Abstract
Background
The purpose of this study was to determine the shifting trends in bacteriology and antimicrobial resistance of infectious specimens isolated from gastrointestinal (GI) fistula patients over eight years in China.
Methods
We retrospectively reviewed the microbial records of intra-abdominal specimens at a teaching hospital from 2008 to 2015. Study period was divided into the first half (2008–2011) and the second half (2012–2015). All isolates underwent antibiotic susceptibility testing by the micro dilution method.
Results
A total of 874 intra-abdominal isolates were consecutively collected from 502 GI fistula patients (mean age, 46.5 years, 71.1% male) during the study period. Patients in the second study period (2012–2015) were older (>65 years) and more likely to have experienced cancer. Over the entire study period, most infections were caused by E. coli (24.2%) and K. pneumonia (14.1%). There was a significant decrease in the proportion E. coli isolates that were extended- spectrum beta-lactamase (ESBL)-positive (P = 0.026). The proportion of E. coli resistant to imipenem increased from 14.3% in 2008–2011 to 25.9% in 2012–2015 (P = 0.037). Imipenem resistance prevalence was higher in ESBL-negative bacteria than ESBL-positive bacteria for both E. coli and K. pneumonia (P < 0.001). In Enterococcus, significant increase in resistance to ampicillin (P = 0.01) and moxifloxacin (P = 0.02) over time were observed. In Staphylococcus and fungi, rates of antibiotic resistance did not significantly change over the study period.
Conclusions
Gram-negative bacteria predominated as causative agents of intra-abdominal infections in GI fistula patients, and there was an increase in levels of resistance to certain antibiotics, particularly carbapenems. Infection control and source control are important tools available to surgeons to prevent the emergence of antibiotic-resistant pathogens.
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2744-7) contains supplementary material, which is available to authorized users.
Keywords: Bacteriology, Antibiotic resistance, Intra-abdominal infections
Background
Gastrointestinal (GI) fistula is a complex and challenging problem associated with intra-abdominal infections (IAIs), leading to high morbidity and mortality worldwide [1, 2]. Effective treatment for IAIs patients involves both source control and antimicrobial therapy [3]. Despite improvements in patient care, therapeutic failure remains common [4].
Selection and prompt initiation of the appropriate empiric antimicrobial therapy play an important role in decreasing morbidity and mortality in GI fistula patients with IAIs [5]. The Infectious Diseases Society of America (IDSA) guidelines recommend use of single agents, such as carbapenems, piperacillin/tazobactam, cephalosporins, fluoroquinolones and aminoglycosides combined with metronidazole to treat IAIs in adults [2]. The distribution of pathogens causing IAIs and their drug susceptibility profiles may change over time, particularly with the spread of antibiotic resistance, making it more challenging for surgeons to select appropriate antibiotic therapies [6, 7]. To improve the outcome of GI fistula patients with IAIs, it is essential for surgeons to be aware of the local bacteriology and antimicrobial resistance trends of the causative pathogens [8].
Large-scale antibiotic susceptibility surveillances have been launched over the past decades which have informed surgeons of current trends in the emergence of antibiotic-resistant bacterial strains involved in IAIs [9–11]. However, these short-term surveillances might put up an incomplete facade pattern as the fluctuations of antibiotic resistance appeared in the shorter time period [12]. Therefore, a longitudinal surveillance is critical as guidance for empiric therapy.
Methods
Patients and samples
Microbiology and antibiotic susceptibility of isolates collected at Jinling Hospital between 2008 and 2015 were retrospectively reviewed using the hospital medical record system. Data extracted from the system for each isolate included demographic characteristics of the patient (age, sex), co-morbidities (hypertension, diabetes, cancer, inflammatory bowel disease, lung injury, renal injury) and fistula location. Upper GI fistula was defined as fistula located in the stomach or duodenum and lower GI fistula was defined as fistula located in the jejunum, ileum or colon [13]. Isolates from tissue, fluid or deep wound cultures obtained during operation, abdominal drains, fluid from paracentesis or percutaneous aspiration of abscesses were included, and those from drain bottles, stool, superficial wounds, or perirectal abscess were excluded.
The study protocol was approved by the Institutional Review Board Ethics Committee of Jinling Hospital, and all research work was in compliance with the Helsinki Declaration.
Pathogenic examination and antibiotic susceptibility determination
Samples were collected with sterile cotton swabs (Zhejiang Gongdong Medical Technology Co. Ltd., Taizhou, Zhejiang, China) and then sent to the microbiology laboratory for processing. Bacteria were isolated and then identified by the Vitek and Analytical Profile Index (API) bacterial identification systems or by traditional manual methods (BioMérieux, Hazelwood, MO, USA).
To assess antimicrobial susceptibility, minimum inhibitory concentrations (MICs) for each antimicrobial agent were determined by the agar dilution method, according to each year’s CLSI guidelines (Clinical Laboratory Standards Institute, USA, as annually updated) [14]. Phenotypic identification of extended-spectrum beta-lactamase (ESBL) production of Escherichia. Coli (E. coli), Klebsiella and Enterobacter species were expanded. If MICs of ceftazidime, cefepime, or ceftriaxone were ≥2 mg/L among E. coli, Klebsiella or Enterobacter species, ESBL production was suspected. For these ESBL-suspected isolates, if the MIC of cefepime was at least eightfold more than that of cefepime in the presence of clavulanic acid, ESBL production was identified [15]. Escherichia coli ATCC 25922, Klebsiella pneumonia ATCC 700603 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains.
Statistical analysis
Descriptive statistics were presented for categorical variables and continuous variables. We divided the study period into two periods for analysis: 2008–2011 and 2012–2015. We use the Mantel–Haenszel linear-by-linear association χ2 test to detect significant differences over time. Continuous variables were analyzed using the student t-test. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (Version 22 IBM, Armonk, NY).
