Abstract
Asymptomatic pulmonary tuberculosis (PTB) mimicking lung cancer is rare and has been documented in few studies. Accurately diagnosing this atypical disease remains an enormous challenge for clinicians. The aim of the present study was to characterize asymptomatic patients with PTB who were initially diagnosed with lung cancer according to their chest computer tomography (CT) or whole-body 18F-fludeoxyglucose-positron emission tomography-computer tomography (PET-CT) presentations. The clinical characteristics and radiographic features of patients with PTB were analyzed and compared to those of patients with lung cancer. In patients with PTB, all lesions exhibited suspected malignant signs on chest CT and the maximum standard uptake value (SUVmax) of PET-CT imaging was between 2.65 and 10.9. Compared with lung cancer, the factors associated with PTB included an age <60 years (82% vs. 46%, P=0.03), being male (77% vs. 51%, P=0.025), the presence of diabetes (55% vs. 16%, P<0.01), spiculated margins (82% vs. 44%, P=0.002) and a lower SUVmax (P=0.036). The optimal cut-off level was SUVmax 8.45 for discriminating between PTB and lung cancer. At this point, the sensitivity and specificity were 63.0 and 88.9%, respectively. The results of the current study revealed methods of distinguishing between the two similar diseases. Furthermore, the results of the current study may increase awareness that although imaging of lesions may resemble lung cancer, a diagnosis of PTB should be considered. Accurate diagnosis of PTB would mean that patients would be able to avoid undergoing unnecessary operations that induce a high financial burden.
Keywords: pulmonary tuberculosis, lung cancer, computed tomography, positron emission tomography, clinical characteristics, early diagnosis
Introduction
Tuberculosis remains a major cause of death worldwide despite a decline in incidence in recent years (1). It is estimated that there were 9.6 million new cases of tuberculosis and 1.5 million cases of tuberculosis-associated mortality in 2014. China, one of nine high-burden countries of tuberculosis, had 10% of the global total TB cases (2). Although the majority of exposed individuals have asymptomatic latent tuberculosis, they are at increased risk of developing active tuberculosis and becoming infectious (3). Asymptomatic pulmonary tuberculosis (PTB) exhibits various clinical and radiological features that resemble a number of other diseases (4,5). Cases of PTB that mimic lung cancer are encountered on occasion. According to a report from National Central Cancer Registry of China, there were 651,053 newly diagnosed cases of lung cancer in 2011 in China, and 529,153 patients died of lung cancer with a crude incidence and mortality of 48.32 per 100,000 population and 39.27 per 100,000 population, respectively (6,7). Due to the increasing incidence and mortality of lung cancer, it has become the leading cause of cancer-related death in China (8). The clinical symptoms and radiologic features of PTB are nonspecific and resemble lung cancer, therefore accurate diagnosis of these two diseases remain a diagnostic dilemma in the clinic (9–13).
Typical manifestations of PTB are generally easy to diagnose due to defined clinical characteristics and radiographic findings. However, many symptoms of PTB, including cough, hemoptysis and weight loss are also common in lung cancer (14). Meanwhile, typical radiographic features indicating lung cancer, including thick-walled cavities and high fluorodeoxyglucose (FDG) uptake are also seen in PTB (11), and so patients exhibiting asymptomatic PTB mimicking lung cancer are often misdiagnosed with lung cancer. The misdiagnosis may result in delayed treatment for PTB and unnecessary operations, further increasing the severity and complications of PTB (15,16). Therefore, the present study was conducted in order to identify the features of this unusual PTB and improve the diagnostic accuracy.
Patients and methods
Patients
A total of 1,487 individuals at Chinese PLA General Hospital (Beijing, China) were consecutively enrolled in the current study between January 2011 and December 2015. These individuals were included as they underwent a periodic health examination and an indeterminate lung nodule or mass was detected by chest X-ray. A total of 241 individuals were initially excluded due to the following reasons: 138 did not undergo further examination, 45 exhibited symptoms of PTB or lung cancer, 39 had a history of cancer or tuberculosis, 16 had undergone thoracic surgery in the past 6 months and 3 were <18 years old. Out of the 1,246 individuals who subsequently underwent chest computer tomography (CT) or whole-body 18F-fludeoxyglucose-positron emission tomography-computer tomography (PET-CT), 226 were presumptively diagnosed with lung cancer. No pathological examinations were performed in 112 individuals, the data for 7 individuals was missing and 4 individuals tested negative for lung cancer or PTB; these patients were all excluded. Therefore, 22 patients with PTB and 81 patients with lung cancer were included in the current study (Fig. 1). PTB diagnosis was established by pathological and acid-fast bacilli examinations. Briefly, tissue samples obtained from pulmonary lobectomy excision, percutaneous transthoracic needle biopsy or transbronchial lung biopsy were excised and fixed with 10% neutral buffered formalin at room temperature for 48 h. After fixation, the tissues were dehydrated and embedded in paraffin. Samples were then cut into 4 µm sections, stained with hematoxylin and eosin and examined using light microscopy (Olympus BX51; Olympus Corp. Tokyo, Japan). PTB lesions exhibited chronic granulomatous inflammation, with or without caseous necrosis. To further identified the mycobacteria in the tissue sections, acid-fast staining kits (Baso Biotechnology Co., Ltd., Wuhan, China) were used according to the manufacturer's protocol. Of the 22 patients with PTB, the mean age was 53.1±10.0 years (age range, 36–72 years) and there were more males (17/22, 77.3%) than females (5/22, 22.7%). The patients with lung cancer consisted of 41 males and 40 females with a mean age of 61±5.4 years (range, 33–79 years). The present study was approved by the Institutional Review Board of Chinese PLA General Hospital and written informed consents were waived as all the data were retrospectively reviewed and analyzed anonymously.
