Abstract
The present study aimed to assess interleukin (IL)-6 expression in a murine model of ulcerative colitis (UC) induced by dextran sulfate sodium (DSS) and its potential association with the anti-colitis effects of triptolide (TL). Serum IL-6 levels were measured by ELISA. IL-6 gene expression levels in colonic mucosa specimens were assessed by reverse-transcription quantitative PCR and protein expression was evaluated by western blot analysis and immunohistochemistry. The expression of IL-6 was weak in mucosa specimens from normal control animals and upregulated in DSS-induced mice. In model mice treated with TL (0.4 and 0.6 mg/kg), dexamethasone or mesalazine, IL-6 expression was significantly reduced compared with that in model mice treated with normal saline or propylene glycol (P<0.05), while TL at 0.2 mg/kg did not elicit any significant inhibitory effect. There was no significant difference among TL (0.4 mg/kg and 0.6 mg/kg), mesalazine and dexamethasone treatments (P>0.05) in terms of IL-6 expression or histological score. The results of the present study indicated that IL-6 was overexpressed in a mouse model of UC and was involved in disease progression. In addition, TL exerted therapeutic effects in UC through inhibition of IL-6 expression.
Keywords: interleukin-6, triptolide, ulcerative colitis
Introduction
Studies have reported that immunological factors have a vital role in ulcerative colitis (UC), with other parameters, such as mental state, environment, irritability and free radical damage, are all involved in UC progression (1,2). Numerous cytokines contribute to non-specific inflammation induced by the above factors in susceptible individuals. Multiple pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8 and IL-17, have been reported to have a pivotal role in the pathogenesis of UC. IL-6 is generated by mononuclear macrophages, fibroblasts, endothelial cells, T cells and B cells, which possess various biological properties (3–7). Previous studies have demonstrated that IL-6 was overexpressed in inflammatory bowel mucosa, accompanied with IL-1, IL-17 and TNF-α, leading to the occurrence of inflammation in UC (8,9).
Triptolide (TL), a diterpenoid, is produced by the plant Tripterygium wilfordii. TL is known as a drug with marked pharmacological activities, including anti-inflammatory and immunomodulatory properties as well as improvement of the microcirculation; it inhibits the proliferation of various types of cancer cell and enhances tumor sensitivity to chemotherapy (10–27). In the present study, the expression patterns of IL-6 in a mouse model of UC model were assessed, as well as association between the anti-colitis effects of TL and IL-6 levels.
Materials and methods
Animals and colitis modeling
A total of 80 female BALB/c mice (age, 4–6 weeks; weight, 20–24 g) were obtained from the Animal Center of the Medical College of Nantong University (Nantong, China). They were raised in the Animal Center of Nantong University (Nantong, China) for 2 weeks at 22–24°C and 49% humidity with a 12-h light/dark cycle and were fed a mixed-feed formula and had ad libitum access to distilled drinking water. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals promulgated by the National Research Council, and supported by the Chinese National Committee for the Use of Experimental Animals for Medical Purposes, Jiangsu Branch (Nanjing, China). The study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University (Nantong, China).
According to the method by Stevceva et al (28) from 2001, a mouse model of UC was established using dextran sulfate sodium (DSS; MP Biomedicals, Santa Ana, CA, USA). A total of 80 female BALB/c mice were stochastically divided into eight groups and housed in separate cages (10 mice per cage). The eight groups were designated as Group A-H. Group A was composed of non-treated sham mice; groups B-H contained DSS-induced model animals. Mice in group B were injected 0.2 ml normal saline intraperitoneally once daily; group C received 0.2 ml 20% propylene glycol as vehicle control daily, also intraperitoneally. Groups D-F were intraperitoneally injected daily with TL (Zelang Medical Science and Technology, Nanjing, China) dissolved in 20% propylene glycol at 0.20 mg/kg (TL1), 0.40 mg/kg (TL2) and 0.60 mg/kg (TL3). Group G was intraperitoneally injected with dexamethasone (Sichuan Guangda Pharmaceutical Co., Ltd., Pengzhou, China) dissolved in normal saline at a daily dose of 0.1 mg/kg, and group H was administered mesalazine (Ipsen Ltd., Slough, UK) dissolved in water by oral gavage at a daily dose of 20 mg/kg.
