Abstract
Atherosclerosis is a chronic inflammatory disease, which is associated with the increased expression of adhesion molecules in vascular smooth muscle cells (VSMCs). Cordycepin is one of the major bioactive components of Ophiocordyceps sinensis that has been demonstrated to exert anti-atherogenic activity; however, its molecular mechanisms are poorly understood. The aim of the present study was to examine the in vitro effects of cordycepin on the tumor necrosis factor (TNF)-α-induced suppression of adhesion molecule expression. The results of the present study demonstrated that cordycepin markedly inhibited the expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) in TNF-α-stimulated human aortic vascular smooth muscle cells (HA-VSMCs). Cordycepin significantly inhibited the TNF-α-induced mitogen-activated protein kinase (MAPK) and protein kinase B (Akt) activation (P<0.05), markedly inhibited the TNF-α-induced expression level of nuclear factor (NF)-κB p65 and markedly prevented the TNF-α-associated degradation of IκBα in HA-VSMCs. The results of the present study suggest that cordycepin inhibits the expression of VCAM-1 and ICAM-1 in TNF-α-stimulated HA-VSMCs via downregulating the MAPK/Akt/NF-κB signaling pathway. Therefore, cordycepin may have a potential therapeutic application for preventing the advancement of atherosclerotic lesions.
Keywords: cordycepin, atherosclerosis, human aortic smooth muscle cells, adhesion molecules
Introduction
Atherosclerosis is a progressive disease characterized by the accumulation of lipids in the vessel wall of arteries (1). As the disease progresses, vascular smooth muscle cells (VSMCs) undergo phenotypic transformation and become activated to secrete pro-inflammatory cytokines and monocyte chemoattractant protein-1, and express cell adhesion molecules that promote leukocyte recruitment, migration and differentiation (2). It has previously been reported that vascular cell adhesion molecule (VCAM)-1 is upregulated in the VSMCs of atherosclerotic lesions (3,4). Additionally, in cultured VSMCs, interleukin-1 and tumor necrosis factor (TNF)-α induced the expression of VCAM-1 and intercellular adhesion molecule (ICAM)-1, as well as monocyte adhesion to VSMCs (5). This indicates that inhibiting the expression of these adhesion molecules in VSMCs may have promising therapeutic applications for the treatment of atherosclerosis.
Cordycepin is one of the major bioactive components of Ophiocordyceps sinensis and has previously been demonstrated to exert a variety of important biological actions, including antioxidant, anti-inflammatory and anti-tumor activities (6–9). Additionally, cordycepin possesses an anti-atherogenic effect (10–12). One study demonstrated that cordycepin is able to inhibit cell growth, induce G1-phase cell-cycle arrest, downregulate the expression of cyclins and cyclin-dependent kinase, and upregulate the expression of p27kip1 in VSMCs (6). However, its underlying molecular mechanisms are poorly understood. The objective of the present study was to examine the in vitro effects of cordycepin on the ability to suppress the TNF-α-induced expression of adhesion molecules. The results of the present study demonstrated that cordycepin is able to inhibit TNF-α-induced adhesion molecule expression through downregulating the mitogen-activated protein kinase (MAPK)/protein kinase B (Akt)/nuclear factor (NF)-κB signaling pathway in VSMCs.
Materials and methods
Cell culture and treatment
Human aortic vascular smooth muscle cell line (HA-VSMCs; cat. no. CRL-1999™) was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified eagle medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 10 ng/ml recombinant human epidermal growth factor (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 2 ng/ml basic fibroblast growth factor (Sigma-Aldrich; Merck KGaA), and 5 µg/ml insulin (Sigma-Aldrich; Merck KGaA) in a humidified atmosphere containing 5% CO2 for 7 d at 37°C. HASMCs were subsequently incubated with TNF-α (10 ng/ml) for different times (30 min or 4 h), with or without pretreatment with, 5 or 10 µM cordycepin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 2 h at 37°C.
