Abstract
The present study aimed to assess the expression of endoplasmic reticulum oxidoreductin-1-like (ERO1L) in gastric cancer and determine its association with patient prognosis. A total of 105 patients with gastric cancer undergoing radical gastrectomy were selected for the current study. Gastric cancer tissues (the observation group) and normal gastric tissue adjacent to the carcinoma (the control group) were resected from patients. Levels of ERO1L mRNA and protein in tumor tissues and adjacent tissues were detected using reverse transcription-quantitative polymerase chain reaction, western blotting and immunohistochemistry. Patients were divided into two groups: A positive group and negative group, according to the expression of ERO1. The expression of ERO1L in gastric cancer and its association with patient prognosis was analyzed. Levels of ERO1 mRNA and protein in gastric cancer were significantly higher than those of adjacent tissues (P<0.05). Immunohistochemical analysis demonstrated that there were 22 patients exhibiting negative expression of ERO1L and 83 patients exhibiting positive expression of ERO1L. The cumulative recurrence rates over 3 years in patients with positive expression of ERO1L were significantly higher than in patients with negative expression of ERO1L (P<0.05); the cumulative survival rates over 3 years in patients with positive expression of ERO1L were significantly lower than those of patients with negative expression of ERO1L (P<0.05). Thus, the current study determined that ERO1L was highly expressed in gastric cancer tissue. The high expression of ERO1L was associated with adverse prognoses in patients with gastric cancer. ERO1L may therefore be a therapeutic target for the prevention of gastric cancer.
Keywords: gastric cancer, endoplasmic reticulum oxidoreductin-1-like, prognosis, cumulative survival rate, cumulative recurrence rate
Introduction
Gastric cancer is the second most common malignant tumor and has the second highest cancer-associated mortality rate in the world and the highest cancer-associated mortality rate in China (1). The morbidity and mortality rates of gastric cancer are increasing, posing an increasing threat to public health (2). The symptoms of gastric cancer are mostly non-specific and this mainly accounts for its delay in presentation. The prognosis of patients with gastric cancer is closely associated with its diagnosis and treatment. The methods used to diagnose gastric cancer include endoscope, ultrasound and immunological detection (3). At present, there are multiple chemotherapy regimens used for gastric cancer, which will be chosen according to the patient's condition and physical fitness (4). If standardized comprehensive treatment is provided when patients are in the earlier stages of gastric cancer, the 5-year survival rate may be >90% and some patients enter remission. By contrast, although standardized comprehensive treatment is performed on patients with advanced gastric cancer, the 5-year survival rate remains <5% (5,6). In China, 70% of patients with gastric cancer are in the middle or advanced stages when a definitive diagnosis is made. These patients are unable to undergo tumor resection and require chemotherapy instead. However, chemotherapy regimens often fail in such patients, as gastric cancer cells readily develop multidrug resistance (7,8). Thus, promoting the early detection, diagnosis and treatment of gastric cancer is key to improving the rates of remissio'n and survival in patients with gastric cancer. Additionally, it is vital to clarify the pathogenesis of gastric cancer to improve the treatment and prognosis of patients.
The endoplasmic reticulum oxidoreductin-1-like protein (ERO1L) gene is located on chromosome 14 in humans and is considered to be the primary source of endoplasmic reticulum (9). ERO1L protein is an oxidase in the endoplasmic reticulum which regulates hypoxia-induced oxidative protein folding. ERO1L may catalyze the formation of a protein folding disulfide bond and is thought to be involved in cellular apoptosis and tumor infiltration, as well as metastasis induced by endoplasmic reticulum stress (ERS) (10–12). The current study assessed the expression of ERO1L in gastric cancer and analyzed the association between ERO1L expression and the prognosis of patients with gastric cancer, so as to elucidate the value of ERO1L protein in postoperative prognosis of gastric cancer.
