Abstract
Human trophoblastic cell-surface marker, tumor-associated calcium signal transducer 2 (TROP2), is a newly identified marker that has a vital role in the proliferation and invasion of various tumors. However, its specific function in ovarian cancer has not been researched. The purpose of the present study was to investigate the role of TROP2 in the formation of ovarian cancer and its possible mechanism. TROP2 was knocked down by small interfering (si)RNA in ovarian cancer cell line, A2780. The expression of TROP2 protein following transfection was detected by western blot analysis. Cell viability was determined using a Cell Counting kit-8. Cancer cell migration and invasion were examined by wound healing and cell invasion assays, respectively. Apoptosis-related proteins, such as B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax), were measured by western blotting. Results demonstrated that the expression levels of TROP2 were markedly downregulated by siRNA in A2780 cells compared with the control groups, which led to strong inhibition of proliferation and invasion. Furthermore, TROP2 downregulation also reduced cell migratory ability. Additionally, in the TROP2-knockout group, Bcl-2 was downregulated and Bax was upregulated compared with the control. The present study suggested that the expression of TROP2 was related to cellular proliferation, migration and invasion. TROP2 may disrupt the balance in the Bax family to participate in apoptosis regulation in A2780 cells. Therefore, the overexpression of TROP2 may have a crucial role in tumorigenesis and tumor progression by disturbing the Bax/Bcl-2 balance in ovarian cancer.
Keywords: tumor-associated calcium signal transducer 2, ovarian cancer, proliferation, migration, small interfering RNA
Introduction
Ovarian cancer is one of the most common malignancies in women. Though its morbidity ranks third after cervical cancer and endometrial carcinoma among female genital system neoplasms, the mortality of ovarian cancer tops the list (1). In 2014, ovarian cancer was responsible for ~21,980 new cases and ~14,270 deaths in the United States (1). Owing to the complexity of the ovary in embryogenesis, the anatomical structure and reproductive endocrine function, early stage ovarian cancer is often hard to detect in patients (2). The majority of ovarian cancer cases are epithelial ovarian carcinomas, which are often diagnosed at the advanced stage, leading to very poor prognosis (3). Tumor-associated calcium signal transducer 2 (TROP2), which is differentially expressed in various types of cancer, is a transmembrane glycoprotein encoded by the TACSTD2 gene (4). TROP2 was initially discovered in trophoblast cells, specifically in chorionic trophoblast cells during placental implantation, and may promote the invasion and metastasis of tumor cells (5,6). Overexpression of TROP2 has been identified in breast cancer (7), colorectal cancer (8), endometrial endometrioid adenocarcinoma (9), cervical cancer (10), laryngeal carcinoma (11) and oral squamous cell cancer (4). Therefore, TROP2 may be a potential prognosis biomarker, as well as a novel therapeutic target. Although research exits on TROP2 expression and poor survival in Italian patients with ovarian carcinoma (12), the biological significance of TROP2 in epithelial ovarian carcinomas remains unclear. It has previously been demonstrated that TROP2 protein was overexpressed in ovarian cancer cases and its expression level was correlated with the clinical outcomes in another previous study (13,14). The present study aimed to investigate the specific function of TROP2 in ovarian cancer cells. The effects of TROP2 expression on proliferation, cell invasion and metastasis were investigated in vitro.
Materials and methods
Cell culture
A2780, HO8910 and SK-OV-3 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 37°C in RPMI 1640 medium (Hyclone, GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% mycoplasma-free fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA USA) and 1% penicillin-streptomycin in a CO2 incubator under standardized conditions.
Immunofluorescence staining
The three cell lines were seeded at 35,000 cells per well onto 15-mm glass slides in a 24-well plate and incubated at 37°C for 24 h. Cells were fixed at room temperature with 4% paraformaldehyde for 15 min and subsequently washed with phosphate-buffered saline (PBS) 3 times for 3 min each. Following this, cells were blocked with 5% normal goat serum (Boster Biological Technology, Ltd., Wuhan, China) for 30 min at room temperature. After thorough washing with PBS 3 times for 3 min each, the cells were incubated with anti-TROP2 monoclonal antibody (1:500; catalogue no. sc-376746; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Subsequent to 3 washes for 3 min each with PBS, the cells were stained with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (1:200; catalogue no. ZF-0312; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 1 h at room temperature. Fluorescence-labeled TROP2 was observed and photographed under a fluorescent microscope.
