Abstract
Previous studies have demonstrated that lipoxin A4 (LXA4) analogs blocked both airway hyper-responsiveness and pulmonary inflammation in a murine model of asthma. The present pilot study investigated the initial efficacy and safety of inhaled 5(S),6(R)-LXA4 methyl ester and BML-111, a LXA4 agonist, in the treatment of asthmatic children with acute episodes. A total of 50 asthmatic children diagnosed with acute moderate asthma were randomly assigned into groups and subjected to an inhalation challenge with pulmicort (n=10), ventolin (n=10), 5(S),6(R)-LXA4 methyl ester (n=10), BML-111 (n=10) or normal saline as a placebo (n=10). Pulmonary function was assessed prior to and following the challenge. Acute toxicity and safety of the inhaled 5(S),6(R)-LXA4 methyl ester and BML-111 in normal BALB/c mice were investigated prior to the current pilot study conducted in patients. Following the inhalation challenge, pulmonary function parameters in all groups with the exception of the normal saline-treated group indicated an improvement. The efficacies of 5(S),6(R)-LXA4 methyl ester and BML-111 were superior to the efficacy of pulmicort but reduced when compared to the efficacy of ventolin with regard to the improvement of pulmonary function following the inhalation challenge. No clinical adverse events were observed in the enrolled patients. All safety parameters in the full blood counts, routine urine and feces examination, electrocardiogram and liver and kidney function tests at baseline and the end of the current study were within normal limits for all patients. No significant differences in kidney or liver function tests were observed in mice treated with 5(S),6(R)-LXA4 methyl ester and BML-111. Light and electron microscopy demonstrated no airway epithelium or alveolar epithelial cell damage in the treated mice. The present preliminary study of a small sample demonstrates the initial efficacy and safety of inhaled 5(S),6(R)-LXA4 methyl ester and BML-111 in the treatment of asthmatic children with acute moderate episodes, and suggests that an inhaled LXA4 analog and LXA4 receptor agonist may exhibit potential as a novel therapeutic strategy for asthma.
Keywords: lipoxin A4, lipoxin A4 receptor agonist, asthma, inhalation, children
Introduction
The prevalence of asthma continues to increase globally and optimal treatment remains a challenge (1). Asthma is a chronic inflammatory disorder of the airways characterized by airway hyper-responsiveness, reversible bronchoconstriction and airway remodeling (2). Some cells that migrate to the bronchial tree from the bloodstream are sources of local inflammation. A number of eosinophils, polymorphonuclear cells (PMNs) and T lymphocytes infiltrate peribronchial tissues in patients with asthma, which are then introduced into the lung. These have an increased capacity to generate proinflammatory mediators, chemokines and cytokines including leukotriene (LT) B4, cysteinyl leukotrienes (CysLTs), T-helper lymphocyte 2 (Th2) cytokines and interleukins (3,4). Innate lymphoid cells, including natural killer (NK) cells, also serve regulatory roles in Th2 cytokine production in asthma (5). Corticosteroid therapy is primarily recommended for patients with asthma (1,2). Corticosteroids have been demonstrated to reduce the number of eosinophils and activated T lymphocytes in bronchoalveolar lavage fluid (BALF) and therefore, reduce the production of proinflammatory cytokines and chemokines (1). However, corticosteroids have no effects on the release of LTB4 by PMNs or isolated populations of blood cells (6,7) and may upregulate the activity of 5-lipoxygenase, thus increasing the production of leukotrienes (LTs) in human mast cells and PMNs (8,9). Oral and inhaled corticosteroids have no effect on the release of LTC4 in BALF obtained from patients with asthma (10). Short-term oral or inhaled corticotherapy has no effect on LT synthesis, whereas long-term oral corticotherapy induced changes in membrane phospholipids and therefore decreased 5-lipoxygenase activity and LT release by PMNs from glucocorticoid-dependent asthmatic subjects (11). LTs are lipids synthesized by mast cells, alveolar macrophages and eosinophils in the lungs from arachidonic acids in the 5-lipoxygenase pathway. They are considered to serve important roles in the pathogenesis of asthma (3). LTB4 acts as a proadhesive agent, chemoattractant and secretagogue for eosinophils, PMNs and T lymphocytes (3,12). CysLTs