Patients and methods
Patients
A total of 105 patients with gastric cancer undergoing radical gastrectomy at The First Affiliated Hospital of Henan University of Science and Technology (Henan, China) were recruited in the current study between October 2013 and October 2015. There were 63 males and 42 females, with mean age of 53.7±12.8 years old (range, 39–75 years). Gastric cancer tissue and normal gastric tissue adjacent to the carcinoma were taken from all patients during radical resection of gastric tumors. Lesions were not detected in normal adjacent tissues following pathological examination. The tumor location in each patient varied. There were 46 cases in the gastric antrum/pylorus, 36 cases in gastric fundus/cardia and 23 cases in the gastric body. Additionally, the tumor-node-metastasis (TNM) stages of all patients were assessed (13). There were 46 patients with TNM stage II and 59 patients had stage III. The current study was conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Henan University of Science and Technology (Henan, China). Written informed consent was obtained from all participants.
RT-PCR
A total of 0.1 g tissue was weighed and TRIzol (Takara, Biotechnology, Co., Ltd., Dalian, China) was added. The tissue was cut into small pieces using surgical scissors and ground using a glass homogenizer. Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. The final precipitation was dissolved in RNase free water, the optical density (OD) value of total RNA and OD260/OD280 were recorded to calculate RNA purity. RNA was transcribed into cDNA using the PrimeScript RT Reagent kit (Takara Biotechnology, Co., Ltd.), following the manufacturer's instructions. GAPDH was used as a reference gene. The primers used were as follows: ERO1L, forward, 5′-CCATTAGTGCTGCCA-ACCAGTA-3′ and reverse, 5′-ATCTGCATCAGCATCACGGTC-3′; GAPDH, forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′. The primer was diluted to 10 µmol/l−1. A PCR reaction system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was prepared with a total reaction volume of 20 µl. The PCR reaction conditions were as follows: Initiation for 3 min at 94°C, degeneration for 30 sec at 94°C, annealing for 30 sec at 60°C and extension for 30 sec at 72°C, for a total of 35 cycles. The data were directly read from the ABI 7500 Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and quantitatively analyzed using the 2−ΔΔCq method (14).
Western blotting
A total of 0.15 g gastric cancer and normal tissue was weighed and cut into pieces using the scissors. The sample was ground using the glass homogenizer, washed using 10X pre-cooled PBS, centrifuged at 3,000 × g for 5 min at 4°C. The supernatant was removed, radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China) was placed in the ice box, lyzed for 30 min and centrifuged at 15,000 × g for 10 min at 4°C. The supernatant was removed and protein concentration was determined using the BCA protein assay kit (Beyotime Institute of Biotechnology), 5X SDS sample buffer was added; the final concentration was 1X. The sample was boiled for 30 min prior to 12% SDS-PAGE (100 ng protein per lane). The protein was then transferred onto PVEF membranes and blocked with 5% nonfat milk powder for 30 min at room temperature. Mouse anti human ERO1L monoclonal antibody (dilution, 1:1,000; cat. no. ab57177; Abcam, Cambridge, UK) was added, incubated overnight at 4°C, prior to washing with PBST three times for 5 min each time. Goat-anti-mouse immunoglobulin G monoclonal antibody (dilution, 1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was added and incubated at room temperature for 1 h. The 3,3′-diaminobenzidine (DAB) reagent (Beyotime Institute of Biotechnology) was used for visualization and photographed. GAPDH antibody (dilution, 1:2,000; cat. no, sc-25778; Santa Cruz Biotechnology, Inc.) was used as an internal reference, and incubated overnight at 4°C. Results were analyzed using the Image J software (version 1.38, National Institutes of Health, Bethesda, MD, USA) and the relative expression of target protein was calculated.