Cell viability assay
Cell proliferation was determined via MTT assay. In brief, HA-VSMCs were seeded in 96-well plates at a density of 1×104 cells/well and cultured in DMEM at 37°C until they reached 80% confluence. Then, MTT (0.2 mg/ml) was added to each well and incubated for 4 h at 37°C. The supernatant was removed and the formazan crystals were dissolved in dimethylsulfoxide. The absorbance was measured using a spectrophotometer at 550 nm (NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). HA-VSMCs treated with DMEM were used as a control.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
For DNase treatment 2 units of DNase I polymerase (Invitrogen; Thermo Fisher Scientific, Inc.) were used per µg of total RNA at 37°C for 30 min. Total RNA was extracted from HA-VSMCs using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Approximately 5 µg total RNA for each sample were reverse-transcribed using an oligo-(dT) primer and M-MLV reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. qPCR was performed in a final volume of 10 µl, which contained 5 µl SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA), 1 µl cDNA (1:50 dilution) and 2 µl the forward and reverse primers (1 mM). qPCR was performed with the ABI Prism 7500 sequence detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a SYBR-Green real-time PCR Master Mix kit (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol. Primers were designed as follows; VCAM-1 forward, 5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse, 5′-GGTGAGCATTATCACCCAGAA-3′; ICAM-1 forward, 5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse, 5′-GGTGAGCATTATCACCCAGAA-3′; and β-actin forward, 5′-AGAAAATCTGGCACCACACC-3′ and reverse, 5′-TAGCACAGCCTGGATAGCAA-3′. The PCR cycling program was 95°C for 5 min, then 35 cycles of 95°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec, and a final extension at 72°C for 5 min. β-actin was used as the internal reference gene. The relative expression levels were calculated using the 2−ΔΔCq method, and the target gene was normalized to β-actin (13).
Western blot analysis
HA-VSMCs were homogenized and lysed with radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.; 100 mM NaCl, 50 mM Tris-HCl pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM β-glycerophosphate, 2 mM sodium vanadate and protease inhibitor). Lysates were sonicated for 5 sec on ice and centrifuged at 6,000 × g for 5 min at 4°C. Supernatants were collected and the protein concentration was detected using a Bio-Rad protein assay kit (cat. no. 500-0002; Bio-Rad Laboratories, Inc.). A total of 30 µg protein was separated by 10% SDS-PAGE followed by electro-blotting-mediated transference onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). After blocking in Tris-buffered saline buffer (50 mmol/l NaCl, 10 mmol/l Tris, pH 7.4) containing 5% nonfat milk for 2 h at room temperature, the membranes were subsequently incubated overnight at 4°C with anti-VCAM-1 (1:2,000; cat. no. ab7224, Abcam, Cambridge, UK), anti-ICAM-1 (1:1,000; cat. no. sc-8439, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-phosphorylated (p)-p38 (1:1,500; cat. no. sc-166182, Santa Cruz Biotechnology, Inc.), anti-p38 (1:1,500; cat. no. sc-81621, Santa Cruz Biotechnology, Inc.), anti-p-extracellular signal-regulated kinase (ERK)1/2 (1:2,000; cat. no. sc-136521, Santa Cruz Biotechnology, Inc.), anti-ERK1/2 (1:2,000; cat. no. sc-514302, Santa Cruz Biotechnology, Inc.), anti-p- c-Jun N-terminal kinase (JNK; 1:3,000; sc-293136, Santa Cruz Biotechnology, Inc.), anti-JNK (1:3,000; cat. no. sc-137020, Santa Cruz Biotechnology, Inc.), anti-p-Akt (1:1,000; sc-52940, Santa Cruz Biotechnology, Inc.), anti-Akt (1:1,000; cat. no. sc-5298, Santa Cruz Biotechnology, Inc.), anti-NF-κB p65 (1:2,000; ca. no. sc-8008, Santa Cruz Biotechnology, Inc.) anti-IκBα (1:1,000; cat. no. sc-1643, Santa Cruz Biotechnology, Inc.) and anti-β-actin (1:1,500; cat. no. sc-8432, Santa Cruz Biotechnology, Inc.) primary antibodies. Membranes were subsequently washed three times with PBS containing 0.1% (v/v) Tween-20 (Invitrogen) for 10 min and incubated with horseradish peroxidase conjugated goat anti-mouse IgG-horse radish peroxidase secondary antibodies (1:3,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature, followed by exposure using enhanced chemiluminescence detection reagents (Thermo Fisher Scientific, Inc.). BandScan 5.0 software (Glyko, Inc., Novato, CA, USA) was used for the quantification of proteins following western blot analysis. All experiments were repeated at least three times.
