Introduction
Intensity modulated radiotherapy (IMRT) has emerged as the favored radiation modality for the definitive and post operative treatment of locally advanced head and neck squamous cell carcinoma (HNSCC) [1, 2]. Numerous prospective studies have demonstrated comparable tumor control rates with lower incidence of acute and late toxicities when comparing IMRT to conventional radiotherapeutic techniques [3-5]. Despite these advances, treatment related morbidity remains significant and strategies to mitigate the late effects of radiotherapy are still needed. Such strategies are especially important for patients with locally advanced oropharyngeal cancer, who are generally younger with longer life expectancy and are often cured of disease. One method whereby toxicity may be reduced is by limitation of the extent of uninvolved nodal basins that are irradiated electively.
The level V nodal group was first described by Rouviere in 1932 [6] and is subdivided into levels Va, Vb, and Vc [7]. Levels Va and Vb contain the lymphatics of the posterior triangle of the neck, located superior to the transverse cervical vessels, while Level Vc contains the lateral supraclavicular lymph nodes [7]. The rate of histopathological involvement of this nodal group in HNSCC has been extensively studied in the surgical literature [8-14], with rates of involvement across carcinomas of different HNSCC subs-sites ranging from 5-19%. The inclusion of LVN in the standard template for elective nodal dissection has been debated [15, 16], however, to our knowledge, there is only a single study investigating the omission of level V nodal irradiation in oropharyngeal cancer [17].
At our center, the majority of patients with locally advanced oropharyngeal carcinoma (OPC) undergo definitive chemoradiation (CRT) [4]. Herein, we analyze regional nodal failures in patients treated for locally advanced OPC to determine whether level V can safely be omitted from elective radiation volumes.
Materials and Methods
Patients
We reviewed radiation treatment plans of 745 consecutive patients with OPC who were treated at our institution between July 2001 to December 2013. Starting in 2004, some radiation oncologists at our center began consistently eliminating coverage of clinically uninvolved LVNs in OPC patients. For the current analysis, all patients with Stage III/IV OPC treated definitively with IMRT and chemotherapy after 2004 were included. 408 patients met the above criteria. None of the patients had radiographically, clinically, or pathologically involved level V lymph nodes at the time of diagnosis.
Workup
All patients underwent a pretreatment staging workup that included history and physical examination by the treating medical, surgical, and radiation oncologists as well as flexible nasopharyngoscopy, and computed tomography (CT) of the head and neck with contrast. The majority of patients underwent fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT imaging. For 165 patients (40.4 %), testing for tumor HPV status was performed with p16 immunohistochemistry (IHC), HPV in-situ hybridization (ISH), and/or polymerase chain reaction for E6/E7 mRNA. A positive result from any of these tests was regarded as HPV positive disease.
Treatment
For each patient, the recommended course of treatment was formulated with the input of a multidisciplinary team including a radiation oncologist, medical oncologist, head and neck surgeon, pathologist and radiologist. In the majority of cases, definitive CRT was undertaken with platinum-based chemotherapy.
Target volumes were delineated as described previously [4, 18] using dose painting IMRT. Briefly, primary gross tumor volume (GTV) included all discernible gross disease appreciated on clinical examination, nasopharyngoscopy, imaging and operative and pathology reports. Primary nodal GTV included all proven or suspicious (>1 cm, necrotic, enhancing, or FDG avid) lymph nodes. The high risk clinical target volume (CTV59.4) at the primary site was defined as GTV plus a margin of 1.0-cm to 1.5-cm. In the node positive neck, CTV59.4 included levels II-IVa+b (with sparing of IB unless there was gross involvement or extension of the primary GTV into the oral cavity) as well as the retropharyngeal lymph nodes. The lower node-positive neck was treated to 50-54 Gy with either an LAN field or IMRT when gross disease was only present in the upper neck. In the node negative neck, CTV54 included levels II-IVa and the RPLNS. CTV70 was typically the same as GTV70 unless uncertainty existed as to gross tumor extent, in which case a margin of 0.5-cm was added. The PTV70 was defined as the CTV70 plus a margin of 0.3-cm to 0.5-cm, and the PTV59.4 and PTV54 were defined as their respective CTVs plus an identical margin range. For radiation planning purposes, the most recent consensus guideline defining the radiologic borders of LVN (Va and Vb) was utilized [7]. Specifically, LVN was defined as extending from the cranial edge of the hyoid bone to the transverse cervical vessels caudally, from the posterior border of the sternocleidomastoid to the anterior edge of the trapezius muscle posteriorly, and from the platysma muscle superficially to the paraspinal muscles medially. In all cases, Level V was either treated bilaterally or excluded bilaterally.