Immunohistochemical analysis
The tissue specimens, including controls and gastric cancer tissue, were embedded in paraffin using embedding agent at room temperature for 1 h, continuously cut into 4 mm slices, adhered on a glass slide processed with polylysine, baked in an oven at 50°C for 1 h, hydrated using xylene and 100, 95, 80 and 75% ethanol and washed three times with distilled water. The slides were then arranged in the buffer solution containing sodium citrate, heated twice for 8 min each time and washed with PBS three times. Slides were then incubated in 3% H2O2 solution at room temperature for 10 min to block endogenous catalase activity and followed by three washes with PBS 5 min each time. The ERO1L antibody (dilution, 1:100; cat. no. ab57177) was added, incubated overnight at 4°C and washed 3 times using PBST for 5 min each time. Subsequently, 10% goat-anti-mouse secondary antibody (dilution, 1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) was added, incubated at room temperature for 1 h and washed for 3 times using PBST for 5 min each time. DAB development, staining with hematoxylin, 0.1% hydrochloric acid differentiation, dehydration, transparency and neutral resin sealing were performed successively. A fluorescent microscope (BX61 Automated Fluorescent Microscope; Olympus Corporation, Tokyo, Japan) was used to observe the above results at magnification, ×200.
Observation index
Microscope observation demonstrated that ERO1L protein was primarily detected in the cytoplasm. The staining was pale yellow and brown and cells were analyzed according to cell staining intensity. For each tissue section, 5 high power fields were selected. A total of 100 target cells were counted under each high power field. The average percentage of 5 fields was judged as the result. Staining evaluation was performed as follows: 0, all negative; 1, positive cells ≤10%; 2, positive cells 11–50%; 3, positive cells 51–75%; 4, positive cells >75%. Staining intensity was evaluated as follows: 0, no staining; 1, pale yellow; 2, brown; 3, deep yellow. The two scores were multiplied and the total score was the demarcation point. <4 was determined to indicate negative ERO1L expression and ≥4 was determined to indicate positive expression of ERO1L. The cumulative survival and recurrence rates were evaluated in the ERO1L positive and negative groups.
Statistical analysis
All data were analyzed using SPSS 17.0 statistical software (SPSS, Inc, Chicago, IL, USA). All measurement data are presented as the mean ± standard deviation. The measurement data among groups were compared using the Student's t-test. The cumulative survival and recurrence rates were analyzed using the log-Rank test. P<0.05 indicated a statistically significant difference.
Results
Comparison of ERO1L mRNA levels between the two groups of tissue
The results of RT-qPCR indicated that ERO1L mRNA levels in gastric cancer tissues were significantly higher compared with normal adjacent tissues and that this difference was statistically significant (P<0.05; Fig. 1).
Figure 1.
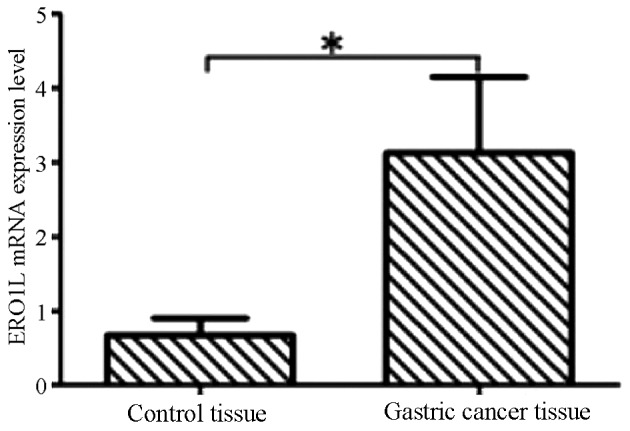
Analysis of ERO1L mRNA levels in control and gastric cancer tissues by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean + standard deviation. *P<0.05. ERO1L, endoplasmic reticulum oxidoreductin-1-like.
Comparison of ERO1L protein expression in the two groups of tissue
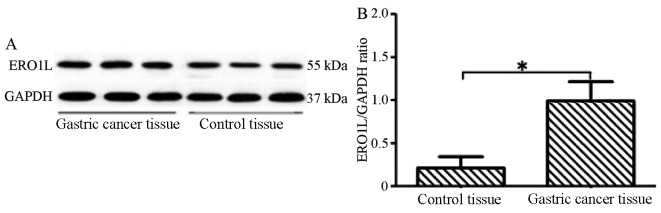
The results of the western blot analysis demonstrated that, compared with adjacent tissues, ERO1L expression in gastric cancer tissue was significantly higher (P<0.05; Fig. 2).