Statistical analysis
Data was analyzed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard error of the mean. Statistical evaluation was performed using Student's t-test or one-way analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of cordycepin on HA-VMSC viability
To determine the effect of cordycepin on cell viability, an MTT assay was performed. The results revealed no significant difference between the experimental and control groups following of HA-VSMCs with 1–10 µM cordycepin (Fig. 1).
Figure 1.
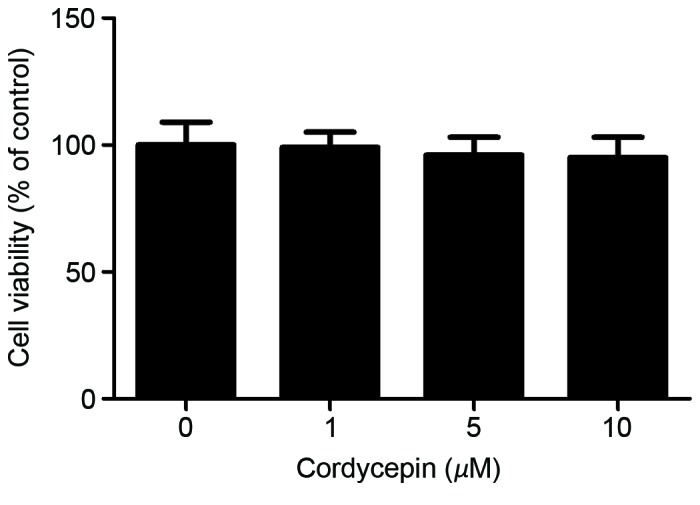
Effect of cordycepin on HA-VMSC viability. HA-VMSCs were treated with various concentrations (1, 5 or 10 µM) of cordycepin for 24 h. The cytotoxicity was then measured using an MTT assay. All experiments were repeated at least three times. HA-VSMC, human aortic vascular smooth muscle cell.
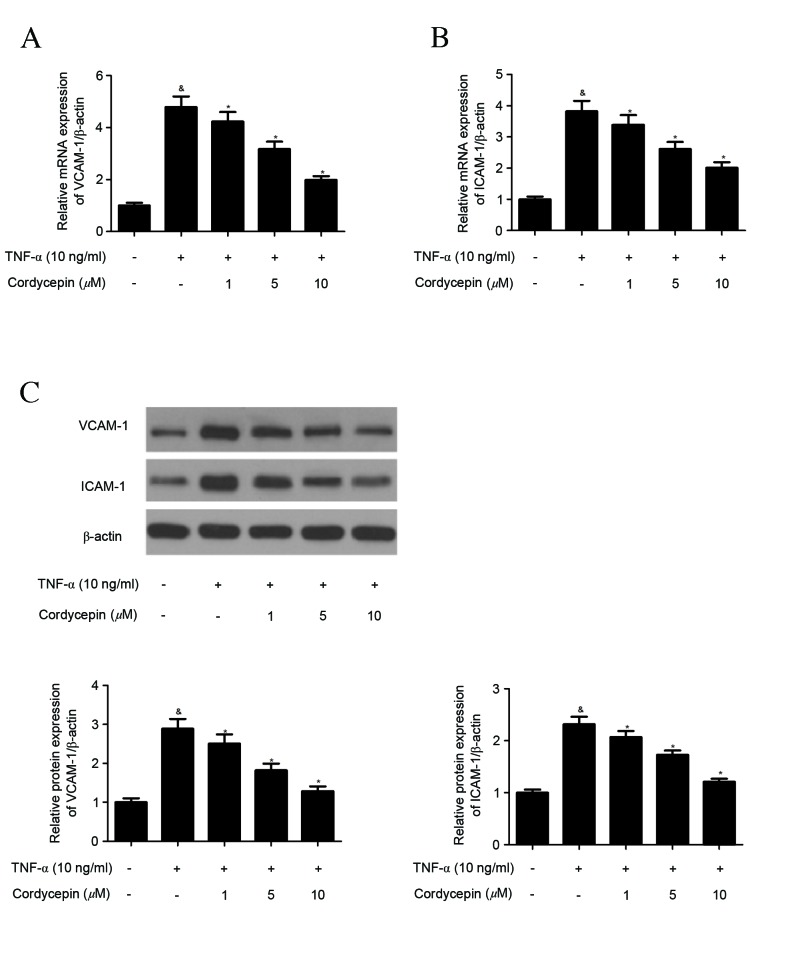
Cordycepin inhibits the TNF-α-induced expression of adhesion molecules in HA-VSMCs
The effect of cordycepin on the expression of adhesion molecules in HA-VSMCs was investigated using RT-qPCR and western blot analysis. Treatment with TNF-α significantly induced the expression of VCAM-1 and ICAM-1 mRNA (Fig. 2A and B). Cordycepin, however, significantly inhibited the TNF-α-induced expression of VCAM-1 and ICAM-1 mRNA in a concentration-dependent manner (Fig. 2A and B). Similarly, western blot analysis indicated that cordycepin significantly suppressed the TNF-α-induced protein expression of VCAM-1 and ICAM-1 in a concentration-dependent manner (Fig. 2C).