cause bronchoconstriction, which contributes to early- and late-phase responses to the inhaled allergen challenge (13) and induce airway remodeling (14). Clinical reports and experimental studies have demonstrated that the LT antagonist montelukast may reduce asthmatic symptoms and prevent airway remodeling when used as an additional treatment to reduce the intake of β2-agonists and corticosteroids in order to decrease the harmful side effects of corticosteroids (15,16). However, LT antagonists may be produced naturally and endogenously in the human body, namely lipoxins (LXs). LXs are lipoxygenases, primarily 15-lipoxygenase-derived eicosanoids that are distinct in function and structure from LTB4 and CysLTs. Lipoxin A4 (LXA4) and its analogs blocked CysLT-mediated airway obstruction (17,18), similar to the action of montelukast. They also inhibited LTB4-induced PMN and eosinophil chemotaxis (19). LXA4 also inhibited the production of LTB4 in PMNs (20) and the secretion of CysLTs in BALF obtained from asthmatic mice (21). In addition, LXs have been demonstrated to inhibit granulocyte activation and block the release of a number of proinflammatory cytokines and chemokines including T lymphocyte cytokines, similar to the action of corticosteroids. LXs may also increase NK cell-mediated apoptosis of both eosinophils and neutrophils (5). Thus, LXs exhibit the anti-inflammatory properties of montelukast and corticosteroids and have been indicated as a potential endogenous ‘braking signal’ in the inflammatory process, and these properties indicate the potential superiority of LXs in the treatment of asthma (5,17,18,22). Indeed, the biological activity of the LXs in asthma has been defined over the past two decades. LXA4 analogs blocked both airway hyper-responsiveness and pulmonary inflammation in a murine model of asthma (21). Furthermore, the powerful anti-inflammatory properties of LX provide a rationale for its application in the treatment of asthma, along with the observations of defective LX generation in uncontrolled asthma (23–25). A previous study also demonstrated that insufficient generation of LXA4 and overproduction of LTs may be responsible for a worsening of asthma in children (26). Thus far, two published human clinical studies with LXs demonstrated noteworthy therapeutic promise for asthma and allergic diseases (17,27). When administered to patients with asthma via nebulization, LXA4 attenuated LTC4-induced bronchoconstriction (17). A previous study indicated that treatment of allergic eczema in infants with topical 15(R/S)-methyl-LXA4 decreased eczema severity and duration, and improved patients' quality of life with a similar efficacy to topical corticosteroids (27). LXA4 exhibits the potential to be a novel therapeutic treatment in asthma (5,28,29). Thus, clinical trials with LXA4 stable analogs are required to determine its efficacy and safety in the treatment of asthmatic patients. This pilot study was undertaken to investigate the efficacy and safety of a LXA4 analog and LXA4 receptor agonist in the treatment of asthmatic children with acute episodes.
Patients and methods
Patients
A total of 50 asthmatic children (aged 5–12 years) were recruited between August 2013 and June 2014 from the Department of Pediatrics, Nanjing First Hospital Affiliated to Nanjing Medical University (Nanjing, China). Diagnosis of asthma and the assessment of severity and asthma management were based on Global Initiative for Asthma guidelines (2). All enrolled patients were classified as having mild persistent asthma with acute moderate asthma attacks, and had not received short-acting β2-agonists for 6 h before the assessment, or long-acting β2-agonists or glucocorticoids or theophylline or leukotriene modifiers 24 h before the examination. Patients who suffered from recent respiratory infection, respiratory disorders other than asthma, hepatic or renal or cardiovascular diseases, recent surgery or systemic inflammatory disorders were excluded from the study. As a control, 10 normal healthy children with similar sex and age distributions were recruited. The baseline clinical profile of the patients prior to the study and of the controls is presented in Table I. Parents or guardians of all enrolled children provided written informed consent for their child to participate in the current study. The protocol was approved by the Ethics Committee of Nanjing First Hospital Affiliated to Nanjing Medical University.
Table I.