Figure 2.
Comparison of ERO1L expression in the two groups of tissue. (A) Western blots indicating ERO1L expression in the gastric cancer and adjacent normal tissue. (B) ERO1L expression in the two groups as determined by quantitative analysis. Data are presented as the mean ± standard deviation. *P<0.05. ERO1L, endoplasmic reticulum oxidoreductin-1-like.
ERO1L protein expression in the two groups of tissues
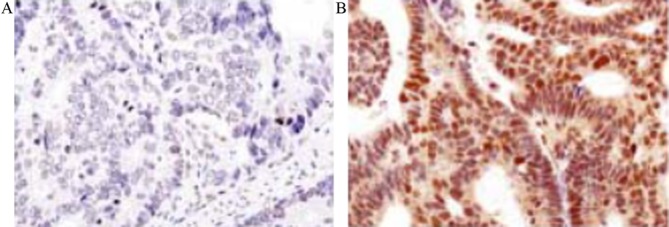
As presented in Fig. 3, the results of immunohistochemistry demonstrated that ERO1L was primarily expressed in the cytoplasm, but was not expressed in the nucleus or cell membrane. Results from immunohistochemistry demonstrated that expression of ERO1L was negative in the adjacent tissues, whereas its expression was markedly increased in gastric cancer tissues. In gastric cancer tissue, the expression of ERO1L was positive in 83 cases and negative in 22 cases.
Figure 3.
Immunochemical assay results of endoplasmic reticulum oxidoreductin-1-like expression in the two groups of tissue. (A) Control tissue and (B) gastric cancer tissue. Magnification, ×200.
Expression of ERO1L and its association with the prognosis of patients
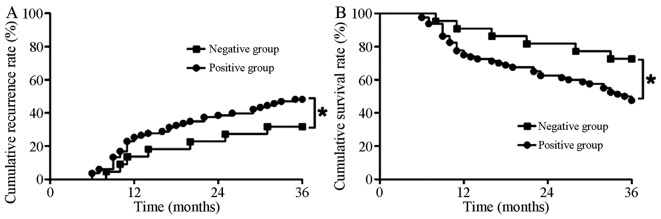
As presented in Fig. 4, all patients were followed up for >36 months. Kaplan-Meier curve indicated that the cumulative recurrence rates at 1, 2 and 3 years were 25.30% (n=21), 38.55% (n=32) and 48.19% (n=40) in ERO1L positive patients; The cumulative recurrence rates within 1, 2 and 3 years were 13.64% (n=3), 22.73% (n=5) and 31.82% (n=7) respectively in ERO1L negative patients. The cumulative recurrence rate of ERO1L positive patients was significantly higher than that of negative patients, and the difference was statistically significant (P<0.05; Fig. 4A). Log-Rank test indicated that the cumulative survival rates for 1, 2 and 3 years were 75.90% (n=63), 63.86% (n=53) and 49.40% (n=41) respectively in patients positive for ERO1L. By contrast, the cumulative survival rates for 1, 2 and 3 years were 90.91% (n=20), 81.82% (n=18) and 72.73% (n=16) respectively, in patients negative for ERO1L. The cumulative survival rate in patients positive for ERO1L was significantly lower than that of patients negative for ERO1L (P<0.05; Fig. 4B).
Figure 4.
The cumulative recurrence rate and survival rate over 12, 24 and 36 months in patients who were ERO1L negative compared with those who were ERO1L positive. (A) Cumulative recurrence rate curve and (B) Cumulative survival rate curve. *P<0.05. ERO1L, endoplasmic reticulum oxidoreductin-1-like.