Evaluation of DSS-induced UC in mice
To assess the severity of colitis, the mice were monitored daily for activity, weight, dietary intake, stool properties and hematochezia. According to the standard by Murano et al (29), the DAI score was calculated as follows: DAI=(score of body weight loss + score of stool properties + score of hematochezia)/3.
Macroscopic score
At day 8, the mice were sacrificed by cervical dislocation and sectioned along the mesenteric junction to expose the intestinal cavity. After washing and drying, the collected intestinal wall was spread and fixed for visual inspection of inflammation and ulcer formation. The disease status was evaluated using the criteria by Ekström (30).
Histological score
Colonic tissue specimens were paraffin-embedded and then stained with hematoxylin and eosin (H&E). The level of inflammation was evaluated by two independent pathologists according to a well-established scoring system (31).
Immunohistochemistry
Tissue samples were stained according to the manufacturer's protocol using and immunohistochemistry kit (cat. no. ab64261; Abcam, Cambridge, MA, USA), and the stained sections were analyzed by microscopy. To avoid possible technical errors, immunostaining data were evaluated by two independent pathologists. IL-6-positive cells (anti-IL-6 antibody; cat. no. ab7737; Abcam) were stained a brown/yellow color, ten high-power fields were randomly selected in each sample, with at least 500 cells counted. The following scoring system was employed for density evaluation: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. In addition, the extent of staining was recorded as follows: 0, <1%; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, >75%. IL-6 expression was evaluated by the two scales. The final score was obtained by multiplication of the two scores.
ELISA
The blood was obtained from the mice following scarification. IL-6 levels in plasma were measured using ELISA kits purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). ELISA was performed according to the manufacturer's instructions. The optical density was measured at a wavelength of 450 nm using a microtiter plate reader (Luwen Biological Technology Co., Ltd., Shanghai, China). The final results were expressed as pg/ml.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA from the colonic mucosa was extracted according to standard TRIzol RNA isolation instructions (Invitrogen; Thermo Fisher Scientific, Inc.) and evaluated with a spectrophotometer for quantity and purity. The RT reaction was performed for 15 min at 37°C. Forward and reverse primer sequences used to amplify IL-6 and β-actin were synthesized by the Sangon Biotech Co., Ltd. (Shanghai, China). The primers for IL-6 were as follows: Forward, 5′-GGAATTCGTGGAAATGAGAA-3′ and reverse 5′-GCACTAGGAAAGCCGAGTAC-3′. The primers for β-actin were as follows: Forward 5′-TCATCACTATTGGCAACGAGC-3′, and reverse 5′-AACAGTCCGCCTAGAAGCAC-3′. The amplification was performed under the following conditions: 95°C for 10 min (initial denaturation), followed by 45 cycles of 95°C for 10 sec (denaturation), 60°C for 20 sec (annealing) and 20°C for 35 sec (extension). Melting curves were generated at 95°C for 15 sec, 60°C for 60 sec and 95°C for 15 sec in order to assess the specificity of each reaction. β-actin mRNA was used as an internal control. The mRNA ratio of target gene to β-actin was calculated as the relative expression level of the target gene using the 2-∆∆Cq method (32).
Western blot analysis
Tissue samples were homogenized in homogenization buffer and centrifuged at 13,000 × g at 4°C for 20 min to collect the supernatants. The supernatants were subjected to BCA protein assay to determine the protein concentrations of the samples. The total tissue protein was extracted and its concentration was measured by UV spectrophotometry (Shanghai Mapada Instruments Co., Ltd., Shanghai, China). Equal amounts of total protein (20 µl) were separated by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). After blocking with 5% nonfat milk for 2 h, the membrane was incubated overnight with anti-IL-6 (1:2,500; cat. no. ab7737; Abcam) and anti-β-actin antibodies (1:2,500; cat. no. ab8226; Abcam) at 4°C. This was followed by incubation with horseradish peroxidase-conjugated secondary anti-mouse antibody (1:5,000; cat. no. A32733; Pierce; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. Band intensities were measured with ImageJ version 1.36b (National Institutes of Health, Bethesda, MA, USA) and normalized to β-actin levels. Experiments were performed three times.