Results
Patient characteristics
A total of 502 GI fistula patients (mean age 46.5 years, 71.1% male) were included. Demographic characteristics of included patients are shown in Table1. Patients in the second study period (2012–2015) exhibited significant enrichment of clinical factors, including advanced age (P = 0.02), cancer (16.0% VS 6.7%, P = 0.001) and renal injury (16.7% VS 9.2%, P = 0.013) than patients from 2008 to 2011. In addition, the 2012–1015 cohort had a significantly higher percentage of lower GI fistula (P = 0.005) and a lower percentage of intensive care unit (ICU) patients (P < 0.001) (Table 1). We did not find the difference change in mortality rates over time (27.6% VS 28.1%, P = 0.896).
Table 1.
Clinical characteristics of patients during 2008 and 2015
| 2008–2012 (n = 239) |
2012–2015 (n = 263) |
P | |
|---|---|---|---|
| Gender | |||
| Male | 169 (70.71%) | 188 (71.48%) | 0.849 |
| Age (years) | 44.85 ± 14.99 | 48.05 ± 15.62 | 0.020 |
| ≤ 16 | 6 (2.51%) | 3 (1.14%) | 0.248 |
| 17–32 | 49 (20.50%) | 41 (15.59%) | 0.152 |
| 33–48 | 88 (36.82%) | 96 (36.50%) | 0.941 |
| 49–64 | 76 (31.80%) | 81 (30.80%) | 0.809 |
| ≥ 65 | 20 (8.37%) | 42 (15.97%) | 0.010 |
| Patient location | |||
| ICU | 150 (62.76%) | 123 (46.77%) | <0.001 |
| Fistula location, | |||
| Upper gastrointestinal | 107 (45.15%) | 98 (37.26%) | 0.073 |
| Lower gastrointestinal | 106 (44.35%) | 150 (57.03%) | 0.005 |
| Both | 19 (7.95%) | 15 (5.70%) | 0.317 |
| Co-morbidities | |||
| Hypertension | 39 (16.32%) | 57 (21.67%) | 0.128 |
| Diabetes | 20 (8.37%) | 30 (11.41%) | 0.256 |
| Cancer | 16 (6.69%) | 42 (15.97%) | 0.001 |
| IBD | 8 (3.35%) | 16 (6.08%) | 0.151 |
| Lung Injury | 33 (13.81%) | 45 (17.11%) | 0.308 |
| Renal Injury | 22 (9.21%) | 44 (16.73%) | 0.013 |
| 30-day mortality | 66 (27.62%) | 74 (28.14%) | 0.896 |
Microbiological profile
During the entire study period, 874 isolates were collected, and the mean number of isolates per year was 109+/−19. Co-infection with multiple microbial strains was identified in 118(49.4%) patients during the first study period and 124(47.2%) during the second period. The distribution of microbial strains, stratified by study period, is shown in Fig. 1. The total number of Gram-negative was 638 (73.0%), which became more common over time (P = 0.024), followed by Gram-positive isolates (188, 25.5%), respectively. Overall, E. coli was the most frequently identified bacterial microorganism (216 isolates, 24.2% of all bacterial growths and 33.9% of Gram-negative isolates), followed by K. pneumonia (123 isolates, 14.1% of all bacterial growths and 19.3% of Gram-negative isolates). A significant decrease in the percentage of E. coli isolates that were ESBL-positive occurred between study periods (P = 0.026), but there was no significant difference in the proportion of K. pneumonia that were ESBL positive between study periods. The common Gram-positive bacteria were Enterococcus and Staphylococcus. (Additional file 1: Table S1).
Fig. 1.
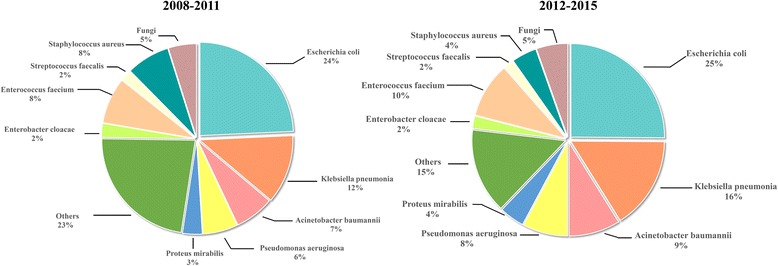
Distribution of strain groups identified in the study periods
Enterobacteriaceae antibiotic resistance
Tables 2 and 3 list the in vitro resistance profiles of E. coli and K. pneumonia, respectively, stratified by ESBL-production ability. There were similar patterns of antibiotic resistance for E. coli and K. pneumonia isolates over the study period, with both displaying high levels of resistance to penicillins, cephalosporins and fluoroquinolones. There was a statistically significant decrease in prevalence of resistance to ceftazidime and cefepime in E. coli isolates (P = 0.042, P = 0.035). No significant change in prevalence of resistance to aztreonam and amikacin was observed in both E. coli and K. pneumonia isolates. Resistance to amikacin was relatively low compared with the other antibiotics mentioned above for both E. coli and K. pneumonia isolates.
Table 2.