Baseline clinical profile of the patients in the five groups and the controls.
| Group | PG | VG | LG | BG | SG | CG | P-value |
|---|---|---|---|---|---|---|---|
| Cases, n | 10 | 10 | 10 | 10 | 10 | 10 | – |
| Female/male | 6/4 | 5/5 | 4/6 | 3/7 | 4/6 | 4/6 | >0.05 |
| Age, years | 8.0±2.3 | 8.1±2.1 | 8.3±2.2 | 9.2±1.8 | 9.0±2.1 | 8.5±2.5 | >0.05 |
| Height, cm | 141±14.1 | 138±19.2 | 143±14.2 | 142±16.1 | 140±16.3 | 139±15.4 | >0.05 |
| Weight, kg | 31.3±10.0 | 32.1±10.2 | 31.5±9.9 | 33.6±8.9 | 31.5±10.9 | 30.2±9.8 | >0.05 |
| PEF predicted, % | 93.6±11.4 | 94.3±10.4 | 92.6±9.9 | 93.7±11.2 | 91.5±12.4 | 102.4±12.4 | >0.05 |
| PEF variability, % | 25.6±4.8 | 24.7±5.7 | 26.1±4.3 | 25.1±6.3 | 24.6±4.3 | 8.2±2.5 | <0.05a |
| Serum total IgE, IU/ml | 226±62.3 | 219±75.5 | 237±64.4 | 231±74.4 | 218±47.3 | 89±25.2 | <0.05a |
| Blood EO, cells/µl | 193±88.4 | 189±92.2 | 201±82.3 | 182±72.2 | 211±89.4 | 72±32.1 | <0.05a |
P<0.05 vs. baseline clinical profile of normal control group. Data are presented as the mean ± standard deviation. PEF, peak expiratory flow; IgE, immunoglobulin E; EO, eosinophils; PG, pulmicort-treated group; VG, ventolin-treated group; LG, lipoxin A4 analog-treated group; BG, BML-111-treated group; SG, normal saline-treated group; CG, normal control group.
Drug preparation
For analysis, 5(S),6(R)-LXA4 methyl ester was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). The solution of 5(S),6(R)-LXA4 methyl ester (50 µg/0.5 ml 100% alcohol) was diluted in 2 ml normal saline (0.9% sodium chloride) as an inhalation solution for each patient in the relevant group. LXA4 receptor agonist BML-111 [5(S),6(R),7-trihydroxyheptanoic acid methyl ester] was purchased from Sigma-Aldrich; Merck KgaA (Darmstadt, Germany). A solution of BML-111 (2 mg/0.5 ml 100% alcohol) was diluted in 2 ml normal saline as an inhalation solution for each patient in the relevant group. Previous studies were used as references for the selection of 5(S),6(R)-LXA4 methyl ester and BML-111 dosages (17,28–29). Pulmicort, an inhaled glucocorticoid budesonide, was obtained from AstraZeneca (London, UK). A nebulization solution of pulmicort (1 mg/2 ml) was mixed with 0.5 ml 100% alcohol for each patient in the relevant group. Ventolin nebules, an inhaled β2-agonist salbutamol sulphate, was purchased from GlaxoSmithKline (Brantford, UK). Nebulization solution of ventolin (0.5 mg/1 ml) was mixed with 0.5 ml 100% alcohol and 1 ml normal saline for each patient in the relevant group. As a placebo, 2 ml normal saline was mixed with 0.5 ml 100% alcohol for each patient in the relevant group. The color, smell and external appearance of the five nebulization solutions were identical. As the 5(S),6(R)-LXA4 methyl ester and BML-111 were stored in 0.5 ml alcohol, it was necessary to exclude the possibility that alcohol itself may have an effect on airway response. Thus, a nebulization solution of pulmicort, ventolin and normal saline was mixed with 0.5 ml alcohol.