Statistical analysis
Values are expressed as the mean ± standard error of the mean. SPSS 21.0 (International Business Machines Corporation, Armonk, NY, USA) was employed for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Disease severity
To determine DAI scores, factors such as activity, body weight, fur color, grooming, diet, stool consistency and hematochezia were taken into account. Mice with no intervention had a normal body weight, shiny fur as well as a regular dietary intake and stool. However, mice treated with DSS (colitis models) were anorexic and the body weight started to decrease at days 2–3 after injection with normal saline or propylene glycol; their hair became darkened and the stool properties were gradually altered. On the fourth day of treatment, hematochezia or fecal occult blood was observed, along with marked body weight loss. However, after treatment with TL (0.40 or 0.60 mg/kg), dexamethasone or mesalazine, the mice showed increased body weight and dietary intake; indeed, their activity was improved, shiny hair was regained, hematochezia was alleviated and their stool gradually regained its normal consistency (Fig. 1). These findings supported the previously suggested notion that TL has a therapeutic benefit in UC.
Figure 1.
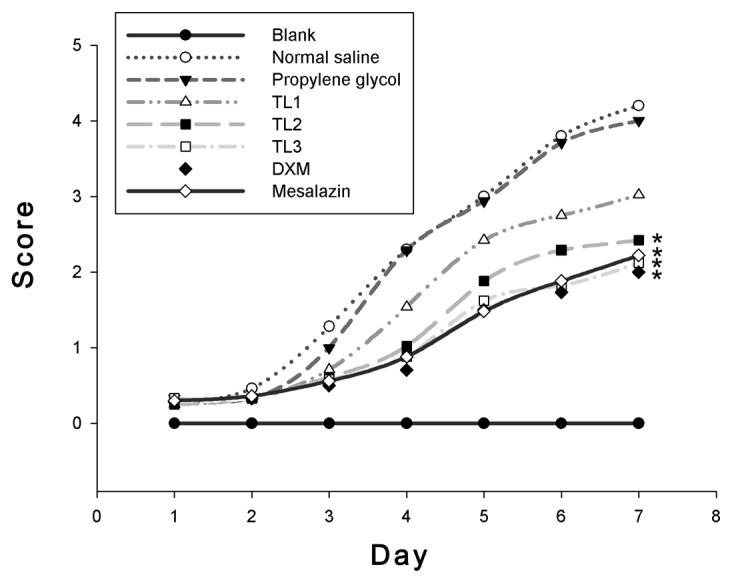
Disease activity indices of mice from the first day of dosing. *P<0.05 vs. normal saline and propylene glycol treatment groups. TL, triptolide; DXM, dexamethasone.
Gross and histological observations
Next, the present study assessed the morphological alterations in the intestinal mucosa of the mice. In the blank control group, no hyperemia, edema or erosion was found. However, after treatment with DSS, obvious erosion and superficial ulceration were observed. The lesions were continuous and developed from the anus; the rectum and distal colon were mostly affected, with severe mucosal hyperemia and edema, while the proximal colon showed mild hyperemia and edema. After treatment with TL (0.40 or 0.60 mg/kg), dexamethasone or mesalazine, the colonic mucosal injury was alleviated; the rectum and distal colon mucosae had mild hyperemia and edema, and no hyperemia or edema was observed in the proximal colon. The associated scores are shown in Table I.
Table I.
Gross score comparison among groups (n=8).
| Group | Gross score |
|---|---|
| Blank control | 0.30±0.08a |
| Normal saline treatment | 2.92±0.30 |
| Propylene glycol | 2.78±0.26 |
| TL1 (0.2 mg/kg) | 2.52±0.20 |
| TL2 (0.4 mg/kg) | 2.12±0.14a |
| TL3 (0.6 mg/kg) | 2.06±0.12a |
| Dexamethasone | 2.00±0.10a |
| Mesalazine | 1.92±0.12a |
Values are expressed as the mean ± standard deviation.
P<0.01 vs. propylene glycol and normal saline groups. TL, triptolide.
For histological observation, H&E-stained colonic mucosa specimens were prepared. The colonic mucosa was intact, without erosion or ulceration; the glands had normal morphology and were well arranged in sham control mice. After treatment with DSS, lesion, erosion and ulceration areas were observed in the colonic mucosa, with massive infiltration of lymphocytes and mononuclear cells as well as a certain number of neutrophils in the mucosa and submucosal. Lymphoid follicles were formed occasionally. The glands were deformed and disordered. However, after treatment with TL (0.40 or 0.60 mg/kg), dexamethasone or mesalazine, the degree of inflammation, erosion and ulceration in the colonic mucosa was significantly alleviated compared with that in the saline- and propylene glycol-treated groups (Table II).