In the definitive setting, dose was typically prescribed in 2.12 Gy, 1.8 Gy, and 1.64 Gy daily fractions over a course of 33 fractions to the PTV70, PTV59.4, and PTV54, respectively. The lower neck was either included in the IMRT fields or included in a low anterior neck field matched to the IMRT fields (approximately 75% of cases).
Event Definitions
The primary end-point of interest was histologically proven regional nodal failure (with or without a synchronous local and/or distant failure). The competing events of interest were failure without a regional component (whether distant, local, or a combination of the two) and death without recurrent disease. Patients who had experienced none of these events were censored at last follow-up. Regional nodal failure was defined as either persistent or new disease in any of the cervical nodal levels consistent with nodal disease based on the AJCC Cancer Staging Manual, 7th edition [19]. Local recurrence was defined as either persistent or new disease anywhere within the PTV70. All failure dates were documented at the time of biopsy; however, if no biopsy results were available and patients were treated for presumed failure, imaging date providing evidence of such was selected.
Statistical Analysis
Age was treated as a continuous variable; stage (III versus IV), tobacco (>=10 pack years versus <10 pack years), HPV status (positive versus negative), and elective inclusion of level V nodes were treated as categorical variables. Pearson Chi-Square test was used to compare differences in smoking, stage, and HPV/p16 status between patients in whom elective level V nodes were treated and patients in whom they were omitted.
Both univariate and multivariate analyses were conducted using the competing risks regression method of Fine and Gray for regional recurrence. For all multivariate analyses, elective level V treatment as a categorical variable was included. Confounding variables were included if and only if they were both significantly associated with the inclusion of elective level V nodes as ascertained by the chi-square test and significantly associated with regional recurrence. Cumulative incidence curves were generated utilizing the Kaplan-Meier method.
All tests were 2-sided and statistical significance was defined as <=0.05. R version 3.1.2 statistical software was used (R Foundation for Statistical Computing, Vienna, Austria) for all statistical analyses.
Results
Patient and Tumor Characteristics
A total of 408 patients with stage III/IV OPC treated with definitive IMRT after 2004 were included in our analysis. 295 (72.3%) patients were treated to level V electively, while 113 (27.7%) were not.
Median follow-up for surviving patients in the group as a whole was 63.6 months (range, 1.3 to 125 months); median follow-up for patients in whom level V was excluded was 51.2 months (range, 1.3 to 125.4 months); median follow-up for patients in whom level V was treated was 65.5 months (range, 6.6 to 124.5 months).
Detailed patient and tumor characteristics are shown in table 1. The median patient age was 59 (range 27-91) for the entire cohort. Sub-sites of primary disease within the oropharynx included 166 tonsil, 222 base of tongue, 16 posterior pharyngeal wall, and 4 soft palate. HPV ISH and/or p16 status were available in 165 patients (40.4%).
Table 1.
Patient and Tumor Characteristics
| LVN Treated | LVN Untreated | p Value | |
|---|---|---|---|
| N=295 (72.3%) | N=113 (27.7%) | ||
| Median (Range) | Median (Range) | ||
| Age | 58 (27 - 84) | 59 (43 - 91) | p=0.16 |
| KPS | 90 (60 - 100) | 90 (70 - 100) | p=0.67 |
| N (%) | N (%) | ||
| Gender | p=0.55 | ||
| Male | 262 (89%) | 98 (87%) | |
| Female | 33 (11%) | 15 (13%) | |
| Tobacco use | p=0.04 | ||
| <10 pack years | 143 (48%) | 65 (57%) | |
| >= 10 pack years | 152 (52%) | 45 (40%) | |
| Unknown | 0 (0%) | 3 (3%) | |
| T Stage | p=0.054 | ||
| T1 | 62 (21%) | 26 (23%) | |
| T2 | 131 (44%) | 63 (56%) | |
| T3 | 58 (20%) | 16 (14%) | |
| T4 | 44 (15%) | 8 (7%) | |
| N Stage | p=0.49 | ||
| N0 | 7 (2%) | 2 (2%) | |
| N1 | 49 (17%) | 26 (23%) | |
| N2a | 27 (9%) | 10 (9%) | |
| N2b | 139 (47%) | 49 (43%) | |
| N2c | 67 (23%) | 26 (23%) | |
| N3 | 6 (2%) | 0 (0%) | |
| Stage Grouping | p=0.006 | ||
| III | 42 (14%) | 29 (26%) | |
| IV | 253 (86%) | 84 (74%) | |
| HPV Status | p=0.97 | ||
| Performed | 124 (42%) | 41 (36%) | |
| Positive | 82 (66%) | 27 (66%) | |
| Negative | 42 (34%) | 14 (34%) |
HPV status was not significantly associated with elective coverage of LVN (HPV positive, p=0.146; HPV negative, p=0.854). There were significant differences in overall stage (p=0.006) and tobacco status (p=0.04) between the two groups.