Figure 2.
Cordycepin inhibits the TNF-α-induced expression of adhesion molecules in HA-VMSCs. HA-VMSCs were preincubated with various concentrations (1, 5 or 10 µM) of cordycepin for 2 h and stimulated with TNF-α (10 ng/ml) for 4 h. (A and B) VCAM-1 and ICAM-1 mRNA expression was determined by reverse transcription-quantitative polymerase chain reaction (C) VCAM-1 and ICAM-1 protein expressions were determined by western blot analysis. Data are presented as the mean ± standard error of the mean. All experiments were repeated at least three times. &P<0.05 vs. the control group; *P<0.05 vs. the TNF-α group. TNF, tumor necrosis factor; HA-VSMC, human aortic smooth muscle cell; VCAM, vascular cell adhesion molecule; ICAM, intracellular adhesion molecule.
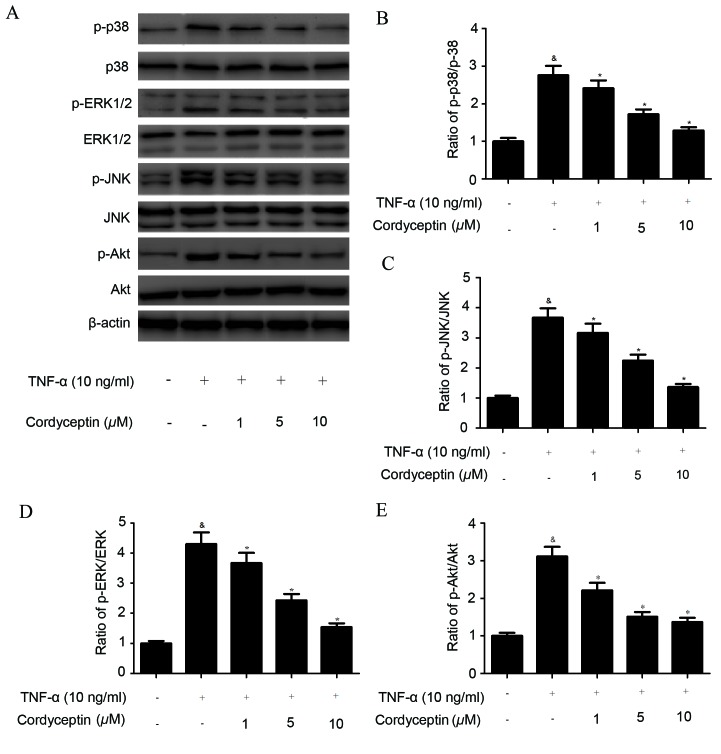
Cordycepin inhibits TNF-α-induced phosphorylation of MAPKs and Akt in HA-VSMCs
MAPK and Akt are two important intracellular pathways involved in cell adhesion (14), and so in the present study various members of the MAPK and Akt signal networks were detected via western blotting (Fig. 3A). It was demonstrated that the phosphorylation levels of p38 MAPK (Fig. 3B), ERK1/2 (Fig. 3C), JNK (Fig. 3D), and Akt (Fig. 3E) were significantly increased in TNF-α-stimulated HA-VMSCs, compared with the control. However, cordycepin treatment significantly inhibited the TNF-α-induced phosphorylation of MAPKs and Akt in HA-VMSCs (Fig. 3B-E).