Table II.
Histological score comparison among groups (n=8).
| Group | Histological score |
|---|---|
| Blank control | 0.40±0.10a |
| Normal saline treatment | 3.36±0.30 |
| Propylene glycol | 3.10±0.26 |
| TL1 (0.2 mg/kg) | 2.62±0.22 |
| TL2 (0.4 mg/kg) | 1.86±0.20a |
| TL3 (0.6 mg/kg) | 1.70±0.20a |
| Dexamethasone | 1.56±0.16a |
| Mesalazine | 1.72±0.12a |
Values are expressed as the mean ± standard deviation.
P<0.01 vs. propylene glycol and normal saline groups. TL, triptolide.
Serum IL-6 levels in mice
Next, the serum levels of IL-6 in mice were analyzed using ELISA. Serum IL-6 levels in the normal saline- and propylene glycol-treated groups were significantly higher than the blank control groups, indicating a significant increase of IL-6 induced by DSS compared with that in the control group (P<0.05). Serum IL-6 levels in the TL (0.40 and 0.60 mg/kg), dexamethasone and mesalazine treatment groups were significantly reduced compared with those in the normal saline and propylene glycol-treated groups (P<0.05; Table III).
Table III.
Serum IL-6 levels among groups (n=8).
| Group | Serum IL-6 levels (pg/ml) |
|---|---|
| Blank control | 35.56±5.22a |
| Normal saline treatment | 93.48±16.20 |
| Propylene glycol | 86.95±15.48 |
| TL1 (0.2 mg/kg) | 75.35±13.42 |
| TL2 (0.4 mg/kg) | 62.45±11.28a |
| TL3 (0.6 mg/kg) | 57.12±9.82a |
| Dexamethasone | 52.99±6.68a |
| Mesalazine | 59.26±9.16a |
Values are expressed as the mean ± standard deviation.
P<0.01 vs. propylene glycol and normal saline groups. TL, triptolide.
Tissue mRNA and protein expression pattern of IL-6 in mice
IL-6 mRNA was barely detected in the colonic mucosa of blank control mice; however, after treatment with DSS, IL-6 gene expression levels were significantly increased (P<0.05). Of note, treatment with TL (0.40 or 0.60 mg/kg), dexamethasone or mesalazine significantly decreased colitis-induced IL-6 mRNA levels (P<0.05). However, there was no significant difference in IL-6 mRNA expression levels among these abovementioned treatment groups E-H (P>0.05; Table IV).
Table IV.
IL-6 mRNA expression levels in each group (n=8).
| Group | IL-6 mRNA expression |
|---|---|
| Blank control | 1.000±0.000a |
| Normal saline treatment | 2.662±0.366 |
| Propylene glycol | 2.522±0.342 |
| TL1 (0.2 mg/kg) | 2.022±0.320 |
| TL2 (0.4 mg/kg) | 1.402±0.202a |
| TL3 (0.6 mg/kg) | 1.382±0.182a |
| Dexamethasone | 1.228±0.142a |
| Mesalazine | 1.326±0.150a |
Values are expressed as the mean ± standard deviation.
P<0.01 vs. propylene glycol and normal saline groups. TL, triptolide.