Transfection
In order to knockdown the expression of endogenous TROP2, lentivirus containing two small interfering (si)RNA sequences targeting TROP2 were designed and synthesized by Shanghai GenePharma Co., Ltd., (Shanghai, China). The siRNA sequences were as follows: TROP2-homo-1100, 5-GCA CGC TCA TCT ATT ACC T-3; and TROP2-homo-550, 5-CCA AGT GTC TGC TGC TCA A-3. The negative scramble control sequence was as follows: 5-TTC TCC GAA CGT GTC ACG T-3. The cells were seeded at ~1.0×105 cells/well into 6-well plates and cultured at 37°C overnight under standard conditions. After 50% confluence was reached, the number of cells in a well was counted using a hemocytometer. TROP2 siRNA were transfected into cells in Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a multiplicity of infection (MOI)=20 [MOI= transducing units per cell (TU) number/cell], according to the manufacturers instructions. The culture medium was replaced after 24 h incubation. A total of 48 h after transfection, the cells were observed and photographed under a fluorescence microscope. After successful transfection, siRNA sequences were stably expressed. Untransfected cells were used as a blank control, while cells transfected with scrambled siRNA were considered as negative control.
Cell viability assay
Cell viability was examined using a cell counting kit-8 (CCK-8) assay based on the optical density (OD) value, according to the manufacturers protocol (Bestbio, Shanghai, China). Cells were seeded at ~3.0×103 cells/well in a 96-well plate and cultured at 37°C in an incubator. After incubation for 24, 48, 72, 96 and 120 h, 10 µl CCK-8 was added into each well, and subsequently incubated for another 3 h at 37°C. The OD value was determined by the microplate reader at a wavelength of 450 nm. The cell viability of each group was calculated by GraphPad Prism software, version 7.00 (GraphPad Software, Inc., La Jolla, CA, USA). The experiment was repeated three times.
Wound healing assay
Untransfected and transfected cells were seeded at 5.0×105 cells/well in 6-well plates and cultured routinely. After reaching 90% confluence, the cell monolayer was scratched with a sterile pipette tip. After washing 3 times with PBS for 5 min each to clear the floating cells, 1.5 ml RPMI 1640 medium supplemented with 1% FBS was added into each well. Photographs were taken by a microscope at 0, 24 and 48 h after scratching. Results were indicated as the relative width of scratch-the distance migrated relative to the original scratched distance. The experiment was conducted three times.
Cell invasion assay
The invasive ability of cells was measured using the Corning® Matrigel® Basement Membrane Matrix (catalogue no. 356234; Corning Inc., Corning, NY, USA) and a 24-well transwell chamber (Corning Inc., Corning, New York, USA) according to the manufacturers protocol. The number of cells that passed through an 8-mm polycarbonate membrane was calculated. The polycarbonate surface of each chamber was covered with 20 µl Matrigel (1:4 dilution) to create an artificial basement membrane. Cells were cultured at 37°C in FBS-free RPMI 1640 medium for 24 h. After serum starvation, the cells were seeded at 1×105 cells/well in the upper Transwell chamber, which contained ~200 µl serum-free RPMI 1640. The lower chamber was filled with 600 µl of RPMI 1640 medium supplemented with 10% FBS. After an incubation of 48 h at 37°C, the chambers were fixed at room temperature with paraformaldehyde for 30 min. Cells attached to the upper surface of the chambers were removed with a sterile cotton swab, and cells that adhered to the lower surface were stained with 0.1% crystal violet (Guangfu Institute of Superfine Chemical Industry, Tianjin, China) for 20 min at room temperature. The numbers of stained cells were counted using an inverted microscope (OLYMPUS IX 70–142; Olympus Corporation, Tokyo, Japan) in eight random fields. The experiment was repeated three times.
Western blot analysis
B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax) are two regulators of cell apoptosis, the former has a vital anti-apoptotic role and the latter has a pro-apoptotic role (15). Cellular proteins were extracted according to the protocol using a Total Protein Extraction kit (catalogue no. BB-3101; Bestbio) after a 48-h culture at 37°C. Equivalent amounts (20 µg) of protein samples were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking with 5% w/v non-fat dried milk for 1 h at room temperature, the blots were incubated with primary antibodies specific to TROP2 (catalogue no. sc-376746; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Bcl-2 (catalogue no. 4223; Cell Signaling Technology, Inc., Danvers MA, USA) and Bax (catalogue no. 5023, Cell Signaling Technology, Inc.; all 1:1,000) overnight at 4°C. Subsequently, after 3 washes with PBS for 5 min, the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (H+L) (catalogue no. ZB 2305; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China; 1:4,000) for 1 h at room temperature. Bands were detected by enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA) using ImageQuant LAS (General Electric Company, USA). The results were analyzed by Quantity One version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). GAPDH was used as an internal control.