Figure 3.
Cordycepin inhibits TNF-α-induced phosphorylation of MAPKs and Akt in HA-VMSCs. HA-VMSCs were pretreated with various concentrations (1, 5 or 10 µM) of cordycepin for 2 h and subsequently incubated with TNF-α (10 ng/ml) for 30 min. (A) The expression of p-p38, p38, p-ERK1/2, ERK1/2, p-JNK, JNK, p-Akt and Akt proteins was detected via western blotting and representative blots were shown. (B-E) Quantification analysis was performed using BandScan 5.0 software. All data are presented as the mean ± standard error of the mean. All experiments were repeated a minimum of three times. &P<0.05 vs. the control group; *P<0.05 vs. the TNF-α group. TNF, tumor necrosis factor; MAPK, mitogen activated protein kinase; HA-VSMC, human aortic vascular smooth muscle cell; p, phosphorylated; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; Akt, protein kinase B.
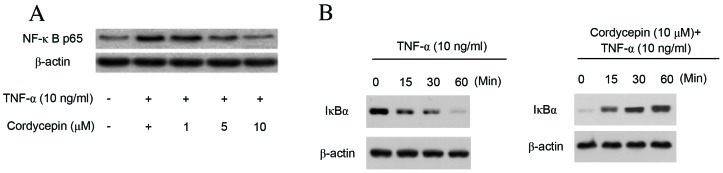
Cordycepin inhibits TNF-α-induced NF-κB activation in HA-VSMCs
Activation of NF-κB serves an important role in the development of vascular damage, and transcription factors have been known to be important mediators of adhesion molecule expression (15). Therefore, in the present study the effect of cordycepin on NF-κB transcriptional activation was investigated. Pre-incubation of HA-VMSCs with cordycepin induced a dose-dependent decrease in NF-κB p65 translocation to the nuclear fraction (Fig. 4A). The effect of cordycepin on IκB protein in TNF-α-stimulated HA-VSMCs was subsequently investigated, suggesting that that the cordycepin only treatment affects the degradation of IκBα. The results demonstrated that cordycepin markedly reduced the TNF-α-induced degradation of IκBα (Fig. 4B).
Figure 4.
Cordycepin inhibits the TNF-α-induced activation of NF-κB in HA-VMSCs. (A) HA-VMSCs were pretreated with various concentrations (1, 5 or 10 µM) of cordycepin for 2 h and subsequently incubated with TNF-α (10 ng/ml) for 4 h. Nuclear expression of NF-κB p65 was detected via western blotting to analyze the translocation of NF-κB in HA-VSMC. (B) IκBα degradation was also analyzed by western blot analysis in HA-VSMCs. All data are presented as the mean ± standard error of the mean. All experiments were repeated at least three times. TNF, tumor necrosis factor; NF, nuclear factor; HA-VSMC, human aortic vascular smooth muscle cell.
Discussion
VSMCs express VCAM-1 and ICAM-1, which have previously been described as prominent in the fibrous caps of advanced atherosclerotic plaques (16). Therefore, pharmacological agents that inhibit the expression of these adhesion molecules may have the potential to inhibit atherosclerosis. In the present study, it was demonstrated that cordycepin inhibits the TNF-α-induced expression of adhesion molecules. Furthermore, cordycepin treatment significantly inhibited the TNF-α-induced phosphorylation of MAPKs and Akt in HA-VSMCs, inhibited the TNF-α-induced expression of NF-κB p65 and inhibited the degradation of IκBα in HA-VMSCs.
It has previously been demonstrated that the expression of adhesion molecules, such as VCAM-1 and ICAM-1, is increased in coronary atherosclerotic tissues (17). TNF-α is a multifunctional cytokine that has previously been demonstrated to be associated with regulating the expression of VCAM-1 and ICAM-1 in VSMCs (18). This is consistent with the findings of the present study that TNF-α significantly increased the expression of VCAM-1 and ICAM-1 in HA-VSMCs, whereas, cordycepin significantly prevented the TNF-α-induced expression of VCAM-1 and ICAM-1. These results suggest that cordycepin has an inhibitory effect on the expression of adhesion molecules in TNF-α-stimulated HA-VSMCs.