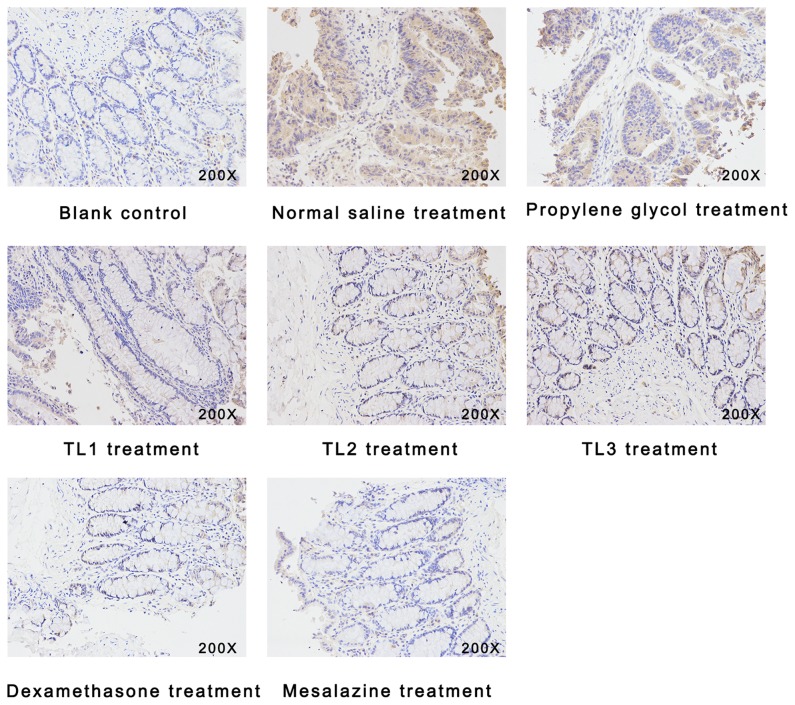
As presented in Fig. 2 and Table V, immunohistological analysis revealed an upregulation of IL-6 in the colonic mucosa tissue samples from UC mice in the vehicle-treated groups, compared with those in the blank control (P<0.05). However, TL (0.40 and 0.60 mg/kg), dexamethasone and mesalazine treatment alleviated this UC-associated upregulation of IL-6 (P<0.05). However, there was no significant difference in IL-6 expression levels among the TL (0.40 and 0.60 mg/kg), dexamethasone and mesalazine treatment groups (P>0.05).
Figure 2.
Immunohistochemical analysis of interleukin-6 (magnification, ×200).
Table V.
Immunohistological score for IL-6 in each of the treatment groups (n=8).
| Group | Immunohistological score |
|---|---|
| Blank control | 1.00±0.00a |
| Normal saline treatment | 3.88±0.38 |
| Propylene glycol | 3.72±0.36 |
| TL1 (0.2 mg/kg) | 2.92±0.26 |
| TL2 (0.4 mg/kg) | 2.34±0.20a |
| TL3 (0.6 mg/kg) | 1.98±0.16a |
| Dexamethasone | 1.76±0.12a |
| Mesalazine | 1.88±0.15a |
Values are expressed as the mean ± standard deviation.
P<0.01 vs. propylene glycol and normal saline groups. TL, triptolide.
To further assess the expression pattern of IL-6 in colonic mucosa tissue samples, IL-6 protein levels were evaluated by western blot analysis. IL-6 protein was weakly expressed in the colonic mucosa tissues of blank control mice; however, significantly higher IL-6 protein levels were found after induction of UC with DSS, and treatment with dexamethasone, mesalazine and TL (0.40 or 0.60 mg/kg) significantly attenuated these effects (Fig. 3).
Figure 3.
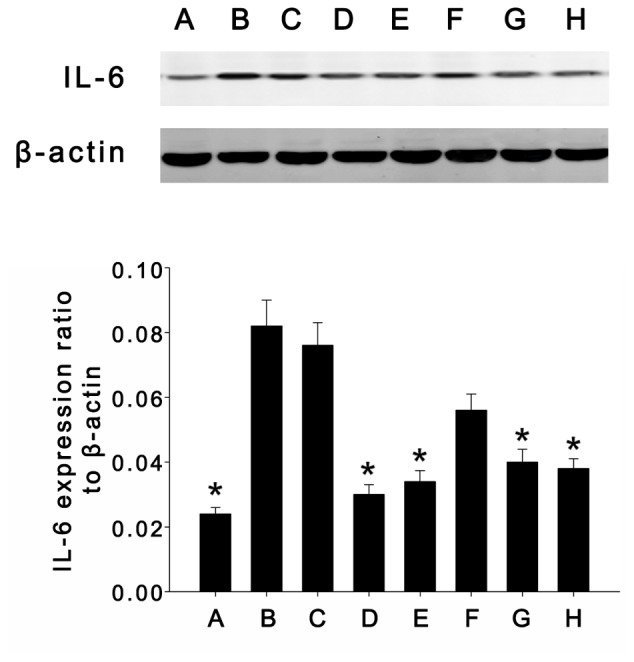
Expression of interleukin-6 (23 kDa) and β-actin (47 kDa) in intestinal tissue samples determined by western blot analysis. (A) Blank control group; (B) normal saline treatment group; (C) propylene glycol treatment group; (D) dexamethasone treatment group; (E) mesalazine treatment group; (F) TL1 treatment group (0.20 mg/kg); (G) TL2 treatment group (0.40 mg/kg); (H) TL3 treatment group (0.60 mg/kg). Values are expressed as the mean ± standard error of the mean. *P<0.05, vs. normal saline and propylene glycol treatment groups. TL, triptolide.