Antimicrobial resistance of Escherichia coli isolates to the tested antibiotics
| Resistance rate (%) | ||||
|---|---|---|---|---|
| Isolate/Antibiotics | 2008–2011 | 2012–2015 | Total | P for Trend Test |
| All Escherichia coli | ||||
| AMK | 7.78 | 14.16 | 11.33 | 0.154 |
| AMP | 97.92 | 96.49 | 97.14 | 0.537 |
| SAM | 81.97 | 87.93 | 85.88 | 0.279 |
| TZP | 23.47 | 25.00 | 24.30 | 0.795 |
| CZO | 92.55 | 91.30 | 91.87 | 0.742 |
| CAZ | 81.82 | 69.83 | 75.35 | 0.042 |
| FEP | 75.51 | 62.07 | 68.22 | 0.035 |
| IPM | 14.29 | 25.86 | 20.56 | 0.037 |
| CIP | 90.16 | 79.13 | 82.95 | 0.064 |
| ESBL+ | ||||
| AMK | 6.78 | 12.33 | 9.85 | 0.287 |
| AMP | 100.00 | 100.00 | 100.00 | – |
| SAM | 77.78 | 93.15 | 88.07 | 0.020 |
| TZP | 20.31 | 6.85 | 13.14 | 0.020 |
| SXT | 84.21 | 73.61 | 77.27 | 0.207 |
| CZO | 100.00 | 100.00 | 100.00 | – |
| CAZ | 89.23 | 68.49 | 78.26 | 0.003 |
| FEP | 81.25 | 60.27 | 70.07 | 0.007 |
| IPM | 12.31 | 8.22 | 10.14 | 0.427 |
| CIP | 86.11 | 80.82 | 82.57 | 0.494 |
| ESBL- | ||||
| AMK | 6.67 | 16.22 | 13.46 | 0.361 |
| AMP | 88.24 | 89.74 | 89.29 | 0.867 |
| SAM | 77.78 | 77.50 | 77.55 | 0.986 |
| ZP | 35.29 | 57.50 | 50.88 | 0.125 |
| SXT | 88.89 | 67.50 | 71.43 | 0.199 |
| CZO | 58.82 | 74.36 | 69.64 | 0.245 |
| CTT | 28.57 | 70.00 | 63.83 | 0.035 |
| CAZ | 52.94 | 70.00 | 64.91 | 0.217 |
| FEP | 52.94 | 62.50 | 59.65 | 0.501 |
| IPM | 43.75 | 57.50 | 53.57 | 0.351 |
| CIP | 88.89 | 77.50 | 79.59 | 0.444 |
| Not all tested antibiotics are listed | ||||
AMK Amikacin, AMP Ampicillin, SAM Ampicillin/Sulbactam, CAZ Ceftazidime, efepime, CTT Cefotetan, CZO Cefazolin, IPM Imipenem, TZP Piperacillin-Tazobactam, CIP Ciprofloxacin
Data in boldface reflected p values < 0.05
Table 3.
Antimicrobial resistance of Klebsiella pneumonia isolates to the tested antibiotics
| Resistance rate (%) | ||||
|---|---|---|---|---|
| Isolate/Antibiotics | 2008–2011 | 2012–2015 | Total | P for Trend Test |
| All Klebsiella pneumonia | ||||
| AMK | 40.91 | 36.11 | 37.93 | 0.605 |
| SAM | 97.14 | 85.14 | 88.99 | 0.061 |
| TZP | 75.00 | 58.11 | 64.75 | 0.056 |
| CTT | 60.61 | 54.79 | 56.60 | 0.576 |
| CAZ | 89.80 | 78.38 | 82.93 | 0.099 |
| FEP | 75.00 | 67.57 | 70.49 | 0.379 |
| IPM | 65.31 | 56.76 | 60.16 | 0.343 |
| CIP | 74.29 | 75.68 | 75.23 | 0.875 |
| ESBL+ | ||||
| AMK | 33.33 | 7.41 | 16.67 | 0.031 |
| SAM | 100.00 | 89.29 | 92.11 | 0.281 |
| TZP | 55.56 | 17.86 | 32.61 | 0.008 |
| CTT | 12.50 | 14.81 | 14.29 | 0.869 |
| CAZ | 94.44 | 71.43 | 80.43 | 0.055 |
| FEP | 83.33 | 53.57 | 65.22 | 0.039 |
| IPM | 38.89 | 14.29 | 23.91 | 0.056 |
| CIP | 50.00 | 71.43 | 65.79 | 0.220 |
| ESBL- | ||||
| AMK | 40.91 | 53.33 | 49.25 | 0.339 |
| SAM | 94.44 | 82.61 | 85.94 | 0.221 |
| TZP | 86.36 | 82.61 | 83.82 | 0.694 |
| CTT | 88.89 | 78.26 | 81.25 | 0.327 |
| CAZ | 82.61 | 82.61 | 82.61 | – |
| FEP | 72.73 | 76.09 | 75.00 | 0.765 |
| PM | 78.26 | 82.61 | 81.16 | 0.663 |
| CIP | 83.33 | 78.26 | 79.69 | 0.650 |
| Not all tested antibiotics are listed | ||||
AMK Amikacin, SAM Ampicillin/Sulbactam, CAZ Ceftazidime, FEP Cefepime, CTT Cefotetan, IPM Imipenem, TZP Piperacillin-Tazobactam, CIP Ciprofloxacin
Data in boldface reflected p values < 0.05
In ESBL-positive E. coli, prevalence of resistance to piperacillin/tazobactam (13.1%) was lower than ampicillin/sulbactam resistance prevalence (88.1%) and both decreased over the study period (P = 0.020). The same resistance trend to piperacillin/tazobactam was also observed in ESBL-positive K. pneumonia (P = 0.008). Imipenem resistance prevalence was higher in K. pneumonia than E. coli. It increased over time in E. coli (14.3% VS 25.9%, P = 0.037) but there was no significant change in K. pneumonia (65.3% VS 56.8%, P = 0.343). Imipenem resistance prevalence was higher in ESBL-negative than ESBL-positive bacteria for both E. coli and K. pneumonia (P < 0.001).