Statistical analysis
Statistical analyses were performed using SPSS v. 17.0 software (SPSS, Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation. One-way analysis of variance and Student-Newman-Keuls tests were used. P<0.05 was considered to indicate a statistically significant difference.
Results
Immunofluorescence staining of TROP2
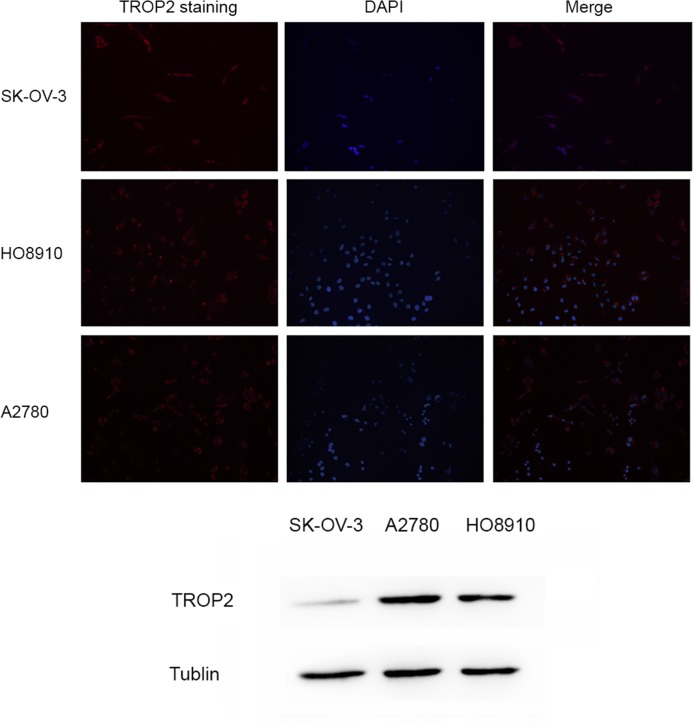
Immunofluorescence analysis demonstrated a layer of red fluorescent staining on the cytomembranes of A2780, HO8910 and SK-OV-3 cells (Fig. 1). Western blotting results (Fig. 1) demonstrated that the A2780 cell line expressed markedly more TROP2 protein than the other two cell lines.
Figure 1.
The expression of TROP2 in ovarian cancer cell lines. Immunofluorescence assay demonstrated a layer of red fluorescent staining on the membrane of SK-OV-3, HO8910 and A2780 cells, and the staining on A2780 cells was markedly brighter than the other two cell lines. Western blot analysis demonstrated the same result. TROP2, tumor-associated calcium signal transducer 2; DAPI, 4, 6-diamidino-2-phenylindole.
Transfections
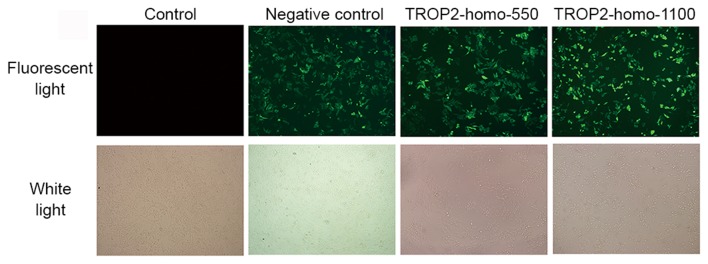
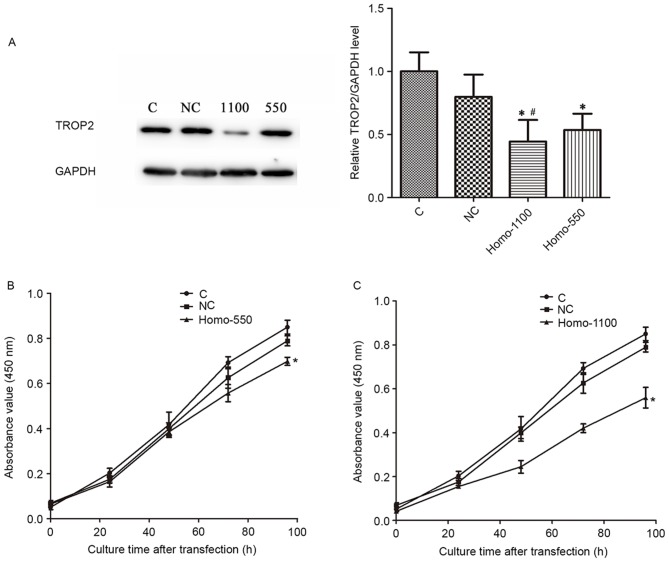
To investigate the specific functions of TROP2, the A2780 cell line was selected to conduct the following experiments. Two independent siRNA sequences were synthesized to downregulate the endogenous expression of TROP2. Cells transfected with scrambled siRNA were considered as the negative control group, and non-transfected cells were used as the blank control. Cells were observed and photographed by a fluorescence microscope 48 h after transfection. Transfected cells demonstrated a bright green stain (Fig. 2). The fluorescent staining indicated expression of green fluorescent protein, which implied successful transfection. A total of 48 h after transfection, the expression of TROP2 protein in each group was measured. As demonstrated in Fig. 3A, the level of TROP2 protein in the transfected cells was significantly decreased, compared with the control groups (P<0.05). After transfection with sequence 1100, the expression of TROP2 was significantly reduced compared with the sequence 550 group (P<0.05).
Figure 2.