Discussion
Inflammatory bowel diseases (IBDs), including UC and Crohn's disease (CD), are characterized by remission and frequent exacerbation. Prior studies point to a profound role of a dysregulated immune system in the immunopathogenesis of IBD. The initial purpose of the immune response in IBD is to protect against foreign antigen invasion; however, as the immune response becomes dysregulated, it may elicit intestinal mucosa tissue damage. In the intestinal mucosa of IBD patients, the expression of pro-inflammatory cytokines, such as IL-1, IL-6, TNF-α and interferon-γ, is significantly upregulated. Aberrant activation of cytokines has a crucial role in the immunological mechanisms underlying IBD pathology (1).
TL is a diterpenoid isolated from the plant Tripterygium wilfordii; it inhibits T-cell proliferation, induces T-cell apoptosis, suppresses tumor angiogenesis and has anti-oxidant properties (10). Lu et al (13) reported that TL may restrain the infiltration of immune cells into corneal fibroblast tissue in virtue of the suppression of IL-6, intercellular adhesion molecule 1 and chemokines induced by lipopolysaccharide. Wang et al (14) demonstrated that arthritic ankle thickness decreased after TL treatment in a rat model of adjuvant-induced arthritis (AA). In synovial tissue samples from rats with AA, the expression of monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-1α was upregulated compared with that in normal control rats. Importantly, TL markedly restrained the AA-associated overexpression of MCP-1 and MIP-1α in a dose-dependent manner. Another study by Yifan et al (15) revealed that in synovial tissue samples from rats with AA, the expression of C-C chemokine receptor type 5 (CCR5) was elevated compared with that in normal rats. Similarly, TL markedly suppressed the overexpression of CCR5 induced by AA. Recently, Xu et al (16) reported that TL inhibited osteoclast differentiation and bone resorption in rheumatoid arthritis, and the repressive effects of T-regulatory cells on bone resorption were enhanced by TL through promoting the secretion of transforming growth factor-β1 and IL-10. A study by Wei et al (19) indicated that TL retarded the Toll-like receptors (TLRs)/nuclear factor (NF)-κB signaling pathway to alleviate experimental colitis. Indeed, TL was demonstrated to inhibit the TLRs/NF-κB pathway in cultured colonic explants from CD patients in vitro. In addition, in IL-10−/− mice, the expression of TLR2 and TLR4 was elevated, with TL inhibiting the TLRs/NF-κB signaling pathway in vivo. Li et al (21) found that TL inhibited the expression of IL-17 to alleviate spontaneous colitis in C3H/HeJBirIL-10−/−mice, likely involving downregulation of the IL-6/signal transducer and activator of transcription 3 signaling pathway. Furthermore, a study by Yu et al (22) demonstrated that TL markedly reduced NF-small ka, Cyrillic B activation in the colon mucosa of IL-10−/− mice. In the latter study, gene expression levels of IL-12 and IL-23 in the colon were also reduced after treatment. The efficacy of TL treatment in reducing intestinal inflammation in IL-10−/− mice is due to its anti-inflammatory as well as immunosuppressive activities. A previous study by our group comparing the effects of TL and dexamethasone in mice with DSS-induced UC indicated that TL ameliorated the disease symptoms and downregulated the expression of solute carrier protein (26). A study by Tao et al (27) indicated triptolide may be a potential therapeutic agent for IBD due to its extracellular matrix protective and anti-inflammatory properties.
The present study demonstrated that after treatment with TL (0.40 and 0.60 mg/kg), dexamethasone and mesalazine, UC in mice was greatly alleviated. Specifically, ulceration and erosion were improved, serum levels of IL-6 were significantly decreased, and the expression of IL-6 in colon tissues was also downregulated. These findings indicated that TL treatment had a similar therapeutic effect to that of dexamethasone and mesalazine, which may be exerted through inhibition of the overexpression of IL-6.