Treatment protocol
This clinical trial was registered with the Chinese Clinical Trial Registry (chictr.org.cn/index.aspx), and the registration number is ChiCTR-TRC-13003279. In this double-blind, placebo-controlled, randomized, prospective, parallel comparative study, the 50 enrolled patients that presented with acute moderate asthma attacks were admitted and randomly divided into five groups according to a computer-generated randomized allocation schedule. Each group consisted of 10 patients. Subsequently, the patients received an inhalation challenge. As outlined in Table I, in the pulmicort-treated group (PG), patients received glucocorticoid pulmicort to inhale; in the ventolin-treated group (VG), patients received β2-agonist ventolin to inhale; in the LXA4 analog-treated group (LG), patients received a LXA4 analog to inhale, 5(S),6(R)-LXA4 methyl ester; in the BML-111-treated group (BG), patients received a LXA4 receptor agonist, BML-111, to inhale; and in the normal saline-treated group (SG), patients received placebo normal saline to inhale. In the healthy control group (CG), the children did not receive any treatment. Inhalation challenges were performed for 15 min using a TurboBOY N (085G1205) nebulizer (PARI GmbH, Starnberg, German). Delivery of air to the nebulizer was regulated to a pressure of 1.5 Pa with an airflow of 5 l/min. All study investigators, parents and caregivers of the enrolled patients remained blinded to the treatment throughout the study period. Pulmonary function was assessed by using a spirometer (Carefusion Microlab; BD Biosciences, Franklin Lakes, NJ, USA) in the enrolled patients 5 min prior to and immediately following the inhalation challenge. The following parameters were recorded: Forced expiratory volume in 1 sec (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), and forced expiratory flow at 25, 50 and 75% expiratory vital capacity (FEF25-75). Blood samples were collected from the enrolled patients prior to the inhalation challenge and on days 1 and 7 following inhalation. Other routine treatment for asthma besides the inhalation challenge was performed immediately following the inhalation challenge to avoid the next asthmatic episode when the inhalation challenge was withdrawn (clinical endpoints). As indicated in Table I, the BG group had more male patients than females, which may have affected the treatment outcome as males tend to have more severe asthma than females at this age. However, as mentioned, only the patients who were diagnosed with mild persistent asthma were enrolled in the current study, and accordingly, all patients had the same severity degree.
Safety observation in patients
Observation and documentation of clinical adverse events, physical examination and laboratory investigations were performed by an investigator prior to the inhalation challenge and on days 1 and 7 following the challenge. Laboratory investigations included a full blood count, routine urine and feces examination, electrocardiogram, and liver and renal function tests, which examined blood aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, urea nitrogen and creatinine measurements.
Safety observations in mice
Acute toxicity and safety of inhaled 5(S),6(R)-LXA4 methyl ester and BML-111 in normal BALB/c mice were investigated prior to the current pilot study conducted in patients. Mice were obtained from Laboratory Animal Center of Nanjing First Hospital, and quarantined for 1 week prior to the experiment and bled to establish that they were virus free. The mice were housed in an animal facility that was maintained at 22–24°C with a 12-h dark/light cycle, and fed with a commercial pelleted mouse food and water ad libitum under specific pathogen-free conditions. The present study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Nanjing First Hospital (Permit no. 2009-0015). A total of 12 male BALB/c mice weighing 19–21 g and aged 6 weeks were randomly divided into three groups (n=4/group): A normal saline-treated control, 5(S),6(R)-LXA4 methyl ester-treated group and BML-111-treated group. Normal saline (50 µl 100% alcohol diluted in 0.5 ml normal saline), 5(S),6(R)-LXA4 methyl ester (1 µg/50 µl alcohol diluted in 0.5 ml normal saline) and BML-111 (50 µg/50 µl alcohol diluted in 0.5 ml normal saline), respectively, were administered once daily for 30 min for 2 days using an atomizer. Mice were sacrificed following the last inhalation. Blood samples were collected in order to determine liver and renal function. The lungs were removed and excised. Lung sections (5-µm thick) were stained with hematoxylin and eosin and observed using light microscopy. Ultrathin sections (50-nm thick) were stained with uranyl acetate and lead citrate. The ultrastructure of bronchiole ciliated epithelial cells and type II alveolar epithelial cells were observed using a transmission electron microscope (EM400; Philips Healthcare, DA Best, The Netherlands).