Figure 1.
Study flow chart.
Methods
The clinical data, radiological features, pathological results and therapeutic strategies of these patients were retrospectively reviewed. Clinical data included age, gender, smoking, medical history, histological type, tumor node metastasis (TNM) staging, performance status (PS) and levels of tumor markers. According to the 2015 World Health Organization (WHO) classifications of lung cancer (17), the histological types of lung cancer in the current study were determined. TNM staging of lung cancers was evaluated using the 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system (18). The PS of lung cancers was assessed using the Eastern Cooperative Oncology Group scale (19). Tumor marker values in the serum including carcinoembryonic antigen (CEA), cytokeratin 19 fragments (CYFRA21-1), neuron-specific enolase (NSE) and cancer antigen (CA)-125 were detected by electrochemiluminescence immunoassay using the Roche COBAS E601 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Squamous cell carcinoma antigen (SCCAg) was assayed using a chemiluminescence microparticle immunoassay (Abbott ARCHITECT i2000SR immuno analyser; Abbott Pharmaceutical Co., Ltd., Lake Bluff, IL, USA). Serum concentrations of CEA (cat. no. 153910-01; Roche Diagnostics GmbH), CYFRA21-1 (cat. no. 146987-02; Roche Diagnostics GmbH), NSE (cat. no. 159459-01; Roche Diagnostics GmbH), CA-125 (cat. no. 16907902; Roche Diagnostics GmbH) and SCCAg (cat. no. 71111LP20; Abbott Pharmaceutical Co., Ltd.) were measured using kits according to the manufacturer's protocol. Levels of CEA >5 ng/ml, CYFRA21-1 >4 ng/ml, NSE >24 ng/ml, CA-125 >35 ng/ml, SCCAg >1.8 ng/ml were considered to be abnormal according to the kit protocol. Radiological findings were based on the initial and rechecked CT or PET-CT manifestation of lesions prior to treatment. The location, largest size and abnormal pulmonary imaging of the lesions were recorded. A 64-row spiral CT with slice thickness of 1.5 mm, 5 mm or high resolution CT was used in all patients and PET-CT was used in a further 36 patients for diagnosis. PET-CT images were obtained 60 min after intravenous injection of FDG. All images were interpreted independently by two experienced physicians; at least one radiologist and another respiratory physician.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. Quantitative variables were presented as the mean ± standard deviation whereas qualitative variables were presented as a percentage of the total number. The χ2 test or Fisher's exact test were used to compare clinical characteristics and imaging features between patients with PTB and those with lung cancer. Receiver operating characteristic (ROC) curves were analyzed to determine an SUVmax cut-off that would provide the optimal sensitivity and specificity for individuals undergoing PET-CT. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical features
The clinical characteristics of the 22 patients with PTB are presented in Table I. A total of 18 patients (81.8%) were <60 years old and 12 patients (54.5%) were smokers. A total of 12 patients (54.5%) had a history of diabetes in the 5 years preceding PTB diagnosis. Out of the 81 cases of lung cancer, the most common histological type was primary lung adenocarcinoma (n=69), followed by squamous cell carcinoma (n=9), pulmonary neuroendocrine carcinoma (n=2) and adenosquamous carcinoma (n=1). Furthermore, 32 (38.3%) patients with lung cancer were stage IA, 19 (23.5%) were stage IB, 25 (30.9%) were stage IIA or IIB and 5 (7.4%) were stage III or IV. Out of all patients with lung cancer, 54 (66.7%) underwent examination of levels of the tumor marker values CEA, CYFRA21-1, NSE, CA-125 and SCCAg prior to biopsy or operation. Based on the CT presentation mimicking lung cancer, 27 (33.3%) underwent operation directly without further examination of tumor marker values. Among these 54 cases, 29 (53.7%) abnormal results were obtained for CEA, 26 (48.1%) for CYFRA21-1, 13 (24.1%) for CA-125, 9 (16.7%) for SCCAg and 2 (3.7%) for NSE. Lung tumor markers were also investigated in the 22 patients with PTB, among which 4 patients exhibited abnormal values: 2 abnormal results were obtained for CYFRA21-1, 1 for CEA, 1 for SCCAg and 1 for NSE. The PS results of lung cancer were as follows: 23 (28.4%) lung cancers were 0, 40 (49.4%) were 1 and 18 (22.2%) were 2. The patients with PTB were compared to those with lung cancer and it was determined that the factors associated with PTB were an age <60 years (P=0.003), being male (P=0.025) and the presence of diabetes (P<0.001). No significant differences between patients with lung cancer and those with PTB were observed regarding smoking history (P=0.247).