A2780 cells were observed and photographed by a fluorescence microscope 48 h after transfection with TROP2-homo-550 and TROP2-homo-1100 small interfering RNA sequences. The bright green stain for the expression of GFP protein implied successful transfection. TROP2, tumor-associated calcium signal transducer 2.
Figure 3.
(A) Western blot analysis measured the effects of siRNA-mediated knockdown of TROP2. GAPDH was used as an internal control. Data are presented as the mean + standard deviation. Cells were transfected with (B) TROP2-homo-550 and (C) TROP2-homo-1100 siRNA and the viability was assessed using Cell counting kit-8 assay at five time points (24, 48, 72, 96 and 120 h). Transfected cells demonstrated a reduction in cell viability, and differences were significant when cells were cultured for 72 h (group 550) and 48 h (group 1100). Data are presented as the mean ± standard deviation. *P<0.05 vs. the control groups, #P<0.05 vs. group 550. TROP2, tumor-associated calcium signal transducer 2; siRNA, small interfering RNA; C, control; NC, negative control.
Cell viability assay
CCK-8 assay was used to investigate the effect of TROP2 on cell viability. Results demonstrated that the knockdown of TROP2 with the 550 and 1100 sequences induced a significant inhibition of the viability of A2780 cells compared with the control and negative control groups (P<0.05; Fig. 3B and C). These results indicated that TROP2 is required for the maintenance of cell viability.
Wound healing assay
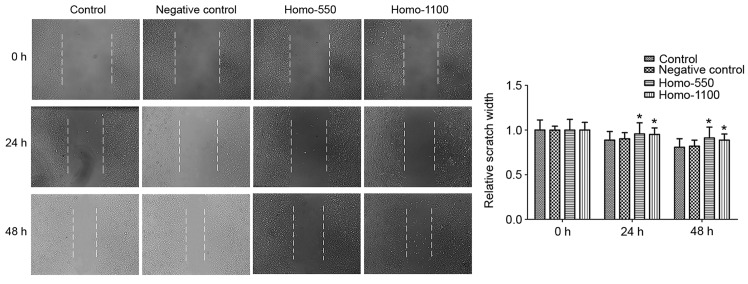
The monolayer wound healing assay was used to assess cell migration ability. As demonstrated in Fig. 4, 24 and 48 h after wounding, transfected cells indicated a significantly slower closing of the scratch, with a greater scratch width, compared with the control groups (P<0.05). These results indicated that TROP2 is required for cell migration.
Figure 4.
The effect of TROP2 expression on cell migration. Cells with TROP2-knockdown demonstrated slower wound recovery compared with the control groups at 24 and 48 h after wounding. Data are presented as the mean + standard deviation. *P<0.05 vs. the control groups at the corresponding time points. TROP2, tumor-associated calcium signal transducer 2.
