long as 21 years in the case of foregut neuroendocrine tumors [33]. Other metachronous tumors present varying levels of delay with a mean of 9.4 years in renal cell carcinoma primaries [34] and 48.2 months [29] in breast primary malignancies. Longer delays in metachronous tumors probably reflect a less aggressive biology and in fact the rarer synchronous metastases to the thyroid are associated with a much poorer prognosis with a mean 5-year survival rate of 7.9% [35].
Most reports of metastases to the thyroid are solitary with Surov and colleagues [4] reporting that thyroid metastases were solitary in 76% of patients in their study. However, Hegerova et al. [7] reported that 79% of their patients had evidence of other metastases at the time of diagnosis of thyroid metastases, which may suggest that the extent of investigations plays a part in determining the identification of other disease.
FNAC is the investigation of choice in the work-up of thyroid nodules. It has been shown to achieve an accuracy of over 90% in the diagnosis of secondary tumors of the thyroid [36]. Unfortunately, as in the case presented, metastatic ductal breast carcinoma involving the thyroid may morphologically mimic primary thyroid malignancy on fine-needle aspiration (FNA) and secondary malignancies of the thyroid may be misdiagnosed.
Outcomes in metastatic thyroid disease tend to be poor since it is a reflection of the aggression and advanced stage of the primary disease [5, 15]. A Mayo Clinic series demonstrated that the mean survival post diagnosis of metastases to the thyroid is 3 years and 6 years from the original diagnosis of a primary malignancy [7].
There are no prospective studies addressing the role of surgery in metastatic disease of the thyroid. Our patient with breast metastasis to the thyroid and coexisting lung and bone metastatic deposits, was managed with a total thyroidectomy with a good outcome. Isolated thyroidectomy has been proposed in previous studies [20, 37] as a local disease control option to palliate and prevent the potential morbidity of tumor extension related to the airway [37]. It has been also suggested that this may be beneficial for a selected group of patients with clinically significant and relatively isolated metastatic disease of the thyroid especially from a renal primary [25]; however, in the absence of prospective trials this is at best speculative.
Conclusions
A past history of a malignancy elsewhere should raise the index of suspicion of metastatic disease in patients presenting with thyroid lumps with or without cervical lymphadenopathy. Detection of metastases to the thyroid generally indicates poor prognosis, obviating the need of surgery in an already compromised patient. An empirical thyroidectomy should be considered in select patients for local disease control.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ contributions
AP drafted the manuscript. AD contributed to the literature search, wrote parts, and revised the first draft of the manuscript. DG is the oncologist of the patient, who provided and wrote the information regarding medical management. RD provided the histopathological images and analysis. FP is the consultant of the patient, who carried out the thyroidectomy and revised the draft of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Agata M. Plonczak, Phone: 0044 7534378695, Email: agata.plonczak@gmail.com
Aimee N. DiMarco, Email: aimee.dimarco@imperial.nhs.uk
Roberto Dina, Email: roberto.dina@imperial.nhs.uk.
Dorothy J. Gujral, Email: dorothy.gujral@imperial.nhs.uk
Fausto F. Palazzo, Email: f.palazzo@imperial.ac.uk
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 3.Moghaddam PA, Cornejo KM, Khan A. Metastatic carcinoma to the thyroid gland: a single institution 20-year experience and review of the literature. Endocr Pathol. 2013;24:116–24. doi: 10.1007/s12022-013-9257-8. [DOI] [PubMed] [Google Scholar]
- 4.Surov A, Machens A, Holzhausen HJ, Spielmann RP, Dralle H. Radiological features of metastases to the thyroid. Acta Radiol. 2016;57:444–50. doi: 10.1177/0284185115581636. [DOI] [PubMed] [Google Scholar]
- 5.Mirallié E, Rigaud J, Mathonnet M, Gibelin H, Regenet N, Hamy A, Bretagnol F, de Calan L, Le Néel JC, Kraimps JL. Management and prognosis of metastases to the thyroid gland. J Am Coll Surg. 2005;200:203–7. doi: 10.1016/j.jamcollsurg.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 6.HooKim K, Gaitor J, Lin O, Reid MD. Secondary tumors involving the thyroid gland: A multi-institutional analysis of 28 cases diagnosed on fine-needle aspiration. Diagn Cytopathol. 2015;43:904–11. doi: 10.1002/dc.23331. [DOI] [PubMed] [Google Scholar]
- 7.Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the Mayo Clinic. Am J Clin Oncol. 2015;38:338–42. doi: 10.1097/COC.0b013e31829d1d09. [DOI] [PubMed] [Google Scholar]
- 8.Salvatore D, Davies T, Schlumberger MJ, Hay ID, Larsen RP, et al. Thyroid Physiology and Diagnostic Evaluation of Patients with Thyroid Disorders. In: Melmed S, Polonsky KS, Larsen RP, et al., editors. Williams Textbook of Endocrinology. Philadelphia: Elsevier; 2016. pp. 334–68. [Google Scholar]
- 9.Harcourt-Webster JN. Secondary neoplasm of the thyroid presenting as a goitre. J Clin Pathol. 1965;18:282–7. doi: 10.1136/jcp.18.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam KY, Lo CY. Metastatic tumors of the thyroid gland: a study of 79 cases in Chinese patients. Arch Pathol Lab Med. 1998;122:37–41. [PubMed] [Google Scholar]