Statistical analysis
Data are expressed as the mean ± standard deviation. Clinical and experimental data were analyzed using Paired-samples t-test or one-way analysis of variance followed by q or Tamhane T2 tests, where appropriate. All analyses were completed using SPSS software (version 11.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Safety observation in mice
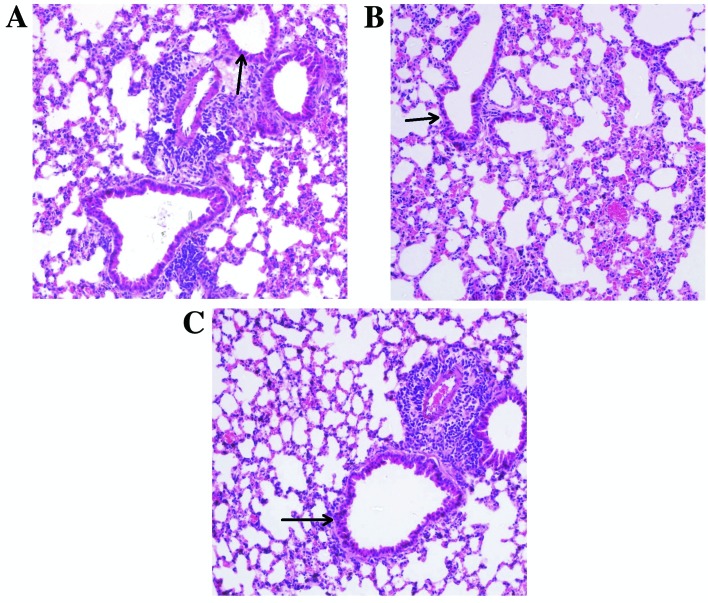
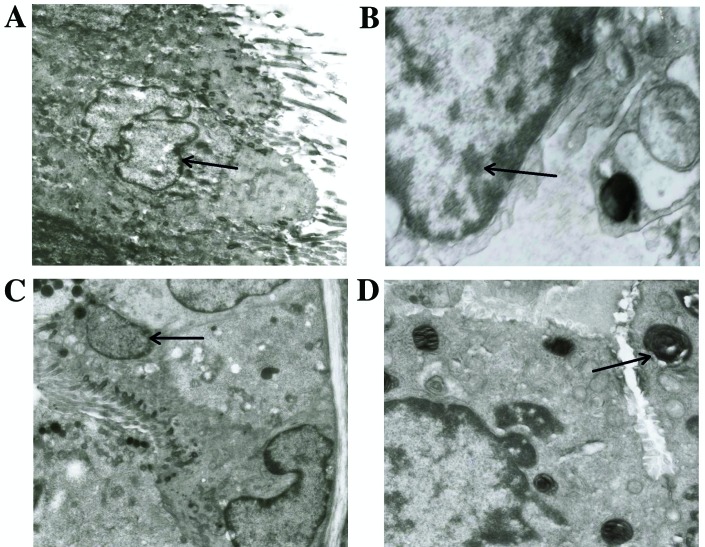
No significant differences in kidney or liver function tests were observed among the mice of the three groups during a pilot study (data not shown). As presented in Fig. 1, light microscopy demonstrated no airway epithelium damage, bronchus or bronchiole wall swelling, hydropic degeneration, hyperplasia or necrosis in lung specimens obtained from the mice of the three groups. As indicated in Fig. 2, treatment with 5(S),6(R)-LXA4 methyl ester and BML-111 did not affect the ultrastructure of bronchiole ciliated epithelial cells and type II alveolar epithelial cells. Under electron microscopy, the ultrastructure of bronchiole ciliated epithelial cells was observed to have maintained a normal appearance without cilium shedding, destruction or poor alignment. The ultrastructure of type II alveolar epithelial cells also demonstrated a normal and clear appearance without evacuation of lamellar bodies and the empty cavity of lamellar bodies was not obvious.
Figure 1.
Light microscopy of lung specimens obtained from normal BALB/c mice following inhalation challenge. Specimens were obtained from mice that had been challenged with (A) normal saline, (B) 5(S),6(R)-LXA4 methyl ester and (C) BML-111. Sections were stained with hematoxylin and eosin and are presented at a magnification of ×400. Small airway epithelial cells have been indicated by arrows.
Figure 2.
Transmission electron microscopy of lung specimens obtained from normal BALB/c mice following inhalation challenge. The ultrastructure of (A) bronchiole ciliated epithelial cell and (B) type II alveolar epithelial cell are presented, in the specimen obtained from a mouse that received inhalation challenge with 5(S),6(R)-LXA4 methyl ester. The ultrastructure of (C) bronchiole ciliated epithelial cell and (B) type II alveolar epithelial cell including multilamellar body (D) from a specimen obtained from a mouse that received inhalation challenge with BML-111. Ultrathin sections (50 nm) are presented at a magnification of ×12,000. Arrows indicate (A and C) Bronchiole ciliated epithelial cells, (B) type II alveolar epithelial cell and (D) multilamellar body.
Safety observation in patients
No clinical adverse events were observed in any of the patients enrolled. All safety parameters in the full blood counts, electrocardiogram, routine urine and feces examination, and kidney and liver function tests (data not shown) at the baseline and the end of the current study were within normal limits for all enrolled patients. No significant differences in routine urine and feces examination, full blood counts, kidney and liver function tests or the electrocardiogram, were observed between the baseline values and the post-treatment values in each group, and between the six groups (P>0.05; Tables II and III).