Cell invasion assay
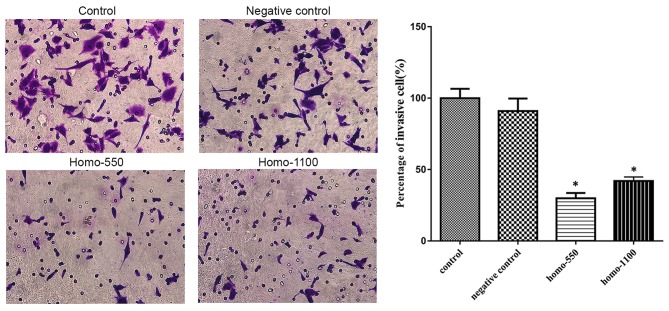
The serum-stimulated Matrigel invasion assay demonstrated that the percentages of A2780 cells that migrated through the polycarbonate membrane in the transfection groups were significantly lower than those in the control groups (P<0.05; Fig. 5). Therefore, downregulation of TROP2 decreased the invasive ability of A2780 cells at 48 h after transfection.
Figure 5.
The influence of TROP2 expression on the invasive capability of A2780 cells. Downregulation of TROP2 inhibited cell invasion. Data are presented as the mean + standard deviation. *P<0.05 vs. the control groups. TROP2, tumor-associated calcium signal transducer 2.
Western blot analysis
To explore the functional mechanism of TROP2, the expression of Bcl-2 and Bax proteins were investigated by western blotting (Fig. 6). The results demonstrated that, following the knockdown of TROP2, the expression of Bax was markedly enhanced compared with the negative control, whereas Bcl-2 protein expression levels were markedly decreased compared with the negative control.
Figure 6.
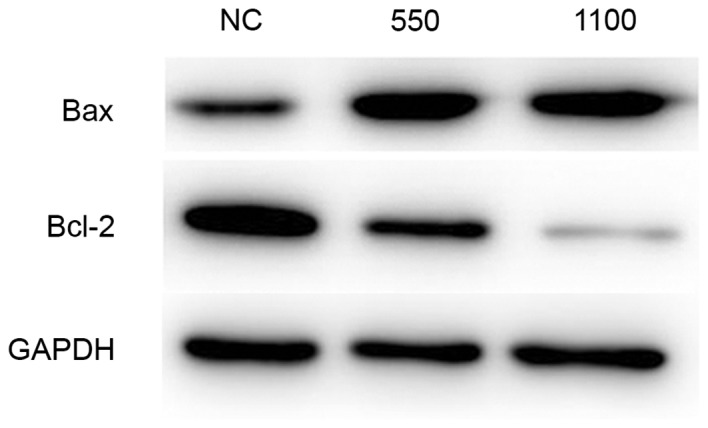
Western blot analysis of expression of Bcl-2 and Bax proteins. GAPDH was used as an internal control. Following the knockdown of TROP2, the expression of Bax was enhanced, whereas Bcl-2 protein expression levels were decreased compared with the NC. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; NC, negative control.
Discussion
Ovarian cancer was the fifth leading cause of cancer-related death among women in the United States in 2014 (1). More than half of the cases of ovarian cancer are highly invasive and insensitive to therapies, and are usually diagnosed at the advanced stages, resulting in a poor prognosis (16). As a type I transmembrane protein with several glycosylation sites, TROP2 is encoded by the single-exon gene, TACSTD2, which is expressed in various human carcinomas (4). However, the role of TROP2 is not well understood. Although TROP2 has previously been suggested to be involved in the adhesion between cancer cells, data has also indicated that it may have a role in cell signal transduction and the growth of cancer cells (17,18).
In the present study, TROP2 expression and localization was investigated by immunofluorescence staining in A2780, HO8910 and SK-OV-3 cells. The results demonstrated that, compared with the other two cell lines, A2780 cells expressed a higher level of TROP2 protein. Therefore, the A2780 cell line was selected to conduct the subsequent experiments. siRNA was utilized to knockdown the endogenous expression of TROP2 to evaluate its effect. In vitro study demonstrated that the downregulation of TROP2 was able to suppress A2780 cell proliferation. By wound healing and Transwell invasion assays, it was indicated that the knockdown of the TROP2 gene was able to decrease the invasion and metastatic capabilities of A2780 cells. Anti-apoptosis was considered as a distinct characteristic of tumor development. Previous research has revealed that the balance between Bcl-2 and Bax has a pivotal role in anti- and pro-apoptosis (15). The present study demonstrated that downregulated expression of TROP2 increased Bax expression and decreased Bcl-2 expression in A2780 cells. The results of the present study therefore suggested that TROP2 may be associated with the invasion, metastasis and resistance to apoptosis of A2780 cells. The effect of TROP2 on the inhibition of apoptosis may work via Bcl-2 family activation. However, there are limitations in the present study, a study by Domcke et al (19) has demonstrated that the A2780 cell line exhibited pronounced differences in molecular profiles vs. tumors, therefore the current research should be completed by subsequent experiments to verify the function and mechanism of TROP2 in an animal tumor-burdened model.