Table I.
Clinical characteristics and imaging features of the 22 patients with PTB.
| No./age/sex | Smoking (years/cigarettes per day) | Diabetes | Largest size (cm) | Location of lesion | Type of biopsy | CT presentation | PET (SUVmax) |
|---|---|---|---|---|---|---|---|
| 1/41/M | 20/20 | Yes | 2.8 | RUL | Pulmonary lobectomy | Ground-glass shadow, spiculated margin, lobulation, vacuole | 2.65 |
| 2/41/M | 20/40 | Yes | 2.5 | RUL | Pulmonary lobectomy | Spiculated margin, vacuole | 8.4 |
| 3/64/M | 20/60 | Yes | 5 | RUL | Pulmonary lobectomy | Spiculated margin, lobulation, pleural indentation | – |
| 4/51/M | 30/50 | Yes | 2.5 | RUL | Pulmonary lobectomy | Spiculated margin, lobulation | – |
| 5/43/F | No | No | 2.6 | RML | Pulmonary lobectomy | Spiculated margin, blood vessel convergency | – |
| 6/50/M | No | Yes | 1.2 | LUL | PTNB | Ground-glass shadow | – |
| 7/59/M | 30/20 | Yes | 2 | LUL | Pulmonary lobectomy | Pleural indentation, vacuole | – |
| 8/55/F | No | No | 1.6 | RUL | TBLB | Spiculated margin, lobulation | 10.9 |
| 9/61/M | 15/40 | Yes | 3.2 | RML | Pulmonary lobectomy | Spiculated margin, lobulation, pleural indentation, cavity | – |
| 10/57/M | No | Yes | 2.3 | RUL | Pulmonary lobectomy | Spiculated margin, lobulation, pleural indentation | 7.7 |
| 11/68/M | 50/20 | Yes | 4 | LUL | Pulmonary lobectomy | Spiculated margin, lobulation, pleural indentation | – |
| 12/39/M | No | No | 1.3 | LUL | Pulmonary lobectomy | Spiculated margin, lobulation | 5.4 |
| 13/72/M | No | No | 1 | RML | Pulmonary lobectomy | Spiculated margin, lobulation | 3.16 |
| 14/63/M | 35/20 | Yes | 1.8 | LUL | Pulmonary lobectomy | Spiculated margin, pleural indentation, blood vessel convergency | – |
| 15/51/F | No | Yes | 3 | LUL | Pulmonary lobectomy | Spiculated margin, lobulation | 4.2 |
| 16/59/M | No | Yes | 2.5 | LLL | Pulmonary lobectomy | Spiculated margin, lobulation, cavity, ground-glass shadow | – |
| 17/56/M | 40/20 | No | 1.5 | LLL | Pulmonary lobectomy | Spiculated margin, lobulation, blood vessel convergency | – |
| 18/41/F | 40/20 | No | 3.5 | RUL | Pulmonary lobectomy | Spiculated margin, vacuole | 6.5 |
| 19/59/M | No | No | 2.7 | RUL | PTNB | Spiculated margin, lobulation, cavity | – |
| 20/36/F | No | No | 2.4 | RUL | Pulmonary lobectomy | Spiculated margin, pleural indentation | – |
| 21/47/M | 10/20 | No | 3.5 | RUL | Pulmonary lobectomy | Lobulation, vacuole | – |
| 22/57/M | 20/40 | No | 2.5 | LUL | Pulmonary lobectomy | Lobulation, blood vessel convergency, pleural indentation | 5.02 |
No, patient number; M, male; F, female; SUVmax, maximum standard uptake value; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; PTNB, percutaneous transthoracic needle biopsy; TBLB, transbronchial lung biopsy; PET, 18F-fludeoxyglucose-positron emission tomography.
Chest CT characteristics of PTB
Chest CT findings demonstrated that PTB lesions involved the left upper lobe in 7 cases (31.8%), left lower lobe in 2 cases (9.1%), right upper lobe in 10 cases (45.5%) and right middle lobe in 3 cases (13.6%). No lesions involved the right lower lobe. The largest size ranged between 1 and 5 cm in diameter and the mean value was 2.51 cm. The suspected malignant signs of lung cancer occurred at PTB lesions and included spiculated margins in 18 cases (81.8%), lobulation in 15 cases (68.2%), blood vessel convergence signs in 4 cases (18.2%), pleural indentation in 8 cases (36.4%), ground-glass shadow in 3 cases (13.6%), cavity in 3 cases (13.6%) and vacuole in 5 cases (22.7%; Table I). Different malignant signs of lung cancer could be detected in a single patient (Fig. 2). Considering the higher risk of malignancy, surgical wedge resection was performed in 17 patients, percutaneous transthoracic needle biopsy in 2 patients and transbronchial lung biopsy in 1 patient. Comparison of the assumed malignancy characteristics in patients with PTB against those with lung cancer (Table II) demonstrated that there were no significant differences between the 2 groups in terms of lobulation, blood vessel convergence signs, pleural indentation and ground-glass opacity (P>0.05). However, there was a significant difference in the frequency of spiculated margins (P=0.002); patients with PTB had a significantly higher frequency of spiculated margins than patients with lung cancer.