Discussion
Gastric cancer is a type of malignant tumor originating from the mucosal epithelial cells of the gastric wall surface layer and can occur in various different areas of the stomach (15). In China, the incidence and mortality rates of gastric cancer are the highest of all malignant tumors. The clinical manifestations of early gastric cancer are mucosal dysplasia and intestinal metaplasia, associated with chronic atrophic gastritis, and have poor specificity, which do not attract the attention of patients and clinicians (16). The majority of patients are in the middle and advanced stages of gastric cancer when they are diagnosed, which is often accompanied by infiltration of adjacent tissues and organs or varying degrees of distant metastasis. In China, most patients generally visit a doctor when marked symptoms occur, which usually occurs when patients are in the middle and advanced stage of gastric cancer. Therefore, the diagnosis rate of patients with early gastric cancer accounts for <10% of all gastric cancer diagnoses in China (17–19). At present, treatments for gastric cancer primarily rely on surgical treatment, combined with radiotherapy and chemotherapy and marked progress has been made in the efficacy of treatments. However, the long-term survival rate of patients remains low and patient quality of life is low. The mechanisms responsible for the occurrence, development and prognosis of gastric cancer are still not fully understood. The present data indicate that they are closely associated with a number of factors. Therefore, it is important to identify a novel molecular target for clinical treatment and curative effect prediction (20,21). Previous studies determined that endoplasmic reticulum oxide (ERO1L) may be used as a potential marker of gastric cancer (22). Therefore, the role of ERO1L in the occurrence and development of gastric cancer was assessed in the current study.
The endoplasmic reticulum is the primary organelle of the cell and its primary function is to be responsible for the folding of protein and to maintain cell homeostasis (23). The stimulation of certain physiological, biochemical and pathological factors can lead to oxidative stress, so as to affect protein folding, the accumulation of unfolded protein and the misfolding of proteins in the endoplasmic reticulum, which leads to ERS (21,24). It was previously determined that ERS was prevalent in tumor tissues (25). ERS was positively correlated with the depth of tumor invasion and degree of metastasis, indicating that ERS might be an important inducement of invasion and metastasis of tumor cells (26). ERS, a type of self-protective reaction of the cells, is involved in the occurrence and development of breast, liver and pancreatic cancer (27–29). ERO1L may promote the formation of a disulfide bond of unfolded protein and can maintain the appropriate oxidation environment of the endoplasmic reticulum as a redox sensor (30). It was determined that the appropriate inhibition of ERO1L may promote the transduction of an anti-severe ERS signal. Thus, ERO1L serves an important role in maintaining the function of ER and comprehensive abilities (10,31). In the present study, the expression of ERO1L in gastric cancer and adjacent normal tissue was determined by RT-qPCR and western blotting. The results determined that the expression of ERO1L in gastric cancer tissue was significantly higher than in adjacent tissues both at the mRNA and protein levels. In addition, immunofluorescence quantitative analysis of ERO1L protein indicated that among the 105 patients, 83 were ERO1L positive and 22 were ERO1L negative. The expression of ERO1L in normal adjacent tissues was negative. Further analysis of the association between ERO1L and patient prognosis demonstrated that the cumulative recurrence rate in ERO1L positive patients was significantly higher than that of patients with negative ERO1L. Furthermore, the cumulative survival rate was significantly lower in patients with positive ERO1L than those with negative ERO1L. The aforementioned results indicate that high ERO1L expression is closely associated with patient prognosis. Further studies are required to further elucidate the role of ERO1L in gastric cancer.
In conclusion, ERO1L is highly expressed in gastric cancer tissue and is associated with poor prognosis in patients with gastric cancer. ERO1L is expected to become a target for the prevention of gastric cancer.