Antimicrobial resistance of non-fermenting bacteria
In A. baumannii isolates, extremely high levels of cephalosporin resistance were observed, which increased to 100% in 2012–2015. In contrast, ceftazidime and cefepime resistance rates were lower in P. aeruginosa isolates (Table 4). Both A. baumannii and P. aeruginosa showed strikingly high resistance rates to imipenem during the study period. Among A. baumannii isolates, imipenem resistance prevalence was 95.6% and did not significantly change during the study period. In contrast, imipenem resistance in P. aeruginosa isolates was much lower. Resistance to fluoroquinolones showed no significant change over time in either pathogen.
Table 4.
Antimicrobial resistance of Acinetobacter baumannii and Pseudomonas aeruginosa isolates to the tested antibiotics
| Resistance rate (%) | ||||
|---|---|---|---|---|
| Isolate/Antibiotics | 2008–2011 | 2012–2015 | Total | P for Trend Test |
| Acinetobacter baumannii | ||||
| AMP | 96.43 | 100.00 | 98.55 | 0.223 |
| SAM | 90.48 | 100.00 | 96.77 | 0.045 |
| TZP | 96.30 | 95.00 | 95.52 | 0.801 |
| SXT | 95.24 | 73.17 | 80.65 | 0.037 |
| CRO | 94.44 | 100.00 | 98.31 | 0.128 |
| CTX | 100.00 | 100.00 | 100.00 | – |
| CAZ | 88.89 | 100.00 | 95.59 | 0.029 |
| FEP | 96.30 | 100.00 | 98.53 | 0.214 |
| IPM | 96.30 | 95.12 | 95.59 | 0.818 |
| LVX | 66.67 | 56.10 | 60.29 | 0.383 |
| CIP | 95.24 | 97.56 | 96.77 | 0.624 |
| Pseudomonas aeruginosa | ||||
| AMK | 31.82 | 22.22 | 25.86 | 0.418 |
| SAM | 100.00 | 100.00 | 100.00 | – |
| TZP | 60.87 | 33.33 | 44.07 | 0.038 |
| ATM | 58.82 | 75.00 | 61.90 | 0.549 |
| SXT | 100.00 | 97.22 | 98.11 | 0.488 |
| CRO | 100.00 | 100.00 | 100.00 | – |
| CTX | 100.00 | 100.00 | 100.00 | – |
| CAZ | 69.57 | 61.11 | 64.41 | 0.508 |
| FEP | 65.22 | 50.00 | 55.93 | 0.251 |
| IPM | 73.91 | 58.33 | 64.41 | 0.223 |
| LVX | 34.78 | 44.44 | 40.68 | 0.461 |
| CIP | 38.89 | 41.18 | 40.38 | 0.873 |
| Not all tested antibiotics are listed | ||||
AMK Amikacin, SAM Ampicillin/Sulbactam, TZP piperacillin-tazobactam, ATM Aztreonam, SXT Trimethoprim/Sulfamethoxazole, CRO Ceftriaxone, CTX cefotaxime, CAZ ceftazidime, FEP cefepime, IPM imipenem, LVX levofloxacin, CIP ciprofloxacin
Data in boldface reflected p values < 0.05
Antimicrobial resistance of gram-positive bacteria and fungi
Antibiotic resistance prevalence rates of Gram-positive bacteria are listed in Table 5. In Enterococcus, resistance to ampicillin increased from 72.2% in 2008–2011 to 92.5% in 2012–2015 (P = 0.01). Resistance to moxifloxacin also increased significantly (P = 0.02). No significant changes in resistance to vancomycin (P = 0.311) and linezolid (P = 0.111) over time were observed.
Table 5.
Antimicrobial resistance of Enterococcus and Staphylococcus isolates to the tested antibiotics
| Resistance rate (%) | ||||
|---|---|---|---|---|
| Isolate/Antibiotics | 2008–2011 | 2012–2015 | Total | P for Trend Test |
| Enterococcus | ||||
| AMP | 72.22 | 92.45 | 84.27 | 0.01 |
| STH | 47.06 | 57.58 | 52.24 | 0.389 |
| GEH | 71.74 | 79.25 | 75.76 | 0.385 |
| ERY | 88.57 | 88.68 | 88.64 | 0.988 |
| CIP | 89.29 | 90.57 | 90.12 | 0.854 |
| CLI | 100.00 | 95.56 | 95.92 | 0.667 |
| MFX | 63.64 | 91.11 | 85.71 | 0.02 |
| PEN | 87.23 | 92.31 | 89.90 | 0.403 |
| TCY | 74.29 | 69.23 | 71.26 | 0.609 |
| VAN | 4.26 | 9.43 | 7.00 | 0.311 |
| LNZ | 4.88 | 0.00 | 2.17 | 0.111 |
| LVX | 77.14 | 90.38 | 85.06 | 0.089 |
| Staphylococcus | ||||
| OXA | 82.69 | 93.10 | 86.42 | 0.190 |
| SXT | 47.62 | 24.14 | 38.03 | 0.045 |
| ERY | 84.62 | 82.76 | 83.95 | 0.827 |
| CIP | 73.68 | 89.66 | 83.33 | 0.147 |
| CLI | 69.57 | 57.14 | 64.86 | 0.278 |
| MFX | 46.34 | 37.93 | 42.86 | 0.484 |
| PEN | 94.34 | 100.00 | 96.34 | 0.192 |
| GEN | 71.15 | 72.41 | 71.60 | 0.904 |
| TCY | 56.82 | 68.97 | 61.64 | 0.296 |
| VAN | 0.00 | 0.00 | 0.00 | – |
| LNZ | 0.00 | 0.00 | 0.00 | – |
| LVX | 68.89 | 79.31 | 72.97 | 0.324 |
| Not all tested antibiotics are listed | ||||
AMP Ampicillin, OXA Oxacillin, STH Streptomycin-High, SXT Trimethoprim/Sulfamethoxazole, ERY erythromycin, GEH gentamicin, CIP ciprofloxacin, CLI Clindamycin, MFX moxifloxacin, PEN penicillin, TCY tetracycline, VAN Vancomycin, LNZ Linezolid, LVX Levofloxacin
Data in boldface reflected p values < 0.05
Methicillin-resistant S. aureus (MRSA) accounted for 94.1% of S. aureus isolates in 2012–2015. There was no significant change in S. aureus resistance to oxacillin over time. All of 51 Staphylococcus were susceptible to vancomycin (Table 5).