Figure 2.
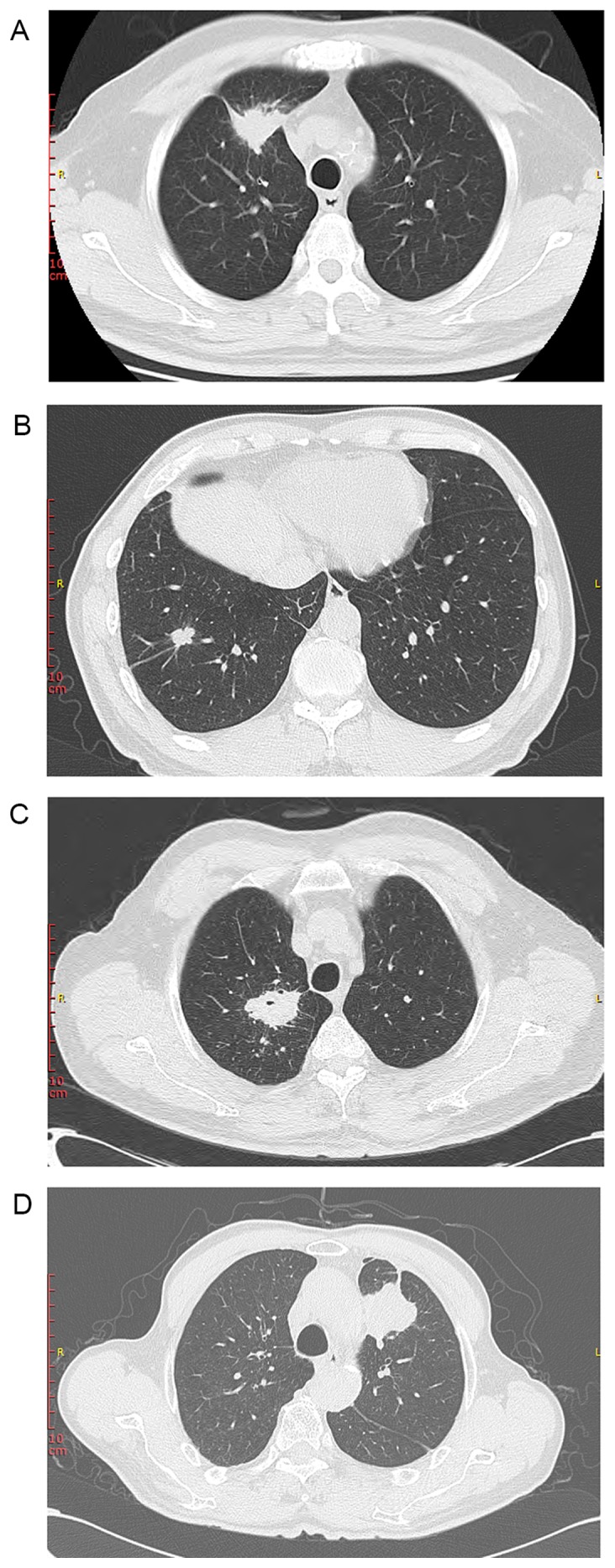
Representative chest computed tomography presentations of asymptomatic pulmonary tuberculosis mimicking lung cancer. Lesions in the 4 cases (from 4 different patients) exhibited the suspected malignant signs of lung cancer including (A) spiculated margins, pleural indentation (B) lobulation, spiculated margins, pleural indentation (C) blood vessel convergency, spiculated margain, pleural indentation (D) pleural indentation and lobulation.
Table II.
Clinical and radiological finding in patients with PTB vs. those with lung cancer.
| Total, no. (%) | Tuberculosis, no. (%) | Lung cancer, no. (%) | P-value | |
|---|---|---|---|---|
| No. | 103 | 22 | 81 | |
| Age, years | 0.003a | |||
| <60 | 55 | 18 | 37 | |
| ≥60 | 48 | 4 | 44 | |
| Sex | 0.025a | |||
| Male | 58 | 17 | 41 | |
| Female | 45 | 5 | 40 | |
| Smoking history | 0.247 | |||
| Smoker | 45 | 12 | 33 | |
| Never smoked | 58 | 10 | 48 | |
| Underlying diseases | <0.001a | |||
| Diabetes | 25 | 12 | 13 | |
| Non-diabetes | 78 | 10 | 68 | |
| Location of lesion | 0.175 | |||
| Upper lobe | 67 | 17 | 50 | |
| Others | 36 | 5 | 31 | |
| Largest size (cm) | 0.210 | |||
| <2 | 40 | 6 | 34 | |
| ≥2 | 63 | 16 | 47 | |
| CT presentation | ||||
| Spiculated margin | 54 | 18 | 36 | 0.002a |
| Lobulation | 63 | 15 | 48 | 0.446 |
| Ground-glass shadow | 31 | 3 | 28 | 0.058 |
| Pleural indentation | 38 | 8 | 30 | 0.922 |
| Blood vessel convergency | 25 | 4 | 21 | 0.452 |
| Cavity | 8 | 3 | 5 | 0.477 |
| Vacuole | 13 | 5 | 8 | 0.212 |
| PET (SUVmax) | ||||
| Mean ± SD | 9.06±5.13 | 5.99±2.66 | 10.09±5.38 | 0.036a |
P<0.05 patients with PTB vs. patients with lung cancer. No, number; PTB, pulmonary tuberculosis; CT, computed tomography; PET, 18F-fludeoxyglucose-positron emission tomography/computer tomography; SUVmax, maximum standard uptake value; SD, standard deviation.