The MAPK signaling pathway serves an important role in the development of atherosclerosis (19–21), and three members of this pathway (ERK, p38, and JNK) have previously been implicated in the mediation of cell adhesion molecule expression in cells in response to external stimuli including TNF-α (22,23). Furthermore, Akt has previously been demonstrated to be associated with the TNF-α-induced activation of NF-κB and expression of adhesion molecules (24). In the present study it was demonstrated that cordycepin treatment significantly inhibited the TNF-α-induced phosphorylation of MAPKs and Akt in HA-VMSCs. These results suggest that cordycepin inhibits TNF-α-induced adhesion molecule expression via the inhibition of MAPK and Akt activation.
It is well known that the NF-κB signaling pathway also serves an important role in the regulation of inflammatory responses (25). Upon stimulation with TNF-α, IκB is proteolytically removed from NF-κB, which results in NF-κB being free to translocate to the nucleus where it binds to specific sequences in the promoter regions of certain genes (26). Additionally, NF-κB is essential to the expression of cell adhesion molecules (27). For example, MacKenzie et al (28) reported that TNF-α-stimulated expression of ICAM-1 and VCAM-1 in HAMSCs is NF-κB-dependent. Furthermore, cordycepin was demonstrated to be associated with regulating the NF-κB signaling pathway. It has previously been reported that cordycepin is able to significantly suppress TNF-α-induced activation of NF-κB and it also inhibited IκBα phosphorylation to suppress the degradation of IκBα in HEK-293T cells (29). Another study demonstrated that cordycepin inhibits lipopolysaccharide-induced inflammation via the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells (30). Similarly, in the present study it was demonstrated that cordycepin inhibited the TNF-α-induced expression of NF-κB p65 as well as the degradation of IκBα in HA-VMSCs. These findings suggest that the inhibitory effects of cordycepin on the expression of adhesion molecules may be associated with the suppression of NF-κB activation in HA-VMSCs.
In conclusion, to our knowledge, this is the first study to demonstrate that cordycepin inhibits the expression of vascular adhesion molecules in TNF-α-stimulated HA-VMSCs via blocking the MAPK/Akt/NF-κB signaling pathway. The results of the present study suggest that cordycepin may be used as a novel therapeutic agent for the treatment of atherosclerosis.
References
- 1.Ross R. Atherosclerosis-an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Braun M, Pietsch P, Schrör K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/S0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- 3.Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Li H. Vascular cell adhesion molecule-1 and smooth muscle cell activation during atherogenesis. J Clin Invest. 1993;92:538–539. doi: 10.1172/JCI116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang Y, Lincoff AM, Plow EF, Topol EJ. Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol. 1994;24:1591–1601. doi: 10.1016/0735-1097(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 6.Jung SM, Park SS, Kim WJ, Moon SK. Ras/ERK1 pathway regulation of p27KIP1-mediated G1-phase cell-cycle arrest in cordycepin-induced inhibition of the proliferation of vascular smooth muscle cells. Eur J Pharmacol. 2012;681:15–22. doi: 10.1016/j.ejphar.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated raW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and nF-κB signaling pathways. Drug Des Dev Ther. 2014;8:1941–1953. doi: 10.2147/DDDT.S71957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Huang C, Fu C, Tian Y, Hu Y, Wang B, Strasner A, Song Y, Song E. Cordycepin (3′-deoxyadenosine) suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. Oncotarget. 2015;6:9834–9853. doi: 10.18632/oncotarget.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Hao LJ, Hung CP, Chen JW, Leu SF, Huang BM. Apoptotic effect of cisplatin and cordycepin on OC3 human oral cancer cells. Chin J integr Med. 2014;20:624–632. doi: 10.1007/s11655-013-1453-3. [DOI] [PubMed] [Google Scholar]