In conclusion, the present study demonstrated that silencing TROP2 expression in A2780 cancer cells suppresses proliferation, invasion and metastasis. Therefore, TROP2 has potential as a novel target for ovarian cancer treatment.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Auersperg N, Ota T, Mitchell GW. Early events in ovarian epithelial carcinogenesis: Progress and problems in experimental approaches. Int J Gynecol Cancer. 2002;12:691–703. doi: 10.1046/j.1525-1438.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 4.Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, Gerhard S, Rasse M, Laimer K. TROP2: A novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191. doi: 10.1038/modpathol.3801001. [DOI] [PubMed] [Google Scholar]
- 5.Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105. doi: 10.18632/genesandcancer.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bignotti E, Zanotti L, Calza S, Falchetti M, Lonardi S, Ravaggi A, Romani C, Todeschini P, Bandiera E, Tassi RA, et al. Trop-2 protein overexpression is an independent marker for predicting disease recurrence in endometrioid endometrial carcinoma. BMC Clin Pathol. 2012;12:22. doi: 10.1186/1472-6890-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Groth J, Sossey-Alaoui K, Hawthorn L, Beall S, Geradts J. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin Cancer Res. 2005;11:4357–4364. doi: 10.1158/1078-0432.CCR-04-2107. [DOI] [PubMed] [Google Scholar]
- 8.Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- 9.Bignotti E, Ravaggi A, Romani C, Falchetti M, Lonardi S, Facchetti F, Pecorelli S, Varughese J, Cocco E, Bellone S, et al. Trop-2 overexpression in poorly differentiated endometrial endometrioid carcinoma: Implications for immunotherapy with hRS7, a humanized anti-trop-2 monoclonal antibody. Int J Gynecol Cancer. 2011;21:1613–1621. doi: 10.1097/IGC.0b013e318228f6da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varughese J, Cocco E, Bellone S, Ratner E, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Buza N, Pecorelli S, Santin AD. Cervical carcinomas overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Am J Obstet Gynecol. 2011;205(567):e1–7. doi: 10.1016/j.ajog.2011.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XD, Wang Q, Chen XL, Huang JF, Yin Y, Da P, Wu H. Trop2 inhibition suppresses the proliferation and invasion of laryngeal carcinoma cells via the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway. Mol Med Rep. 2015;12:865–870. doi: 10.3892/mmr.2015.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bignotti E, Todeschini P, Calza S, Falchetti M, Ravanini M, Tassi RA, Ravaggi A, Bandiera E, Romani C, Zanotti L, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46:944–953. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Varughese J, Cocco E, Bellone S, Bellone M, Todeschini P, Carrara L, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. High-grade, chemotherapy-resistant primary ovarian carcinoma cell lines overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Gynecol Oncol. 2011;122:171–177. doi: 10.1016/j.ygyno.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bignotti E, Todeschini P, Calza S, Falchetti M, Ravanini M, Tassi RA, Ravaggi A, Bandiera E, Romani C, Zanotti L, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46:944–953. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Chen CR, Xia YH, Yao SY, Zhang Q, Wang Y, Ji ZN. Virosecurinine induces apoptosis by affecting Bcl-2 and Bax expression in human colon cancer SW480 cells. Pharmazie. 2012;67:351–354. [PubMed] [Google Scholar]
- 16.Jordan S, Green A, Webb P. Benign epithelial ovarian tumours-cancer precursors or markers for ovarian cancer risk? Cancer Causes Control. 2006;17:623–632. doi: 10.1007/s10552-005-0370-y. [DOI] [PubMed] [Google Scholar]
- 17.Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76:671–676. doi: 10.1002/(SICI)1097-0215(19980529)76:5<671::AID-IJC10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Fornaro M, Dell'Arciprete R, Stella M, Bucci C, Nutini M, Capri MG, Alberti S. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer. 1995;62:610–618. doi: 10.1002/ijc.2910620520. [DOI] [PubMed] [Google Scholar]
- 19.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]