PET-CT findings
Of the 36 patients that underwent PET-CT, 9 patients (25%) were confirmed as PTB and 27 (75%) were diagnosed with lung cancer. Although an SUVmax >2.5 was generally considered to be a diagnostic threshold strongly indicating malignancy, the SUVmax values in all 36 patients were >2.5 (Fig. 3). Comparatively, patients with PTB had a lower mean SUVmax than those with lung cancer; 5.99±2.66 vs. 10.09±5.38 (P=0.036; Table II). An ROC curve with a statistically significant area under the curve was obtained (0.759; 95% confidence interval 0.595–0.924; P=0.021). From the ROC curve, an SUVmax could be obtained that provided optimal sensitivity and specificity for the current study (Fig. 4). With an SUVmax cut-off of 8.45, the sensitivity and specificity of PET-CT in distinguishing between PTB and lung cancer were 63.0 and 88.9%, respectively (Fig. 4). These analyses indicated that an SUVmax cut-off of 8.45 improved the specificity of PET-CT for differentiating between PTB and lung cancer than the conventional SUVmax cut-off value of 2.5.
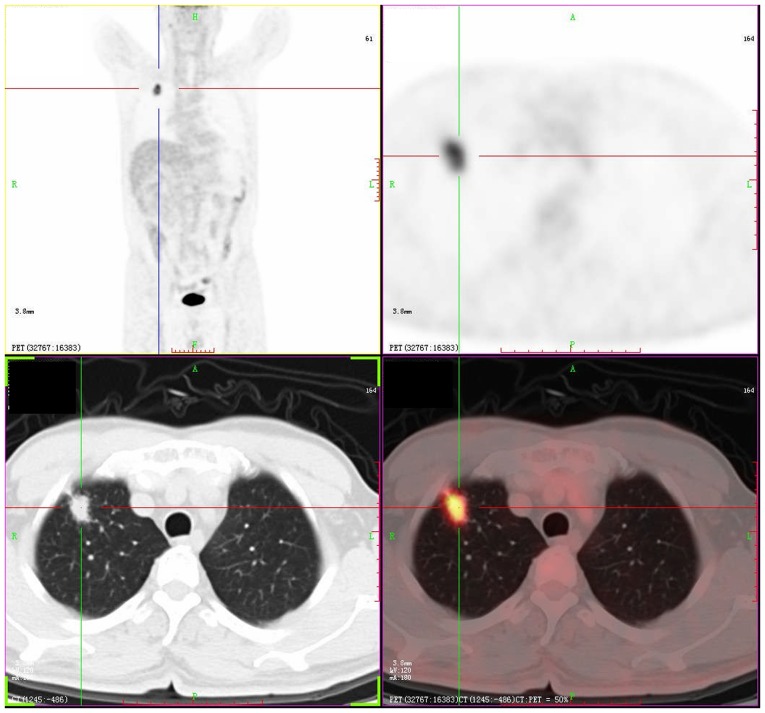
Figure 3.
PET-CT imaging of asymptomatic pulmonary tuberculosis mimicking lung cancer. PET-CT revealed a 2.3×1.3 cm lesion with malignancy features and the SUVmax was 7.7 in the right upper lobe. PET-CT, 18F-fludeoxyglucose-positron emission tomography-computer tomography; SUVmax, maximum standard uptake value.
Figure 4.
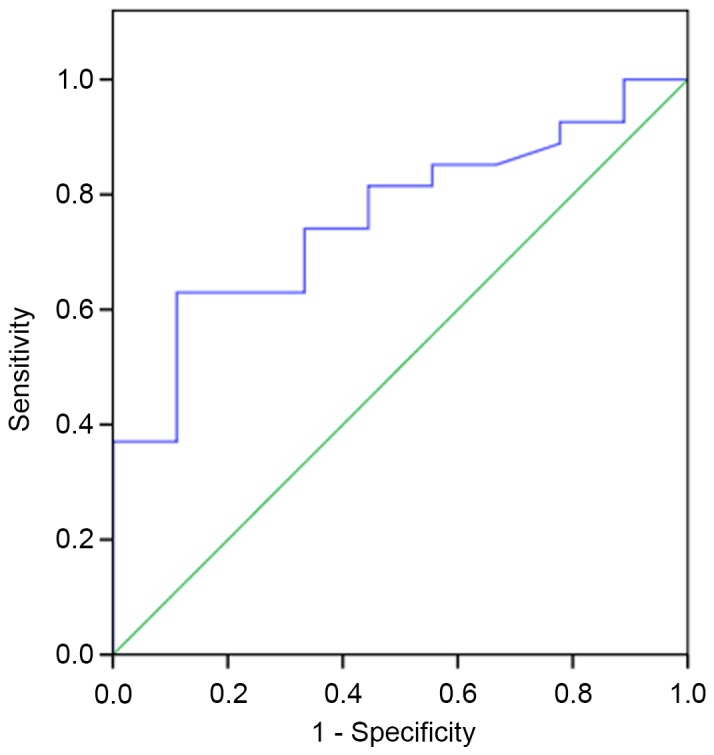
The ROC curve represented the capability of SUVmax to differentiate PTB from lung cancer. ROC, receiver operating characteristic; SUVmax, the maximum standard uptake value.