References
- 1.Ye XS, Yu C, Aggarwal A, Reinhard C. Genomic alterations and molecular subtypes of gastric cancers in Asians. Chin J Cancer. 2016;35:42. doi: 10.1186/s40880-016-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikuchi S, Kagawa S, Ohara T, Kubota T, Kuwada K, Kagawa T, Kuroda S, Shirakawa Y, Nishizaki M, Fujiwara T. Recurrence after endoscopic curative resection of mucosal gastric cancer associated with an adjacent neoplastic precursor lesion. Acta Med Okayama. 2016;70:213–216. doi: 10.18926/AMO/54421. [DOI] [PubMed] [Google Scholar]
- 3.Davidson M, Okines AF, Starling N. Current and future therapies for advanced gastric cancer. Clin Colorectal Cancer. 2015;14:239–250. doi: 10.1016/j.clcc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Oikawa K, Saito A, Kiyuna T, Graf HP, Cosatto E, Kuroda M. Pathological diagnosis of gastric cancers with a novel computerized analysis system. J Pathol Inform. 2017;8:5. doi: 10.4103/2153-3539.201114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Han H, Fu S, Yang P, Gu Z, Zhou Q, Cao Z. Dehydroeffusol inhibits gastric cancer cell growth and tumorigenicity by selectively inducing tumor-suppressive endoplasmic reticulum stress and a moderate apoptosis. Biochem Pharmacol. 2016;104:8–18. doi: 10.1016/j.bcp.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Lan F, Zhu M, Qi Q, Zhang Y, Liu Y. Prognostic value of serum tumor abnormal protein in gastric cancer patients. Mol Clin Oncol. 2016;5:216–220. doi: 10.3892/mco.2016.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo Q, Liu J, Zhang J, Wu M, Guo L, Liao W. Development of trastuzumab-resistant human gastric carcinoma cell lines and mechanisms of drug resistance. Sci Rep. 2015;5:11634. doi: 10.1038/srep11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Zhao J, Li A, Gao P, Sun J, Song Y, Liu J, Chen P, Wang Z. Clinicopathological significance of claudin 4 expression in gastric carcinoma: A systematic review and meta-analysis. Onco Targets Ther. 2016;9:3205–3212. doi: 10.2147/OTT.S99461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q, Zhu X, Wu Y, Feng B, Jin D, Huang J, Lei T, Gan L, Yang Z. Molecular cloning and characterization of the porcine Ero1L and ERp44 genes: Potential roles in controlling energy metabolism. Gen Comp Endocrinol. 2011;173:259–269. doi: 10.1016/j.ygcen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Seol SY, Kim C, Lim JY, Yoon SO, Hong SW, Kim JW, Choi SH, Cho JY. Overexpression of endoplasmic reticulum oxidoreductin 1-α (ERO1L) is associated with poor prognosis of gastric cancer. Cancer Res Treat. 2016;48:1196–1209. doi: 10.4143/crt.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Periyasamy P, Guo ML, Buch S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy. 2016;12:1310–1329. doi: 10.1080/15548627.2016.1183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZZ, Yuan K, Yue HT, Yuan FH, Bi HT, Weng SP, He JG, Chen YH. Identification and functional characterization of an endoplasmic reticulum oxidoreductin 1-α gene in Litopenaeus vannamei. Dev Comp Immunol. 2016;57:10–19. doi: 10.1016/j.dci.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Bando E, Makuuchi R, Tokunaga M, Tanizawa Y, Kawamura T, Terashima M. Impact of clinical tumor-node-metastasis staging on survival in gastric carcinoma patients receiving surgery. Gastric Cancer. 2017;20:448–456. doi: 10.1007/s10120-016-0637-x. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Yeldan E, Oguz S, Usta U, Ilhan E, Senlikci A. Risk factors for peritoneal dissemination of gastric cancer. Minerva Chir. 2015;70:91–96. [PubMed] [Google Scholar]
- 16.Zhang WH, Chen XZ, Yang K, Liu K, Guo DJ, Wang W, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Risk factors and survival outcomes for postoperative pulmonary complications in gastric cancer patients. Hepatogastroenterology. 2015;62:766–772. [PubMed] [Google Scholar]