Fungi isolates showed lower antibiotic resistance rates than bacteria isolates, and rates did not significantly vary over time (Table 6).
Table 6.
Antimicrobial resistance of Fungi isolates to the tested antibiotics
| Resistance rate (%) | ||||
|---|---|---|---|---|
| Antibiotics | 2008–2011 | 2012–2015 | Total | P for Trend Test |
| FLU | 18.18 | 8.33 | 11.43 | 0.395 |
| VOR | 0.00 | 8.33 | 5.71 | 0.324 |
| ITR | 18.18 | 5.56 | 10.34 | 0.279 |
FLU Fluconazole, VOR Voriconazole, ITR Itraconazole
Discussion
To our knowledge, this is the first study to examine the shifting trends in bacteriology and antimicrobial resistance among GI fistula patients in China. Our findings indicate a significant increase in the percentage of IAIs attributable to Gram-negatives bacteria, with a corresponding decrease in the percentage attributable to Gram-positive isolates. There was a trend for increased resistance prevalence levels to certain antibiotics for Gram-negative bacteria, especially carbapenems.
K. pneumonia and A. baumannii have gained notoriety as important pathogens because of their increasing resistance to antibiotics and a rise in the number of severe infections caused by these micro-organisms in surgical settings [16]. We found an increase in IAIs attributable to K. pneumonia and A. baumannii infection over time, although this increase did not reach statistical significance. Colonization with these bacteria have been described as the reason for high incidence in surgical wards and this could be prevented through effective infection control [17, 18]. Therefore, we must heighten our awareness of the importance of infection control.
ESBL production which can hydrolyze β-lactam antibiotics has been increasingly identified worldwide amongst the Enterobacteriaceae family, particularly E. coli and K. pneumonia [19]. In the present study, the overall prevalence of ESBL-positive strains of E. coli was 63.9%, which decreased significantly over time, and the overall prevalence of ESBL-positive strains of K. pneumonia was 37.3%, which did not significant change over the study periods. These levels are somewhat lower than those reported by SMART research in 2012 and 2013 [10]. Carbapenems and piperacillin-tazobactam are the most potent and reliable antibiotics for the treatment of ESBL-producing infection [20]. In our study, we found that resistance to piperacillin-tazobactam decreased over time both ESBL-producing E. coli (P = 0.02) and K. pneumonia (P = 0.008). It suggests that piperacillin-tazobactam is a suitable treatment option for these infections [21].
Resistance to carbapenems is associated with high mortality and has been an emerging concern worldwide [22, 23]. The overall prevalence of imipenem resistance in E. coli isolates was 20.6%, which significantly increased over time. Prevalence in K. pneumonia was 60.2%, which did not change over time. Both these prevalence levels are higher than previous reports [9, 24–26]. This may be because the majority of our patients have transferred from other hospitals and have been treated with antibiotics for a number of days, which has been shown to be a risk factor for carbapenem resistance [27]. High resistance prevalence has also resulted from its spread in surgical wards and ICUs [12]. Standard infection control practice (basic hand hygiene, active surveillance cultures of patients, staff, and the environment) should be carried out to prevent the colonization and spread of resistant bacteria [8, 28, 29].
The prevalence of multidrug resistance amongst A. baumannii isolates makes carbapenem the most effective treatment [30]. Carbapenem resistance has become a serious problem, with prevalence reaching a remarkable 95.6% of all isolates in our study. Similarly high levels have been reported in blood stream infections [23]. Once carbapenem resistant A. baumannii emerges, the infected patient has little chance of effective treatment [31]. Therefore, we need to pay attention to source control and limiting the spread of carbapenem-resistant bacteria.
P. aeruginosa is another a common Gram-negative non-fermenting pathogen causing IAIs. In this study, the most efficient antimicrobial agent for P. aeruginosa was found to be amikacin, as has been reported elsewhere [32]. However, we rarely treat patients with amikacin because of its renal toxicity. In our study we observed a significant decrease in resistance to piperacillin-tazobactam over time, suggesting that piperacillin- tazobactam could be the first choice treatment option for patients infected by P. aeruginosa, as recommended by several studies [33, 34].
The proportion of Gram-positive bacterial isolates that were Enterococcus increased over time. Antibiotic resistance rates for this group of pathogens also increased. Staphylococcus isolates had high levels of penicillin G, macrolide, and clindamycin resistance, but no resistance to vancomycin or linezolid was observed. Antibiotics resistance levels were lower among Gram-positive than Gram-negative bacteria. we therefore recommend focusing on Gram-negative bacteria with high antibiotic resistance in GI fistula patients.