Discussion
The present study illustrates the clinical and radiographic presentations of patients with asymptomatic PTB mimicking lung cancer. The clinical characteristics of patients with PTB were compared with those of patients with lung cancer and it was determined that the clinical associated risk factors associated with PTB included age <60 years, being male and having a history of diabetes. The risk factors of PTB have been identified in previous studies and include overcrowding, poverty and undernutrition, alcohol misuse, diabetes, tobacco smoking and immune-suppressive therapy (20,21). Thus, the WHO has recommended screening for diabetes in all patients diagnosed with TB (22). People with diabetes are three times more susceptible to active PTB than those without and it has been demonstrated that diabetes increases the severity of PTB and predicts poor outcomes in patients with PTB (23–25). One of the reasons for increased PTB susceptibility in patients with diabetes is that diabetes adversely affects host innate and adaptive immunity. This impairment of the immune system delays the recruitment of immunocytes, including macrophages and T helper 1 cells, to the lesions and reduces cytokine expression (26). Due to substantial increases in the prevalence of diabetes (27), effectively controlling PTB will become more of a challenge. This means that the coexistence of tuberculosis and diabetes presents a serious threat to global public health. Typical characteristics suggestive of PTB, including cough, hemoptysis, fever, night sweats and chest pain (28), were rare in patients recruited in the current study, making the early diagnosis of PTB more difficult. The results of the present study indicated that the incidence of diabetes in PTB was significantly higher than in lung cancer, thus a diagnosis of PTB should be taken into consideration for people with diabetes even if they present with CT or PET-CT images that suggest the presence of lung cancer.
Tumor markers have been extensively used to distinguish patients with lung cancer from those with benign diseases. High levels of tumor markers are considered to indicate the presence of lung cancer rather than nonmalignant diseases (29). CEA, cancer antigen 125, CYFRA 21-1 and SCCAg are reliable markers in the diagnosis of non-small cell lung cancer whereas pro-gastrin-releasing peptide and NSE are commonly used to diagnose small-cell lung cancer (30–32). However, it has been reported that abnormal concentrations of tumor markers are detected in benign diseases, including PTB (33,34). In the current study, abnormal levels of CEA, CYFRA21-1, NSE and SCCAg were identified in patients with PTB, indicating that positive tumor markers are not specific for lung cancer.
It has been well documented that various morphological features, including spiculated margins, lobulation, blood vessel convergence signs, pleural indentation, ground-glass opacity and cavity with a thick and irregular wall, detected by chest CT scans, are usually associated with malignancy (35–37). By contrast, nodules with smooth margins, bronchus signs and a round shape were considered to be predictors of benign lesions. A recent study analyzing 84 patients diagnosed with PTB by percutaneous transthoracic needle biopsy, identified nodules with spiculated margins in 26 cases and lobulation in 8 (38). In the current study, morphological characteristics of PTB meant that it exhibited one or more signs of malignancy. Furthermore, it was determined that patients with asymptomatic PTB had a significantly higher frequency of spiculated margins than patients with lung cancer. Consequently, there are overlaps between PTB and lung cancer on the chest CT. Although morphological characteristics may be helpful in identifying whether lesions are PTB or lung cancer, the results may not always be conclusive.
As a precise imaging tool, PET-CT has been widely used to distinguish between malignant and benign lesions (39). PET-CT imaging relies on radiopharmaceutical FDG accumulation in increased glucose metabolic areas, so may be used to detect active TB and evaluate the response to anti-tuberculosis therapy in patients (40). Generally, malignant lesions have a significantly higher FDG uptake than benign lesions. The SUVmax cut-off of 2.5 was considered to be a diagnostic threshold for differentiating benign and malignant lesions (41). However, TB has an extensive range of SUVs, which may be due to its varying degree of granulomatous inflammation. It is not uncommon for TB lesions have an SUVmax >2.5, thus false-positive diagnoses may be made, particularly in tuberculosis-endemic areas. In clinical practice, false-positive diagnoses can also result from the presence of other benign conditions including sarcoidosis and rheumatoid nodule (42). The utility of PET-CT in the evaluation of pulmonary lesions has been assessed, and it has been suggested that an SUVmax of 5.0 may be a more appropriate cut-off value in areas with a high prevalence of TB (43). Hence, the diagnostic accuracy of PET-CT in TB-endemic areas was reduced using the conventional SUVmax cut-off value. From the ROC curve constructed in the current study, it was suggested that an SUVmax cut-off of 8.45 may be more effective at distinguishing between PTB and lung cancer in patients. This cut-off value is much higher than the more commonly used threshold of 2.5. Although an SUVmax cut-off value of 8.45 may result in lung cancers with low metabolic activity being falsely reported as PTB, it may prompt clinicians to be more cautious when interpreting results from PET-CT. Although a biopsy is able to differentiate between PTB and lung cancer, it is not technically possible for some patients. To increase the diagnostic accuracy in the assessment of asymptomatic PTB, SUV values as well as other clinical and radiological results should be integrated. After anti-tuberculosis therapy, high SUV values may rapidly decrease and clinical symptoms are ameliorated in PTB patients (44). In contrast, malignant lesions are unlikely to change after anti-tuberculosis therapy. Therefore, a biopsy as well as the clinical and radiological changes resulting from anti-tuberculosis treatment response may be used to differentiate these two similar diseases.