Table II.
Levels of liver function of the asthmatic children (n=50).
| Level | Alanine transaminase (U/l) | Aspartate transaminase (U/l) | Alkaline phosphatase (U/l) |
|---|---|---|---|
| Before inhalation | 34.27±3.45 | 32.2±5.47 | 129.5±23.11 |
| 1 day after inhalation | 32.13±4.65a | 31.3±6.52a | 133.02±20.30a |
| 7 days after inhalation | 31.75±5.42a | 30.7±7.01a | 131.45±21.33a |
| Normal limits | 9–50 | 15–40 | <500 |
P>0.05 vs. liver function levels before inhalation. Data are presented as the mean ± standard deviation.
Table III.
Levels of kidney function of the patients in the five groups (n=50).
| Serum creatinine (µmol/l) | Urea nitrogen (mmol/l) |
|---|---|
| 45.23±6.82 | 4.10±1.02 |
| 46.03±6.45a | 3.81±1.34a |
| 46.39±5.87a | 3.72±1.41a |
| 39.8–134.4 | 2.86–8.20 |
P>0.05 vs. kidney function levels before inhalation. Data are presented as the mean ± standard deviation.
Efficacy observations
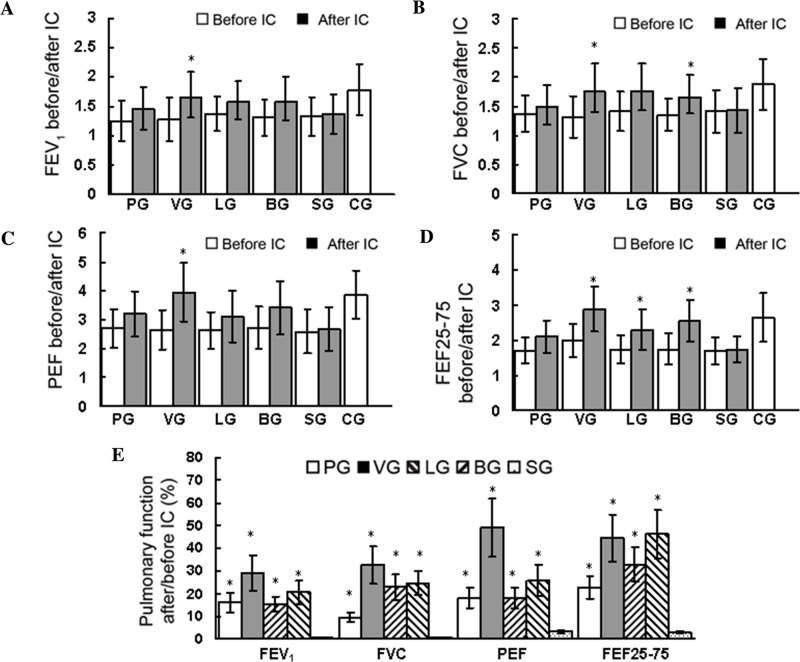
Treatment efficacy was evaluated via pulmonary function tests (P<0.05; Fig. 3). No significant differences in baseline pulmonary function were observed among the five groups of patients (P>0.05; Fig. 3E). However, as indicated in Fig. 3A-D, treatment with pulmicort, ventolin, 5(S),6(R)-LXA4 methyl ester and BML-111 all partially or slightly improved the post-treatment values of pulmonary function. Treatment with ventolin significantly increased the post-treatment values of FEV1, FVC, PEF and FEF25-75 (P<0.05; Fig. 3A-D). Treatment with 5(S),6(R)-LXA4 methyl ester significantly increased the post-treatment values of FEF25-75 (P<0.05; Fig. 3) but not FEV1, FVC or PEF. Treatment with BML-111 significantly increased the post-treatment values of FVC (P<0.; Fig. 3B) and FEF25-75 (P<0.05; Fig. 3D) but not FEV1 or PEF. Treatment with pulmicort did not significantly increase the post-treatment values of FEV1, FVC, PEF or FEF25-75. The inhalation challenge with normal saline did not improve the post-treatment values of pulmonary function. In Fig. 3E, the values were calculated via the following formula: [(post-treatment values- pre-treatment values)/pre-treatment values]x100, in order to reflect the enhanced extent of post-treatment values relative to the pre-treatment values. Notably, when compared with the value of the normal saline-treated patients, treatment with pulmicort, ventolin, 5(S),6(R)-LXA4 methyl ester and BML-111 significantly improved the values of pulmonary function (P<0.05; Fig. 3E). The aforementioned observations suggest that the efficacies of 5(S),6(R)-LXA4 methyl ester and BML-111 were superior to the efficacy of pulmicort but weaker than the efficacy of ventolin for improving pulmonary function following inhalation challenge. In addition, expiratory wheezing identified by auscultation prior to the inhalation challenge was undetectable following the inhalation challenge with pulmicort, ventolin, 5(S),6(R)-LXA4 methyl ester and BML-111, but not normal saline. However, the values of blood eosinophils and immunoglobulin (Ig) E measured 1 h after all inhalation challenges (data not shown) were not changed when compared with the baseline values measured prior to the inhalation challenge (Table I).