- 17.Ji JZ. Review of research on prevention and treatment of gastric cancer in China in thirty years. Chin J Clin Oncol. 2013;40:1345–1351. [Google Scholar]
- 18.Mégraud F, Bessède E, Varon C. Helicobacter pylori infection and gastric carcinoma. Clin Microbiol Infect. 2015;21:984–990. doi: 10.1016/j.cmi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Wu P, Liu B, Du J. Successful personalized chemotherapy for metastatic gastric cancer based on quantitative BRCA1 mRNA expression level: A case report. Oncol Lett. 2016;11:4183–4186. doi: 10.3892/ol.2016.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li QJ, Shan BE, Li H, Su JW, Zhang C, Li QX. Imbalance of Th17/Treg in patients with gastric cancer. Chin Gen Pract. 2015;18:3596–3601. [Google Scholar]
- 21.Bu X, Zhao Y, Zhang Z, Wang M, Li M, Yan Y. Recombinant Newcastle disease virus (rL-RVG) triggers autophagy and apoptosis in gastric carcinoma cells by inducing ER stress. Am J Cancer Res. 2016;6:924–936. [PMC free article] [PubMed] [Google Scholar]
- 22.Seo M, Ryou HJ, Yun EY, Goo TW. Molecular characterization of endoplasmic reticulum Oxidoreductin 1 from Bombyx mori. Int J Mol Sci. 2015;16:26520–26529. doi: 10.3390/ijms161125977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S. Defective Anks1a disrupts the export of receptor tyrosine kinases from the endoplasmic reticulum. BMB Rep. 2016;49:651–652. doi: 10.5483/BMBRep.2016.49.12.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Xu H, Hao Y, Zhao L, Cai X, Tian J, Zhang M, Han X, Ma S, Cao J, Jiang Y. Endoplasmic reticulum oxidoreductin 1α mediates hepatic endoplasmic reticulum stress in homocysteine-induced atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 2014;46:902–910. doi: 10.1093/abbs/gmu081. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Zhang J, Chen H, Chen K, Lai F, Luo J, Wang Z, Bu H, Zhang R, Li H, Tong H. Involvement of endoplasmic reticulum stress in capsaicin-induced apoptosis of human pancreatic cancer cells. Evid Based Complement Alternat Med. 2013;2013:629750. doi: 10.1155/2013/629750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang ZL, Chen RP, Zhou XT, Zhan HL, Hu MM, Liu B, Wu GD, Wu LF. Long non-coding RNA MEG3 induces cell apoptosis in esophageal cancer through endoplasmic reticulum stress. Oncol Rep. 2017;37:3093–3099. doi: 10.3892/or.2017.5568. [DOI] [PubMed] [Google Scholar]
- 27.Conti S, Petrungaro S, Marini ES, Masciarelli S, Tomaipitinca L, Filippini A, Giampietri C, Ziparo E. A novel role of c-FLIP protein in regulation of ER stress response. Cell Signal. 2016;28:1262–1269. doi: 10.1016/j.cellsig.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Hosoi T, Ozawa K. The mechanisms and pharmacological strategy for treatment of ER stress-induced metabolic syndrome. Yakugaku Zasshi. 2016;136:827–830. doi: 10.1248/yakushi.15-00292-5. [DOI] [PubMed] [Google Scholar]
- 29.Kawada K, Iekumo T, Kaneko M, Nomura Y, Okuma Y. ER stress-induced aberrant neuronal maturation and neurodevelopmental disorders. Yakugaku Zasshi. 2016;136:811–815. doi: 10.1248/yakushi.15-00292-3. [DOI] [PubMed] [Google Scholar]
- 30.Enyedi B, Várnai P, Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid Redox Signal. 2010;13:721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- 31.Zou P, Chen M, Ji J, Chen W, Chen X, Ying S, Zhang J, Zhang Z, Liu Z, Yang S, Liang G. Auranofin induces apoptosis by ROS-mediated ER stress and mitochondrial dysfunction and displayed synergistic lethality with piperlongumine in gastric cancer. Oncotarget. 2015;6:36505–36521. doi: 10.18632/oncotarget.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]