In conclusion, patients with asymptomatic PTB mimicking lung cancer usually exhibit abnormal chest CT presentation and increased glucose uptake, which is extremely similar to lung cancer. Compared with lung cancer, the factors associated with asymptomatic PTB include an age of <60 years, being male, having diabetes, spiculated margins and a lower SUVmax. The optimal cut-off level was SUVmax 8.45 for discriminating between asymptomatic PTB and lung cancer. Comprehensively considering the associated risk factors and recognizing the various imaging manifestations of this uncommon PTB may increase the diagnostic accuracy of asymptomatic PTB. An increased threshold of SUVmax may markedly improve the specificity of PET-CT of diagnosing PTB in TB-endemic area and further multicenter studies involving larger samples sizes are required.
Acknowledgements
The present study was supported by the National Science and Technology Major Project of China (grant no. 2015ZX09J15105-004). The authors wish to thank all of the patients involved in the present study.
References
- 1.Dheda K, Barry CE, III, Maartens G. Tuberculosis. Lancet. 2016;387:1211–1226. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global tuberculosis report 2015. World Health Organization; Geneva: 2014. [Google Scholar]
- 3.Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 4.Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: A radiologic review. Radiographics. 2007;27:1255–1273. doi: 10.1148/rg.275065176. [DOI] [PubMed] [Google Scholar]
- 5.Lenaerts A, Barry CE, III, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.1186/s40880-015-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng R, Zeng H, Zuo T, Zhang S, Qiao Y, Zhou Q, Chen W. Lung cancer incidence and mortality in China, 2011. Thorac Cancer. 2016;7:94–99. doi: 10.1111/1759-7714.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C. Lung cancer molecular epidemiology in China: Recent trends. Transl Lung Cancer Res. 2014;3:270–279. doi: 10.3978/j.issn.2218-6751.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitlik SD, Fainstein V, Bodey GP. Tuberculosis mimicking cancer-a reminder. Am J Med. 1984;76:822–825. doi: 10.1016/0002-9343(84)90993-8. [DOI] [PubMed] [Google Scholar]
- 10.Boyaci H, Basyigit I, Baris SA. Positron emission tomography/computed tomography in cases with tuberculosis mimicking lung cancer. Braz J Infect Dis. 2013;17:267–269. doi: 10.1016/j.bjid.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammen I. Tuberculosis mimicking lung cancer. Respir Med Case Rep. 2015;16:45–47. doi: 10.1016/j.rmcr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty N, Noronha V, Joshi A, Rangarajan V, Purandare N, Mohapatra PR, Prabhash K. Diagnostic and treatment dilemma of dual pathology of lung cancer and disseminated tuberculosis. J Clin Oncol. 2014;32:e7–e9. doi: 10.1200/JCO.2012.46.0667. [DOI] [PubMed] [Google Scholar]
- 13.Prapruttam D, Hedgire SS, Mani SE, Chandramohan A, Shyamkumar NK, Harisinghani M. Tuberculosis-the great mimicker. Semin Ultrasound CT MR. 2014;35:195–214. doi: 10.1053/j.sult.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Kang KH, Koh Y, Chang J, Chung HS, Park SK, Yoo K, Song JS. Characteristics of lung cancer in Korea, 1997. Lung Cancer. 2000;30:15–22. doi: 10.1016/S0169-5002(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 15.Shiels MS, Albanes D, Virtamo J, Engels EA. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2011;20:672–678. doi: 10.1158/1055-9965.EPI-10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CY, Hu HY, Pu CY, Huang N, Shen HC, Li CP, Chou YJ. Pulmonary tuberculosis increases the risk of lung cancer: A population-based cohort study. Cancer. 2011;117:618–624. doi: 10.1002/cncr.25616. [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th. International Agency for Research on Cancer; Lyon: 2015. pp. 9–96. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Theron G, Jenkins HE, Cobelens F, Abubakar I, Khan AJ, Cohen T, Dowdy DW. Data for action: Collection and use of local data to end tuberculosis. Lancet. 2015;386:2324–2333. doi: 10.1016/S0140-6736(15)00321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: A systematic review and meta-analysis. Arch Intern Med. 2007;167:335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 22.WHO Guidelines approved by the Guidelines Review Committee. WHO, Geneva: 2011. World Health Organization (WHO): Collaborative Framework for Care and Control of Tuberculosis and Diabetes. [PubMed] [Google Scholar]