- 11.Mayo CW, Schlicke CP. Exogenous tumors of the thyroid gland. Am J Pathol. 1941;17:283–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott RH, Jr, Frantz VK. Metastatic carcinoma masquerading as primary thyroid cancer: a report of authors’ 14 cases. Ann Surg. 1960;151:551–61. doi: 10.1097/00000658-196004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wychulis AR, Beahrs OH, Woolner LB. Metastasis of carcinoma to the thyroid gland. Ann Surg. 1964;160:169–77. doi: 10.1097/00000658-196408000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillay SP, Angorn IB, Baker LW. Tumour metastasis to the thyroid gland. S Afr Med J. 1977;51:509–12. [PubMed] [Google Scholar]
- 15.Lin JD, Weng HF, Ho YS. Clinical and pathological characteristics of secondary thyroid cancer. Thyroid. 1998;8:149–53. doi: 10.1089/thy.1998.8.149. [DOI] [PubMed] [Google Scholar]
- 16.Chacho MS, Greenebaum E, Moussouris HF, Schreiber K, Koss LG. Value of aspiration cytology of the thyroid in metastatic disease. Acta Cytol. 1987;31:705–12. [PubMed] [Google Scholar]
- 17.Rosen IB, Walfish PG, Bain J, Bedard YC. Secondary malignancy of the thyroid gland and its management. Ann Surg Oncol. 1995;2:252–6. doi: 10.1007/BF02307032. [DOI] [PubMed] [Google Scholar]
- 18.De Ridder M, Sermeus AB, Urbain D, Storme GA. Metastases to the thyroid gland—a report of six cases. Eur J Intern Med. 2003;14:377–9. doi: 10.1016/S0953-6205(03)90005-7. [DOI] [PubMed] [Google Scholar]
- 19.Russell JO, Yan K, Burkey B, Scharpf J. Nonthyroid metastasis to the thyroid gland: case series and review with observations by primary pathology. Otolaryngol Head Neck Surg. 2016;155:961–8. doi: 10.1177/0194599816655783. [DOI] [PubMed] [Google Scholar]
- 20.Cichon S, Anielski R, Konturek A, Barczyński M, Cichon W. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbecks Arch Surg. 2006;391:581–7. doi: 10.1007/s00423-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 21.Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–8. doi: 10.1002/(SICI)1097-0142(19970201)79:3<574::AID-CNCR21>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J Surg Oncol. 2004;30:583–8. doi: 10.1016/j.ejso.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Sugitani I, Toda K, Yamada K, Fujimoto Y. Metastatic thyroid tumors: ultrasonographic features, prognostic factors and outcomes in 29 cases. Surg Today. 2014;44:55–61. doi: 10.1007/s00595-013-0492-x. [DOI] [PubMed] [Google Scholar]
- 24.Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Miraglia A, Carani C, Pontecorvi A. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf) 2007;66:565–71. doi: 10.1111/j.1365-2265.2007.02773.x. [DOI] [PubMed] [Google Scholar]
- 25.Calzolari F, Sartori PV, Talarico C, Parmeggiani D, Beretta E, Pezzullo L, Bovo G, Sperlongano P, Monacelli M, Lucchini R, Misso C, Gurrado A, D’Ajello M, Uggeri F, Puxeddu E, Nasi P, Testini M, Rosato L, Barbarisio A, Avenia N. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res. 2008;28:2885–8. [PubMed] [Google Scholar]
- 26.Kim TY, Kim WB, Gong G, et al. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf) 2005;62:236–41. doi: 10.1111/j.1365-2265.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi SH, Baek JH, Ha EJ, et al. Diagnosis of metastasis to the thyroid gland: comparison of core-needle biopsy and fine-needle aspiration. Otolaryngol Head Neck Surg. 2016;154:618–25. doi: 10.1177/0194599816629632. [DOI] [PubMed] [Google Scholar]
- 28.Diaconescu MR, Costea I, Glod M, Grigorovici M, Diaconescu S. Unusual malignant tumors of the thyroid gland. Chirurgia. 2013;108:482–9. [PubMed] [Google Scholar]
- 29.Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22:258–68. doi: 10.1089/thy.2010.0154. [DOI] [PubMed] [Google Scholar]
- 30.Zamora-Ros R, et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: The EPIC study. Int J Cancer. 2015;136:1218–27. doi: 10.1002/ijc.29067. [DOI] [PubMed] [Google Scholar]
- 31.Joseph KR, Edirimanne S, Eslick GD. The association between breast cancer and thyroid cancer: a meta-analysis. Breast Cancer Res Treat. 2015;152:173–81. doi: 10.1007/s10549-015-3456-6. [DOI] [PubMed] [Google Scholar]
- 32.Fei X, Christakos G, Lou Z, Ren Y, Liu Q, Wu J. Spatiotemporal Co-existence of Female Thyroid and Breast Cancers in Hangzhou, China. Sci Rep. 2016;6:2–11. doi: 10.1038/s41598-016-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattavelli F, Collini P, Pizzi N, Gervasoni C, Pennacchioli E, Mazzaferro V. Thyroid as a target of metastases. A case of foregut neuroendocrine carcinoma with multiple abdominal metastases and a thyroid localization after 21 years. Tumori. 2008;94:110–3. doi: 10.1177/030089160809400119. [DOI] [PubMed] [Google Scholar]
- 34.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95:1869–78. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 35.Chen JY, Chen IW, Hsueh C, Chao TC, Gao BR, Lin JD. Synchronous diagnosis of metastatic cancer to the thyroid is associated with poor prognosis. Endocr Pathol. 2015;26:80–6. doi: 10.1007/s12022-015-9357-8. [DOI] [PubMed] [Google Scholar]
- 36.Aron M, Kapila K, Verma K. Role of fine-needle aspiration cytology in the diagnosis of secondary tumors of the thyroid—twenty years’ experience. Diagn Cytopathol. 2006;34:240–5. doi: 10.1002/dc.20329. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Nicol TL, Udelsman R. Clinically significant, isolated metastatic disease to the thyroid gland. World J Surg. 1999;23:177–80. doi: 10.1007/PL00013162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