In conclusion, the presents study revealed that treatment with TL, dexamethasone and mesalazine attenuated bowel tissue inflammation; thus, TL may represent an efficient means for treating UC. Its therapeutic effect may involve restraining IL-6 expression by reducing various types of chemokines, which may offer an alternative option for UC patients. However, further studies are warranted to clarify the molecular mechanisms underlying the therapeutic role of TL in UC.
Acknowledgements
This study was supported by a grant from the Social Application Research Plans Foundation of Nantong (grant no. 2012074).
References
- 1.Hur SJ, Kang SH, Jung HS, Kim SC, Jeon HS, Kim IH, Lee JD. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 2012;32:801–816. doi: 10.1016/j.nutres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Powell N, Lo JW, Biancheri P, Vossenkämper A, Pantazi E, Walker AW, Stolarczyk E, Ammoscato F, Goldberg R, Scott P, et al. Interleukin 6 increases production of cytokines by colonic innate lymphoid cells in mice and patients with chronic intestinal inflammation. Gastroenterology. 2015;149:456–467.e15. doi: 10.1053/j.gastro.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaker A, Gargus M, Fink J, Binkley J, Darwech I, Swietlicki E, Levin MS, Rubin DC. Epimorphin(−/−) mice are protected, in part, from acute colitis via decreased interleukin 6 signaling. Transl Res. 2014;164:70–83. doi: 10.1016/j.trsl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasinathan NK, Subramaniya BR, Pandian I, Sivasithamparam ND. Aegle marmelos fruit extract abates dextran sodium sulfate induced acute colitis in mice: Repression of pro-inflammatory cytokines during colonic inflammation. Biomed Prev Nutr. 2014;4:307–317. doi: 10.1016/j.bionut.2014.03.002. [DOI] [Google Scholar]
- 5.Blumberg R, Cho J, Lewis J, Wu G. Inflammatory bowel disease: An update on the fundamental biology and clinical management. Gastroenterology. 2011;140:1701–1703. doi: 10.1053/j.gastro.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts-Thomson IC, Fon J, Uylaki W, Cummins AG, Barry S. Cells, cytokines and inflammatory bowel disease: A clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5:703–716. doi: 10.1586/egh.11.74. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: Relation to circulating interleukin-6. Gut. 1995;36:45–49. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wine E, Mack DR, Hyams J, Otley AR, Markowitz J, Crandall WV, Leleiko N, Muise AM, Griffiths AM, Turner D. Interleukin-6 is associated with steroid resistance and reflects disease activity in severe pediatric ulcerative colitis. J Crohns Colitis. 2013;7:916–922. doi: 10.1016/j.crohns.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- 11.Lu Y, Fukuda K, Nakamura Y, Kimura K, Kumagai N, Nishida T. Inhibitory effect of triptolide on chemokine expression induced by proinflammatory cytokines in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46:2346–2352. doi: 10.1167/iovs.05-0010. [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Zhou R, Yang Y, Li YC, Yang YF, Zuo JP. Suppression of (5R)-5-hydroxytriptolide (LLDT-8) on allograft rejection in full MHC-mismatched mouse cardiac transplantation. Transplantation. 2006;81:927–933. doi: 10.1097/01.tp.0000203299.39843.d2. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Liu Y, Fukuda K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of chemokine, proinflammatory cytokine, and adhesion molecule expression induced by lipopolysaccharide in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:3796–3800. doi: 10.1167/iovs.06-0319. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wei D, Lai Z, Le Y. Triptolide inhibits CC chemokines expressed in rat adjuvant-induced arthritis. Int Immunopharmacol. 2006;6:1825–1832. doi: 10.1016/j.intimp.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Yifan W, Dengming W, Zheng L, Yanping L, Junkan S. Triptolide inhibits CCR5 expressed in synovial tissue of rat adjuvant-induced arthritis. Pharmacol Rep. 2007;59:795–799. [PubMed] [Google Scholar]