- 23.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, Ottmani SE, Goonesekera SD, Murray MB. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hongguang C, Min L, Shiwen J, Fanghui G, Shaoping H, Tiejie G, Na L, Zhiguo Z. Impact of diabetes on clinical presentation and treatment outcome of pulmonary tuberculosis in Beijing. Epidemiol Infect. 2015;143:150–156. doi: 10.1017/S095026881400079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44:617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen MV, Jenny-Avital ER, Burger S, Leibert EM, Achkar JM. Clinical and radiographic manifestations of sputum culture-negative pulmonary tuberculosis. PLoS One. 2015;10:e0140003. doi: 10.1371/journal.pone.0140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013;80:45–49. doi: 10.1016/j.lungcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Molina R, Filella X, Augé JM, Fuentes R, Bover I, Rifa J, Moreno V, Canals E, Viñolas N, Marquez A, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24:209–218. doi: 10.1159/000074432. [DOI] [PubMed] [Google Scholar]
- 31.Cedrés S, Nuñez I, Longo M, Martinez P, Checa E, Torrejón D, Felip E. Serum tumor markers CEA, CYFRA21-1 and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12:172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Shibayama T, Ueoka H, Nishii K, Kiura K, Tabata M, Miyatake K, Kitajima T, Harada M. Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC) Lung Cancer. 2001;32:61–69. doi: 10.1016/S0169-5002(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 33.Molina R, Auge JM, Filella X, Viñolas N, Alicarte J, Domingo JM, Ballesta AM. Pro-gastrin-releasing peptide (proGRP) in patients with benign and malignant diseases: Comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 2005;25:1773–1778. [PubMed] [Google Scholar]
- 34.Kagohashi K, Satoh H, Kurishima K, Kadono K, Ishikawa H, Ohtsuka M, Sekizawa K. Squamous cell carcinoma antigen in lung cancer and nonmalignant respiratory diseases. Lung. 2008;186:323–326. doi: 10.1007/s00408-008-9108-4. [DOI] [PubMed] [Google Scholar]
- 35.Siegelman SS, Khouri NF, Leo FP, Fishman EK, Braverman RM, Zerhouni EA. Solitary pulmonary nodules: CT assessment. Radiology. 1986;160:307–312. doi: 10.1148/radiology.160.2.3726105. [DOI] [PubMed] [Google Scholar]
- 36.Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: High-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–476. doi: 10.1148/radiology.179.2.2014294. [DOI] [PubMed] [Google Scholar]
- 37.Woodring JH, Fried AM. Significance of wall thickness in solitary cavities of the lung: A follow-up study. AJR Am J Roentgenol. 1983;140:473–474. doi: 10.2214/ajr.140.3.473. [DOI] [PubMed] [Google Scholar]
- 38.Choo JY, Lee KY, Kim MY, Kang EY, Oh YW, Lee SH, Seo BK, Je BK. Pulmonary tuberculosis confirmed by percutaneous transthoracic needle biopsy: Analysis of CT findings and review of correlations with underlying lung disease. Balkan Med J. 2014;31:208–213. doi: 10.5152/balkanmedj.2014.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dabrowska M, Krenke R, Korczynski P, Maskey-Warzechowska M, Zukowska M, Kunikowska J, Orłowski T, Chazan R. Diagnostic accuracy of contrast-enhanced computed tomography and positron emission tomography with 18-FDG in identifying malignant solitary pulmonary nodules. Medicine (Baltimore) 2015;94:e666. doi: 10.1097/MD.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen RY, Dodd LE, Lee M, Paripati P, Hammoud DA, Mountz JM, Jeon D, Zia N, Zahiri H, Coleman MT, et al. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci Transl Med. 2014;6:265ra166. doi: 10.1126/scitranslmed.3009501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sim YT, Goh YG, Dempsey MF, Han S, Poon FW. PET-CT evaluation of solitary pulmonary nodules: Correlation with maximum standardized uptake value and pathology. Lung. 2013;191:625–632. doi: 10.1007/s00408-013-9500-6. [DOI] [PubMed] [Google Scholar]
- 42.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–e120S. doi: 10.1378/chest.12-2351. (5 Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.du Toit R, Shaw JA, Irusen EM, von Groote-Bidlingmaier F, Warwick JM, Koegelenberg CF. The diagnostic accuracy of integrated positron emission tomography/computed tomography in the evaluation of pulmonary mass lesions in a tuberculosis-endemic area. S Afr Med J. 2015;105:1049–1052. doi: 10.7196/SAMJ.2015.v105i12.10300. [DOI] [PubMed] [Google Scholar]
- 44.Verbeeck RK, Günther G, Kibuule D, Hunter C, Rennie TW. Optimizing treatment outcome of first-line anti-tuberculosis drugs: The role of therapeutic drug monitoring. Eur J Clin Pharmacol. 2016;72:905–916. doi: 10.1007/s00228-016-2083-4. [DOI] [PubMed] [Google Scholar]