Figure 3.
FEV1, FVC, PEF, FEF25-75 and pulmonary function assessed prior to and following IC in all enrolled patients. Data are presented as the mean ± standard deviation. (A-D) *P<0.05 vs. value prior to IC in the same group. (E) Value=[(after IC-before IC)/before IC]x100; *P<0.05 vs. the same value in SG. IC, inhalation challenge; PG, pulmicort-treated group (n=10); VG, ventolin-treated group (n=10); LG, 5(S),6(R)-LXA4 methyl ester-treated group (n=10); BG, BML-111-treated group (n=10); SG, normal saline-treated group (n=10); CG, healthy control group (n=10 without IC); FEV1, forced expiratory volume in one second (L); FVC, forced vital capacity (L); PEF, peak expiratory flow (l/sec); FEF25-75, combined forced expiratory flow at 25, 50 and 75% expiratory vital capacity (l/sec).
Discussion
The current pilot clinical trial is, to the best of our knowledge, the first to investigate the efficacy and safety of LXA4 analog and LXA4 receptor agonist in the treatment of children with acute episodes of asthma. The results of the present study indicate that the efficacies of 5(S),6(R)-LXA4 methyl ester and BML-111 were superior to the efficacy of pulmicort but weaker than the efficacy of ventolin with regard to the improvement of pulmonary function following an inhalation challenge. Ventolin is a short-acting β2-agonist salbutamol sulphate, the treatment of choice for acute bronchospasm, which is inhaled as this route is faster and more effective than the oral route (2). Pulmicort, an inhaled corticosteroid budesonide, reduces expiratory airflow limitation and airway responsiveness in asthma, and subsequently improves the spirometric values and other measures of lung function in order to control the symptoms of asthma (1). In the present study, inhaled 5(S),6(R)-LXA4 methyl ester and BML-111 was more effective than pulmicort, this suggests that LX possesses powerful anti-inflammatory properties. However, the anti-inflammatory properties of 5(S), 6(R)-LXA4 methyl ester and BML-111, which were inhaled in a short-course only in the present study, may be confined within lungs and not generalized, as the inhalation challenge with 5(S),6(R)-LXA4 methyl ester and BML-111 did not alter the blood eosinophils and IgE, as outlined in the results.
In the current primary short study, no clinical adverse events occurred in the patients of the five groups, and the inhalation solution of 5(S),6(R)-LXA4 methyl ester and BML-111 was well-tolerated, without side-effects. Side-effects were evaluated via full blood counts, routine urine and feces examination, electrocardiogram, and liver and kidney function tests on day 7 following the inhalation challenge. These results indicate the safety of 5(S),6(R)-LXA4 methyl ester and BML-111 when used as a short-course inhalation treatment. Furthermore, the pilot study conducted in normal BALB/c mice also demonstrated that inhaled 5(S),6(R)-LXA4 methyl ester and BML-111 were safe without damage to the liver and kidney function or the ultrastructure of bronchiole ciliated epithelial cells and type II alveolar epithelial cells.
In conclusion, the current preliminary study in a small sample size demonstrated the initial efficacy and safety of inhaled 5(S),6(R)-LXA4 methyl ester and BML-111 in the treatment of asthmatic children with acute moderate episodes. Future studies are required to investigate whether 15(R/S)-methyl LXA4 is more effective than 5(S),6(R)-LXA4 methyl ester (27) in the treatment of asthma in children with acute episodes, and to determine the efficacy and safety of a larger dose of LXA4 stable analogs in the treatment of large samples of asthmatic children.