In an attempt to identify factors that might influence antibiotic resistance emergence, we analyzed the clinical characteristics of patients. We found that patients in the second study period were older (aged >65 years) and were more likely to suffer cancer, both of which have been demonstrated as risk factors for antibiotic resistance [8, 18, 35]. We also found more IAIs caused by lower GI fistula in the second study period. A recent study by Mu et al. reported that antibiotic intervention exerts location-specific effects on antibiotic resistance genes (increased in the lower GI tract) [36]. Most of our patients were transferred from other hospitals, which means they had been previously treated with antibiotics and were therefore at increased risk of antibiotic resistance. Excessive antibiotic use has been linked with the development of resistance, which is a common practice in many developing countries [18]. Combined, these factors at least partly explain the increase in antibiotic resistance that we have observed. We found that smaller ICU patients showed higher antibiotic resistance. And that again underlines the serious antibiotic resistance.
Inappropriate use of antibiotics and inadequate source control were found to be independent predictors of mortality in a previous analysis [37]. High levels of antibiotic resistance have left few treatment options available to surgeons [8]. However, we found no change in mortality rates over time. This could partially be attributable to the effective management of source control. Newer IAI treatment guidelines recommend intravenous antimicrobial agents as a supplement to source control, and source control may be an available option for surgeons to prevent the emergence of antibiotic-resistant microbial strains [38].
There are some limitations to our study. First, it is a retrospective and single-center surveillance study, which may explain the higher resistance levels observed in our study than other reports from China [23]. However, the critically ill patients at our center were transferred from other hospitals throughout the country, so our study may represent the bacteriology and antimicrobial resistance profiles of severely infected GI fistula patients in China more generally. Second, we did not perform polymerase chain reaction (PCR) and DNA sequencing of isolates. Third, we did not use the unified CLSI breakpoints, as annually updated. In fact, our microbiology laboratory updated determinations according to the newest CLSI documents and 2008–2015 isolates were determined by each year’s documents. Change of breakpoints might cause fluctuations of antimicrobial resistance in short-term surveillances [12]. But there is not a large difference between CLSI breakpoints. And a longitudinal surveillance spanning over 8 years is of great significance for monitoring resistance, which may minimize referral bias.
Conclusions
This study illustrates the shifting trends in bacteriology and antimicrobial resistance in GI fistula patients in China over time. Gram-negative bacteria have become a more significant cause of IAIs in these patients. Currently, carbapenem resistances in Gram-negative bacteria is a serious problem in this patient group. Our findings confirm the urgent need to continue surveillance studies that monitor bacteriology and antimicrobial resistance trends. Infection control and source control are important tools for surgeons to use to prevent the emergence of isolated antibiotic-resistant pathogens.
Acknowledgments
The authors would like to declare that this report is exclusively based on epidemiological findings and we are very thankful to all staff at the Department of Microbiology, Jinling Hospital. We thank Rebecca Baggaley, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
The work was supported by grants from the Natural Science Foundation of China (81571881) and the Key Project of Jiangsu Social Development (BE2016752).
Availability of data and materials
The data supporting the findings of this study are contained within the manuscript.
Abbreviations
- AMK
Amikacin
- AMP
Ampicillin
- ATCC
American Type Culture Collection
- ATM
Aztreonam
- CAZ
Ceftazidime
- CIP
Ciprofloxacin
- CLI
Clindamycin
- CLSI
Clinical Laboratory Standards Institute
- CRO
Ceftriaxone
- CTT
Cefotetan
- CTX
Cefotaxime
- CZO
Cefazolin
- ERY
Erythromycin
- ESBL
extended-spectrum beta-lactamase
- FEP
Cefepime
- FLU
Fluconazole
- GEH
Gentamicin
- GEN
Gentamicin
- GI fistula
gastrointestinal fistula
- IAIs
intra-abdominal infections
- IDSA
Infectious Diseases Society of America
- IPM
Imipenem
- ITR
Itraconazole
- KPC
Carbapenem-resistant K. pneumonia
- LNZ
Linezolid
- LVX
Levofloxacin
- MFX
Moxifloxacin
- MICs
minimum inhibitory concentrations
- MRSA
Methicillin-resistant S. aureus
- OXA
Oxacillin
- PCR
Polymerase chain reaction;
- PEN
Penicillin G
- QDA
Quinupristin/Dalfopristin
- SAM
Ampicillin/Sulbactam
- SMART
Study for Monitoring Antimicrobial Resistance Trends
- STH
Streptomycin-High
- SXT
Trimethoprim/Sulfamethoxazole
- TCY
Tetracycline
- TOB
Tobramycin
- TZP
Piperacillin/Tazobactam
- VAN
Vancomycin
- VOR
Voriconazole
Additional file
Bacterial identification of isolates from intra-abdominal infections in a Tertiary-Care Hospital during 2008 and 2015. (DOCX 15 kb)
Authors’ contributions
Qinjie Liu and Jianan Ren devised and designed the study. Qinjie Liu, Zhiwei Wang, Jie Wu, Tianyu Lu, and Jinjian Huang were responsible for the collection of clinical data and resolved problems by discussion if any occurred. Qinjie Liu was the primary statistician for the study and drafted the manuscript. All authors reviewed the manuscript, which was revised in response to comments by Qinjie Liu and Xiuwen Wu. Gefei Wang monitored the project and Jieshou Li was responsible for the paper as a whole. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The entire study protocol was approved by the Institutional Review Board Ethics Committee of Jinling Hospital, and all research work was conducted in compliance with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2744-7) contains supplementary material, which is available to authorized users.
Contributor Information
Qinjie Liu, Email: a261666943@sina.com.
Jianan Ren, Phone: +08613605169808, Email: JiananR@gmail.com.
Xiuwen Wu, Email: lygwxw@163.com.
Gefei Wang, Email: wgfwang@gmail.com.
Zhiwei Wang, Email: wangzhw824@gmail.com.
Jie Wu, Email: airlamb@foxmail.com.
Jinjian Huang, Email: 932840102@qq.com.