- 10.Imamura K, Asai M, Sugamoto K, Matsumoto T, Yamasaki Y, Kamei I, Hattori T, Kishimoto M, Niisaka S, Kubo M, et al. Suppressing effect of cordycepin on the lipopolysaccharide-induced nitric oxide production in RAW 264.7 cells. Biosci Biotech Bioch. 2015;79:1012–1015. doi: 10.1080/09168451.2015.1008977. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Yang M, Gong S, Yang Y, Chen B, Cai Y, Zheng S, Yang Y, Xia P. Cordyceps sinensis extracts attenuate aortic transplant arteriosclerosis in rats. J Surg Res. 2012;175:123–130. doi: 10.1016/j.jss.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Yang SW, Lim L, Ju S, Choi DH, Song H. Effects of matrix metalloproteinase 13 on vascular smooth muscle cells migration via Akt-ERK dependent pathway. Tissue Cell. 2015;47:115–121. doi: 10.1016/j.tice.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, Liu K. Formation of E-cadhein-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phospharidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol. 2005;19:2564–2578. doi: 10.1210/me.2004-0342. [DOI] [PubMed] [Google Scholar]
- 15.Lindner V. The NF-kappaB and IkappaB system in injured arteries. Pathobiology. 1998;66:311–320. doi: 10.1159/000028039. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Zhang L, Ren Y, Gao Y, Kang L, Lu S. Matrine inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Mol Med Rep. 2016;13:2313–2319. doi: 10.3892/mmr.2016.4767. [DOI] [PubMed] [Google Scholar]
- 17.Yang PY, Rui YC, Lu L, Li TJ, Liu SQ, Yan HX, Wang HY. Time courses of vascular endothelial growth factor and intercellular adhesion molecule-1 expressions in aortas of atherosclerotic rats. Life Sci. 2005;77:2529–2539. doi: 10.1016/j.lfs.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Choi KW, Park HJ, Jung DH, Kim TW, Park YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK, Pyo S. Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vasc Pharmacol. 2010;53:273–280. doi: 10.1016/j.vph.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Lin FY, Chen YH, Tasi JS, Chen JW, Yang TL, Wang HJ, Li CY, Chen YL, Lin SJ. Endotoxin induces toll-like receptor 4 expression in vascular smooth muscle cells via NADPH oxidase activation and mitogen-activated protein kinase signaling pathways. Arterioscl Throm Vas. 2006;26:2630–2637. doi: 10.1161/01.ATV.0000247259.01257.b3. [DOI] [PubMed] [Google Scholar]
- 20.Muslin A. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin Sci. 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, Lee IS. Wogonin suppresses TNF-alpha-induced MMP-9 expression by blocking the NF-kappaB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2006;351:118–125. doi: 10.1016/j.bbrc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Ju JW, Kim SJ, Jun CD, Chun JS. p38 kinase and c-Jun N-terminal kinase oppositely regulates tumor necrosis factor alpha-induced vascular cell adhesion molecule-1 expression and cell adhesion in chondrosarcoma cells. IUBMB Life. 2002;54:293–299. doi: 10.1080/15216540215674. [DOI] [PubMed] [Google Scholar]
- 23.Ho AW, Wong CK, Lam CW. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology. 2008;213:533–544. doi: 10.1016/j.imbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Kang JS, Yoon YD, Han MH, Han SB, Lee K, Lee KH, Park SK, Kim HM. Glabridin suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking sphingosine kinase pathway: Implications of Akt, extracellular signal-regulated kinase, and nuclear factor-κB/Rel signaling pathways. Mol Pharmacol. 2006;69:941–949. doi: 10.1124/mol.105.017442. [DOI] [PubMed] [Google Scholar]
- 25.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 27.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and E-selectin through nuclear factor-kappaB activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie CJ, Ritchie E, Paul A, Plevin R. IKKalpha and IKKbeta function in TNFalpha-stimulated adhesion molecule expression in human aortic smooth muscle cells. Cell Signal. 2007;19:75–80. doi: 10.1016/j.cellsig.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Ren Z, Cui J, Huo Z, Xue J, Cui H, Luo B, Jiang L, Yang R. Cordycepin suppresses TNF-α-induced NF-κB activation by reducing p65 transcriptional activity, inhibiting IκBα, and blocking IKKγ ubiquitination. Int Immunopharmacol. 2012;14:698–703. doi: 10.1016/j.intimp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]