- 16.Xu H, Zhao H, Lu C, Qiu Q, Wang G, Huang J, Guo M, Guo B, Tan Y, Xiao C. Triptolide inhibits osteoclast differentiation and bone resorption in vitro via enhancing the production of IL-10 and TGF-β1 by regulatory T cells. Mediators Inflamm. 2016;2016:8048170. doi: 10.1155/2016/8048170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Chen Y, Liu FQ, Lamb JR, Tam PK. Combined treatment with triptolide and rapamycin prolongs graft survival in a mouse model of cardiac transplantation. Transpl Int. 2008;21:483–494. doi: 10.1111/j.1432-2277.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 18.Tao X, Fan F, Hoffmann V, Gao CY, Longo NS, Zerfas P, Lipsky PE. Effective therapy for nephritis in (NZB × NZW)F1 mice with triptolide and tripdiolide, the principal active components of the Chinese herbal remedy Tripterygium wilfordii Hook F. Arthritis Rheum. 2008;58:1774–1783. doi: 10.1002/art.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei X, Gong J, Zhu J, Wang P, Li N, Zhu W, Li J. The suppressive effect of triptolide on chronic colitis and TNF-alpha/TNFR2 signal pathway in interleukin-10 deficient mice. Clin Immunol. 2008;129:211–218. doi: 10.1016/j.clim.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Kizelsztein P, Komarnytsky S, Raskin I. Oral administration of triptolide ameliorates the clinical signs of experimental autoimmune encephalomyelitis (EAE) by induction of HSP70 and stabilization of NF-kappaB/IkappaBalpha transcriptional complex. J Neuroimmunol. 2009;217:28–37. doi: 10.1016/j.jneuroim.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N, Li JS. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol. 2010;47:2467–2474. doi: 10.1016/j.molimm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Yu C, Shan T, Feng A, Li Y, Zhu W, Xie Y, Li N, Li J. Triptolide ameliorates Crohn's colitis is associated with inhibition of TLRs/NF-κB signaling pathway. Fitoterapia. 2011;82:709–715. doi: 10.1016/j.fitote.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Xia Y, Wang P, Lu L, Zhang F. Triptolide protects mice from ischemia/reperfusion injury by inhibition of IL-17 production. Int Immunopharmacol. 2011;11:1564–1572. doi: 10.1016/j.intimp.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Shi ZQ, Xu X, Xin GZ, Chen J, Qi LW, Li P. Triptolide inhibits amyloid-β production and protects neural cells by inhibiting CXCR2 activity. J Alzheimers Dis. 2013;33:217–229. doi: 10.3233/JAD-2012-120841. [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Li Y, Guo Z, Gong J, Zhu W, Li N, Li J. Triptolide ameliorates ileocolonic anastomosis inflammation in IL-10 deficient mice by mechanism involving suppression of miR-155/SHIP-1 signaling pathway. Mol Immunol. 2013;56:340–346. doi: 10.1016/j.molimm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Gong C, Qu L, Ding X, Cao W, Chen H, Zhang B, Zhou G. Therapeutic effects of triptolide via the inhibition of IL-1β expression in a mouse model of ulcerative colitis. Exp Ther Med. 2016;12:1279–1286. doi: 10.3892/etm.2016.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Q, Wang B, Zheng Y, Li G, Ren J. Triptolide ameliorates colonic fibrosis in an experimental rat model. Mol Med Rep. 2015;12:1891–1897. doi: 10.3892/mmr.2015.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevceva L, Pavli P, Husband AJ, Doe WF. The inflammatory infiltrate in the acute stage of the dextran sulphate sodium induced colitis: B cell response differs depending on the percentage of DSS used to induce it. BMC Clin Pathol. 2001;1:3. doi: 10.1186/1472-6890-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–58. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekström GM. Oxazolone-induced colitis in rats: Effects of budesonide, cyclosporin A, and 5-aminosalicylic acid. Scand J Gastroenterol. 1998;33:174–179. doi: 10.1080/00365529850166914. [DOI] [PubMed] [Google Scholar]
- 31.Boirivant M, Fuss IJ, Ferroni L, De Pascale M, Strober W. Oral administration of recombinant cholera toxin subunit B inhibits IL-12-mediated murine experimental (trinitrobenzene sulfonic acid) colitis. J Immunol. 2001;166:3522–3532. doi: 10.4049/jimmunol.166.5.3522. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]