Tianyu Lu, Email: 790333988@qq.com.
Jieshou Li, Email: jieshou_li@163.com.
References
- 1.Ren J, Liu S, Wang G, Gu G, Ren H, Hong Z, Li J. Laparoscopy improves clinical outcome of gastrointestinal fistula caused by Crohn’s disease. J Surg Res. 2016;200(1):110–116. doi: 10.1016/j.jss.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 2.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 3.Wong PF, Gilliam AD, Kumar S, Shenfine J, O'Dair GN, Leaper DJ. Antibiotic regimens for secondary peritonitis of gastrointestinal origin in adults. The Cochrane database of systematic reviews. 2005;2:CD004539. doi: 10.1002/14651858.CD004539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelsberg J, Berger A, Schell S, Mallick R, Kuznik A, Oster G. Economic consequences of failure of initial antibiotic therapy in hospitalized adults with complicated intra-abdominal infections. Surg Infect. 2008;9(3):335–347. doi: 10.1089/sur.2006.100. [DOI] [PubMed] [Google Scholar]
- 5.Deresinski S. Principles of antibiotic therapy in severe infections: optimizing the therapeutic approach by use of laboratory and clinical data. Clin Infect Dis. 2007;45(Suppl 3):S177–S183. doi: 10.1086/519472. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Y, Wei Z, Shen P, Ji J, Sun Z, Yu H, Zhang T, Ji P, Ni Y, Hu Z, et al. Bacterial-resistance among outpatients of county hospitals in China: significant geographic distinctions and minor differences between central cities. Microbes Infect. 2015;17(6):417–425. doi: 10.1016/j.micinf.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–354. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Di Carlo P, Vitale F, O'Suilleabhain C, Casuccio A. Management of Intra-abdominal Infections due to Carbapenemase-Producing Organisms. Curr Infect Dis Rep. 2014;16(10):428. doi: 10.1007/s11908-014-0428-7. [DOI] [PubMed] [Google Scholar]
- 9.Biedenbach DJ, Bouchillon SK, Hoban DJ, Hackel M, Phuong DM, Nga TT, Phuong NT, Phuong TT, Badal RE. Antimicrobial susceptibility and extended-spectrum beta-lactamase rates in aerobic gram-negative bacteria causing intra-abdominal infections in Vietnam: report from the Study for Monitoring Antimicrobial Resistance Trends (SMART 2009-2011) Diagn Microbiol Infect Dis. 2014;79(4):463–467. doi: 10.1016/j.diagmicrobio.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Yang Q, Liao K, Ni Y, Yu Y, Hu B, Sun Z, Huang W, Wang Y, Wu A, et al. Antimicrobial Susceptibilities of Aerobic and Facultative Gram-Negative Bacilli from Intra-abdominal Infections in Patients from Seven Regions in China in 2012 and 2013. Antimicrob Agents Chemother. 2015;60(1):245–251. doi: 10.1128/AAC.00956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zalacain M, Biedenbach DJ, Badal RE, Young K, Motyl M, Sahm DF. Pathogen Prevalence and Antimicrobial Susceptibility Among Enterobacteriaceae Causing Hospital-Associated Intra-abdominal Infections in Adults in the United States (2012-2013) Clin Ther. 2016;38(6):1510–1521. doi: 10.1016/j.clinthera.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Xie J, Peters BM, Li B, Li L, Yu G, Xu Z, Shirtliff ME. Clinical features and antimicrobial resistance profiles of important Enterobacteriaceae pathogens in Guangzhou representative of Southern China, 2001-2015. Microb Pathog. 2017;107:206–211. doi: 10.1016/j.micpath.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Ren J, Li X, Liu S, Wu Q, Wang G, Gu G, Ren H, Li J. Assessment of Microbiota and Their Drug Resistance in Chronic Fistulous Tracts. Surg Infect. 2015;16(3):236–240. doi: 10.1089/sur.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Politis M, Wajnsztajn D, Rosin B, Block C, Solomon A. Trends of Bacterial Keratitis Culture Isolates in Jerusalem; a 13- Years Analysis. PLoS One. 2016;11(11):e0165223. doi: 10.1371/journal.pone.0165223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko WC, Hsueh PR. Increasing extended-spectrum beta-lactamase production and quinolone resistance among Gram-negative bacilli causing intra-abdominal infections in the Asia/Pacific region: data from the Smart Study 2002-2006. J Infect. 2009;59(2):95–103. doi: 10.1016/j.jinf.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo P, Gulotta G, Casuccio A, Pantuso G, Raineri M, Farulla CA, Bonventre S, Guadagnino G, Ingrassia D, Cocorullo G, et al. KPC - 3 Klebsiella pneumoniae ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: analysis of a case series of 30 patients. BMC Anesthesiol. 2013;13(1):13. doi: 10.1186/1471-2253-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Carlo P, Pantuso G, Cusimano A, D'Arpa F, Giammanco A, Gulotta G, Latteri AM, Madonia S, Salamone G, Mammina C. Two cases of monomicrobial intraabdominal abscesses due to KPC--3 Klebsiella pneumoniae ST258 clone. BMC Gastroenterol. 2011;11:103. doi: 10.1186/1471-230X-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esen S, Leblebicioglu H. Prevalence of nosocomial infections at intensive care units in Turkey: a multicentre 1-day point prevalence study. Scand J Infect Dis. 2004;36(2):144–148. doi: 10.1080/00365540410019156. [DOI] [PubMed] [Google Scholar]