Treatment regimens
One patient received induction chemotherapy without concurrent chemotherapy (0.2%); 11 patients received induction chemotherapy and concurrent chemotherapy (2.7%); the remaining 396 patients (97.1%) received concurrent chemotherapy only. The most commonly used agents were cisplatin (57.3%)—administered at 100 mg/m2 once every three weeks up to a maximum of 3 doses—and cetuximab (11%)—administered with a loading dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. The remainder of patients were treated with various regimens of concurrent systemic therapy including carboplatin and 5-fluorouracil, carboplatin and paclitaxel, cisplatin and bevacizumab or cetuximab and nab-paclitaxel. There were no significant differences between the two groups with regards to treatment regimen.
Outcomes
Zero patients in either group experienced LVN recurrence. There were 26 cases of regional recurrence in the entire cohort, 4 in the LVN untreated group and 22 in the LVN treated group. Among these 26 patients with nodal failures, 51 sites of failure were identified, enumerated in table 2.
Table 2.
Regional Recurrence Locations
| Level V Treated | Level V Untreated | |||
|---|---|---|---|---|
| Location | Ipsilateral | Contralateral | Ipsilateral | Contralateral |
| Ia | 1 | 0 | 0 | 0 |
| Ib | 1 | 0 | 0 | 1 |
| II | 17 | 2 | 4 | 2 |
| III | 8 | 1 | 3 | 3 |
| IV | 5 | 0 | 1 | 1 |
| V | 0 | 0 | 0 | 0 |
| Other | 2a | 0 | 0 | 0 |
other sites include one failure in a subcutaneous nodule adjacent to parotid and one failure in a retropharyngeal lymph node
Failures without a nodal regional recurrence (isolated local failure, isolated distant failure, or synchronous local and distant failure) occurred in 57 patients, 16 in the LVN-untreated group and 41 in the LVN-treated group. The 2-year cumulative rate of regional failure (RF) was 4.5% (95% CI=2.9-6.6) in the overall cohort, 2.2% (95% CI = 0.1-5.9) in the LVN-untreated group, and 5.4% (95% CI = 3.4-8.1) in the LVN-treated group (see figure 1). After adjusting for stage and tobacco status, the two potentially confounding variables that were significantly associated with both regional recurrence risk and LVN inclusion, there was no significant difference between the two groups in the rate of regional failure (HR=1.75 95% CI = (0.61-5.07), p=0.30).
Figure 1.