- 19.Nijssen S, Florijn A, Bonten MJ, Schmitz FJ, Verhoef J, Fluit AC. Beta-lactam susceptibilities and prevalence of ESBL-producing isolates among more than 5000 European Enterobacteriaceae isolates. Int J Antimicrob Agents. 2004;24(6):585–591. doi: 10.1016/j.ijantimicag.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE, Antibacterial Resistance Leadership G Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis. 2015;60(9):1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YT, Coombs G, Ling T, Balaji V, Rodrigues C, Mikamo H, Kim MJ, Rajasekaram DG, Mendoza M, Tan TY, et al. Epidemiology and trends in the antibiotic susceptibilities of Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region, 2010-2013. Int J Antimicrob Agents. 2017;49(6):734–739. doi: 10.1016/j.ijantimicag.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Gu B, Mei Y, Wen Y, Xia W. Increasing resistance rate to carbapenem among blood culture isolates of Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa in a university-affiliated hospital in China, 2004-2011. J Antibiot B. 2015;68(2):115–120. doi: 10.1038/ja.2014.119. [DOI] [PubMed] [Google Scholar]
- 24.Jean SS, Hsueh PR, Group SA-P. Distribution of ESBLs, AmpC beta-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008–14: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) J Antimicrob Chemother. 2017;72(1):166–171. doi: 10.1093/jac/dkw398. [DOI] [PubMed] [Google Scholar]
- 25.Babinchak T, Badal R, Hoban D, Hackel M, Hawser S, Lob S, Bouchillon S. Trends in susceptibility of selected gram-negative bacilli isolated from intra-abdominal infections in North America: SMART 2005-2010. Diagn Microbiol Infect Dis. 2013;76(3):379–381. doi: 10.1016/j.diagmicrobio.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Lee YL, Chen YS, Toh HS, Huang CC, Liu YM, Ho CM, Lu PL, Ko WC, Chen YH, Wang JH, et al. Antimicrobial susceptibility of pathogens isolated sfrom patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2006 to 2010. Int J Antimicrob Agents. 2012;40(Suppl):S29–36. doi: 10.1016/S0924-8579(12)70007-9. [DOI] [PubMed] [Google Scholar]
- 27.Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, Zotou A, Spyropoulou A, Koutsileou K, Vamvakopoulou S, Sioulas N, Karamouzos V, Anastassiou ED, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis. 2017;36(7):1125–1131. doi: 10.1007/s10096-017-2899-6. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Wang M, Wang G, Wu X, Guan W, Ren J. Microbial Characteristics of Nosocomial Infections and Their Association with the Utilization of Hand Hygiene Products: A Hospital-Wide Analysis of 78,344 Cases. Surg Infect. 2017;18(6):676–683. doi: 10.1089/sur.2017.037. [DOI] [PubMed] [Google Scholar]
- 29.Ulger F, Dilek A, Esen S, Sunbul M, Leblebicioglu H. Are healthcare workers’ mobile phones a potential source of nosocomial infections? Review of the literature. J Infect Dev Ctries. 2015;9(10):1046–1053. doi: 10.3855/jidc.6104. [DOI] [PubMed] [Google Scholar]
- 30.Pournaras S, Markogiannakis A, Ikonomidis A, Kondyli L, Bethimouti K, Maniatis AN, Legakis NJ, Tsakris A. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J Antimicrob Chemother. 2006;57(3):557–561. doi: 10.1093/jac/dkl004. [DOI] [PubMed] [Google Scholar]
- 31.Yoon J, Urban C, Terzian C, Mariano N, Rahal JJ. In vitro double and triple synergistic activities of Polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2004;48(3):753–757. doi: 10.1128/AAC.48.3.753-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu A, Zheng B, Xu YC, Huang ZG, Zhong NS, Zhuo C. National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infect. 2016;22(Suppl 1):S1–S8. doi: 10.1016/j.cmi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Tan SH, Teng CB, Ng TM, Lye DC. Antibiotic therapy and clinical outcomes of Pseudomonas aeruginosa (PA) bacteraemia. Ann Acad Med Singap. 2014;43(11):526–534. [PubMed] [Google Scholar]
- 34.Cotrina-Luque J, Gil-Navarro MV, Acosta-Garcia H, Alfaro-Lara ER, Luque-Marquez R, Beltran-Garcia M, Bautista-Paloma FJ. Continuous versus intermittent piperacillin/tazobactam infusion in infection due to or suspected pseudomonas aeruginosa. Int J Clin Pharm. 2016;38(1):70–79. doi: 10.1007/s11096-015-0208-y. [DOI] [PubMed] [Google Scholar]
- 35.Olearo F, Albrich WC, Vernaz N, Harbarth S, Kronenberg A, Swiss Centre For Antibiotic Resistance A Staphylococcus aureus and methicillin resistance in Switzerland: regional differences and trends from 2004 to 2014. Swiss Med Wkly. 2016;146:w14339. doi: 10.4414/smw.2016.14339. [DOI] [PubMed] [Google Scholar]
- 36.Mu C, Yang Y, Su Y, Zoetendal EG, Zhu W. Differences in Microbiota Membership along the Gastrointestinal Tract of Piglets and Their Differential Alterations Following an Early-Life Antibiotic Intervention. Front Microbiol. 2017;8:797. doi: 10.3389/fmicb.2017.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tellor B, Skrupky LP, Symons W, High E, Micek ST, Mazuski JE. Inadequate Source Control and Inappropriate Antibiotics are Key Determinants of Mortality in Patients with Intra-Abdominal Sepsis and Associated Bacteremia. Surg Infect. 2015;16(6):785–793. doi: 10.1089/sur.2014.166. [DOI] [PubMed] [Google Scholar]
- 38.Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, Chang PK, O'Neill PJ, Mollen KP, Huston JM, et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg Infect. 2017;18(1):1–76. doi: 10.1089/sur.2016.261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are contained within the manuscript.