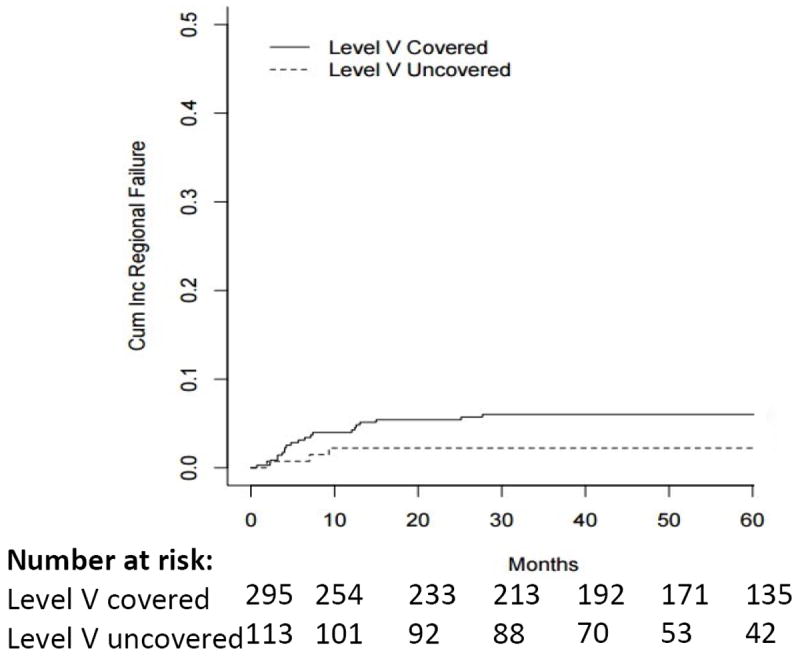
Cumulative Incidence of Regional Failure
There was no significant difference in cumulative mortality risk comparing the LVN-treated and LVN-untreated groups, 16.7% (95% CI, 6.6-18.6) vs 11.8% (95% CI, 12.6-21.2) (p=0.34).
Incidental dose to level V
In order to estimate the dose delivered to level V when it was not explicitly targeted, LVN was contoured in 3 cases where a low anterior neck field was used and 3 cases where IMRT was used to treat the lower neck. In all cases, the dose to LVN was <30 Gy. The mean dose in the LAN group was 23.6 Gy and the mean dose in the IMRT group was 16.4 Gy.
Discussion
Within this cohort of 408 locally advanced OPC patients, no regional nodal failures occurred in level V whether it was covered electively or not. No significant relationship between the cumulative incidence of regional nodal failure and the elective treatment of LVN was identified on either univariate or multivariate analysis. To our knowledge, this is the largest study demonstrating the safety of level V omission in the definitive treatment of OPC.
The cervical lymph nodes are the first site of metastasis in OPC, and the question of whether to omit LVN as part of the standard neck dissection has been extensively studied in the surgical literature [8-14]. These studies have served to inform the radiation treatment approach for OPC and the design of elective nodal fields based on risk of pathologic involvement [15, 16]. Shah and colleagues [13] studied 1119 patients who underwent neck dissection for HNSCC (1081 of which were at the time of primary treatment) and found a 5% rate of level V involvement in the entire cohort with an 11% rate of involvement in OPC patients. Additional data published by Davidson and colleagues in a similarly large cohort demonstrated a 3.6% overall rate and a 6.1% OPC subset rate [8]. Based on these data and other relevant studies [20, 21], omission of ipsilateral LVN was variably recommended in N0-1 OPC patients [15] or in patients without clinical involvement of levels II-IV [16]. However, most of the studies influencing these guidelines were published in the 1970s-1990s, before the modern era of HPV-driven disease which displays unique biology, prognosis, patterns of spread and response to treatment. Our study provides a unique contribution to this literature in that nearly half of our patient population had HPV/p16 testing available and, of those tested, approximately 66% were HPV positive, more closely reflecting the current landscape of OPC epidemiology in the United States [22]. Despite this, no level V failures were seen in our cohort of patients, irrespective of HPV status.
Further contemporary surgical reports on neck dissection results have shown LVN metastasis rates in HNSCC ranging from 5.8% to 19.1% [9-12, 14]. However, these rates appear to be largely driven by non-OPC HNSCC. Combining results of the three studies that report OPC specific rates, [9, 10, 12] 14 of 136 patients (10.3%) were found to have LVN metastases on neck dissection. Our data demonstrate lower rates of LVN metastases when compared to the above surgical literature. These differences may be attributed to differences in study population, lower sensitivity of clinical and radiographic staging compared to surgical staging as well as the possibility of subclinical disease that may be eradicated by incidental radiation dose to LVN. Furthermore, in the above studies, level V metastasis was found to correlate with involvement of multiple other cervical nodal levels (II-IV). Our population is likely composed of patients with earlier stage disease than the above studies as most of our patients were of nodal stage N2b or less. None of our OPC patients demonstrated clinical level V involvement at the time of presentation whereas a portion of the patients in the above studies were found to have clinically positive level V nodes, again suggesting earlier nodal stage in our population. Our analysis of dose to level V in a small subset of our cohort indicated that dose to level V, when it is not explicitly targeted, is under 30 Gy and would be expected to be subtherapeutic.
Mohindra et al. recently reported their experience with omission of level V lymph nodes in OPC in selected patients with N0-N2b disease [17]. Though their study demonstrated no significant difference in regional failure rate between omitted and treated groups as well as no RFs in the LVN regardless of elective coverage, the study population was limited and only included 35 patients with bilateral LVN exclusion. As expected, reduction in integral dose was seen with LVN omission. In the current study, nearly 25% of patients were of advanced nodal stage (N2c-N3), a cohort not included in the above study, and still no LVN failures were observed.
The ultimate goal of limiting treated nodal volumes is reduction in treatment related morbidity without a sacrifice in tumor control. As an example, significant toxicity benefits have been observed with omission of other cervical lymph node regions. Omission of retropharyngeal and high level II nodal stations in OPC patients has been shown to improve dysphagia outcomes [23], as well as minimize parotid dose [24]. While sparing of level V may not result in the same impact as sparing upper neck nodal basins, potential benefits include lower risks of dermatitis, brachial plexopathy [25], neck fibrosis and spasm [26], and shoulder dysfunction [27], particularly as the spinal accessory nerve runs superficially through level V and surgical manipulation can result in significant shoulder impairment [28, 29]. Because most of these events are late toxicities, detection of a difference with LVN omission would require both large sample sizes and extended follow-up. Although potential toxicity benefits of LVN omission have yet to be demonstrated definitively, any decrease in irradiated volumes that can be safely implemented should be encouraged, particularly as improved survival is expected with the increasing proportion of HPV+ OPC cases in the modern era [30, 31].
This study is limited by its retrospective, single-institution nature. A dosimetric comparison between the LVN treated and LVN untreated groups would be informative, though as mentioned above, structures at risk in this region are not routinely contoured for avoidance. Comparison of late patient reported outcomes between the two groups will be an important avenue of future study to determine the true clinical benefit of LVN sparing. It is also important to keep in mind that these results may or may not be applicable to other HNSCC subsites given differences in patterns of spread and lymphatic drainage. In spite of these shortcomings, to our knowledge this study represents the largest cohort of OPC patients reported in the literature treated with LVN omission, demonstrating a clear equivalence of outcome and no increased risk of failure. In light of these findings, elective coverage of level V is now omitted in treatment of OPC at our center in virtually all cases.
Conclusions
Our report demonstrates that in a large cohort of patients, there is no impact on regional control in OPC when elective LVN irradiation is omitted. No regional recurrences occurred in level V when it was omitted from radiation treatment fields. Our data strongly suggest that LVN can be safely excluded from elective treatment volumes in locally advanced OPC.
Highlights.
Analysis of 408 stage III/IV oropharyngeal cancers treated with IMRT.
Bilateral level V nodes were treated in 295 patients and omitted in 113 patients.
With a median follow-up of 63.6 months, there were no LVN failures in either group.
There was no increase in regional failure in the LVN untreated group.
LVN can be safely omitted from the clinical target volume in locally advanced OPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister DG, S S NCCN Clinical Practice Guidelines in Oncology. Version 2. NCCN; 2014. Head and Neck Cancers. [DOI] [PubMed] [Google Scholar]
- 3.Chao KS, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312–21. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]
- 4.Setton J, Caria N, Romanyshyn J, Koutcher L, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–8. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, Amdur RJ, Morris CG, Kirwan JM, Li JG. Intensity-modulated radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2010;120:2218–22. doi: 10.1002/lary.21144. [DOI] [PubMed] [Google Scholar]
- 6.Rouviere H. Systems of the Head and Neck. Ann Arbor, MI: Edwards Brothers; 1938. [Google Scholar]
- 7.Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110:172–81. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Davidson BJ, Kulkarny V, Delacure MD, Shah JP. Posterior triangle metastases of squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1993;166:395–8. doi: 10.1016/s0002-9610(05)80340-x. [DOI] [PubMed] [Google Scholar]
- 9.Kainuma K, Yano T, Kitoh R, Naito T, Usami S. Prevalence of level V metastasis in head and neck squamous cell carcinoma. Acta Otolaryngol. 2013;133:218–24. doi: 10.3109/00016489.2012.726742. [DOI] [PubMed] [Google Scholar]
- 10.Lim YC, Koo BS, Lee JS, Choi EC. Level V lymph node dissection in oral and oropharyngeal carcinoma patients with clinically node-positive neck: is it absolutely necessary? Laryngoscope. 2006;116:1232–5. doi: 10.1097/01.mlg.0000224363.04459.8b. [DOI] [PubMed] [Google Scholar]
- 11.McDuffie CM, Amirghahari N, Caldito G, Lian TS, Thompson L, Nathan CO. Predictive factors for posterior triangle metastasis in HNSCC. Laryngoscope. 2005;115:2114–7. doi: 10.1097/01.mlg.0000182475.49177.72. [DOI] [PubMed] [Google Scholar]
- 12.Naiboglu B, Karapinar U, Agrawal A, Schuller DE, Ozer E. When to manage level V in head and neck carcinoma? Laryngoscope. 2011;121:545–7. doi: 10.1002/lary.21468. [DOI] [PubMed] [Google Scholar]
- 13.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–9. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 14.Wiegand S, Zimmermann AP, Sesterhenn AM, Werner JA. Prevalence of level V metastases in node-positive head and neck squamous cell carcinoma. Anticancer Res. 2011;31:3959–61. [PubMed] [Google Scholar]
- 15.Gregoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135–50. doi: 10.1016/s0167-8140(00)00202-4. [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, Foote RL, O’Sullivan B, Beitler JJ, Vikram B. Intensity-modulated radiation therapy for head and neck cancer: emphasis on the selection and delineation of the targets. Semin Radiat Oncol. 2002;12:238–49. doi: 10.1053/srao.2002.32435. [DOI] [PubMed] [Google Scholar]
- 17.Mohindra P, Urban E, Pagan JD, Geye HM, Patel VB, Bayliss RA, et al. Selective omission of level v nodal coverage for patients with oropharynx cancer: Clinical validation of intensity modulated radiotherapy experience and dosimetric significance. Head Neck. 2014 doi: 10.1002/hed.23924. [DOI] [PubMed] [Google Scholar]
- 18.Target Volume Delineation for Conformal and Intensity-Modulated Radiation Therapy. Springer International Publishing; Switzerland: 2015. [Google Scholar]
- 19.AJCC Cancer Staging Manual. 7. Springer-Verlag; New York: [Google Scholar]
- 20.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–9. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Bataini JP, Bernier J, Brugere J, Jaulerry C, Picco C, Brunin F. Natural history of neck disease in patients with squamous cell carcinoma of oropharynx and pharyngolarynx. Radiother Oncol. 1985;3:245–55. doi: 10.1016/s0167-8140(85)80033-5. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer CR, Gay HA, Haughey BH, Nussenbaum B, Adkins DR, Wildes TM, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120:3994–4002. doi: 10.1002/cncr.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Truong MT, Romesser PB, Qureshi MM, Kovalchuk N, Orlina L, Willins J. Radiation dose to the brachial plexus in head-and-neck intensity-modulated radiation therapy and its relationship to tumor and nodal stage. Int J Radiat Oncol Biol Phys. 2012;84:158–64. doi: 10.1016/j.ijrobp.2011.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter KU, Worden F, Bradford C, Prince M, McLean S, Wolf G, et al. Neck spasm after chemoradiotherapy for head and neck cancer: natural history and dosimetric correlates. Head Neck. 2014;36:176–80. doi: 10.1002/hed.23284. [DOI] [PubMed] [Google Scholar]
- 27.Chen AM, Daly ME, Farwell DG, Vazquez E, Courquin J, Lau DH, et al. Quality of life among long-term survivors of head and neck cancer treated by intensity-modulated radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140:129–33. doi: 10.1001/jamaoto.2013.5988. [DOI] [PubMed] [Google Scholar]
- 28.Cappiello J, Piazza C, Giudice M, De Maria G, Nicolai P. Shoulder disability after different selective neck dissections (levels II-IV versus levels II-V): a comparative study. Laryngoscope. 2005;115:259–63. doi: 10.1097/01.mlg.0000154729.31281.da. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh A, Shallwani H, Ghaffar S. Postoperative shoulder function after different types of neck dissection in head and neck cancer. Ear Nose Throat J. 2014;93:E21–6. [PubMed] [Google Scholar]
- 30.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]