Acknowledgements
The present study was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu, China (grant no. JX10231801).
References
- 1.Sutherland ER, James MK. Diagnosis and treatment of asthma. In: Carpo JD, Glassroth J, Karlinsky JB, King TE, editors. Baum's Textbook of Pulmonary Diseases. 7th. Lippincott Williams & Wilkins; Philadelphia, PA: 2004. pp. 179–202. [Google Scholar]
- 2.WHO/NHLBI Workshop report: Glabal strategy for asthma management and prevention. NIH Publication No. 02–3659p, corp-author. National Institutes of Health/National Heart, Lung and Blood Institute. Bethesda, MD: 2005. [Google Scholar]
- 3.Leff AR. Role of leukotrienes in bronchial hyperresponsiveness and cellular responses in airways. Am J Respir Crit Care Med. 2000;161:S125–S132. doi: 10.1164/ajrccm.161.supplement_1.ltta-25. [DOI] [PubMed] [Google Scholar]
- 4.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-Q. [DOI] [PubMed] [Google Scholar]
- 5.Barnig C, Levy BD. Lipoxin A4: A new direction in asthma therapy? Expert Rev Clin Immunol. 2013;9:491–493. doi: 10.1586/eci.13.36. [DOI] [PubMed] [Google Scholar]
- 6.Freeland HS, Pipkom U, Schleimer RP, Bascom R, Lichtenstein LM, Naclerio RM, Peters SP. Leukotriene B4 as a mediator of early and late reactions to antigen in human: The effect of systemic glucocorticoid treatment in vivo. J Allergy Clin Immunol. 1989;83:634–642. doi: 10.1016/0091-6749(89)90076-6. [DOI] [PubMed] [Google Scholar]
- 7.Schieimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranuation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. 1989;250:598–605. [PubMed] [Google Scholar]
- 8.Colamorea T, Di Paola R, Macchia F, Guerrese MC, Tursi A, Butterfield JH, Caiaffa MF, Haeggström JZ, Macchia L. 5-Lipoxygenase upregulation by dexamethasone in human mast cells. Biechem Biophys Res Commun. 1999;265:617–624. doi: 10.1006/bbrc.1999.1732. [DOI] [PubMed] [Google Scholar]
- 9.Thomas E, Leroux JL, Blotman F, Descomps B, Chavis C. Enhancement of leukotriene A4 biosynthesis in neutrophils from patients with rheumatoid arthritis after a single glucocorticoid dose. Biochem Pharmacol. 1995;49:243–248. doi: 10.1016/S0006-2952(94)00403-X. [DOI] [PubMed] [Google Scholar]
- 10.Bisgaard H. Leukotriene modifiers in pediatric asthma management. Pediatrics. 2001;107:381–390. doi: 10.1542/peds.107.2.381. [DOI] [PubMed] [Google Scholar]
- 11.Vachier I, Chavis C, Majori M, Farce M, Bousquet J, Godard P, Chanez P. Effects of glucocorticoids on endogenous and transcellular metabolism of eicosanoids in asthma. J Allergy Clin Immunol. 2001;107:824–831. doi: 10.1067/mai.2001.113868. [DOI] [PubMed] [Google Scholar]
- 12.Luster AD, Tager AM. T-cell trafficking in asthma: Lipid mediators grease the way. Nat Rev Immunol. 2004;4:711–724. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- 13.O'Byrne PM. Leukotriene bronchoconstriction induced by allergen and exercise. Am J Respir Crit Care Med. 2000;161:S68–S72. doi: 10.1164/ajrccm.161.supplement_1.ltta-14. [DOI] [PubMed] [Google Scholar]
- 14.Parameswaran K, Cox G, Radford K, Janssen LJ, Sehmi R, O'Byrne PM. Cysteinyl leukotrienes promote human airway smooth muscle migration. Am J Respir Crit Care Med. 2002;166:738–742. doi: 10.1164/rccm.200204-291OC. [DOI] [PubMed] [Google Scholar]
- 15.Simons FE, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, Laessig W, Schster A, Perez-Frias J, Sekerel BE, et al. Montelukast added to budesonide in children with persistent asthma: A randomized, double-blind, crossover study. J Pediatr. 2001;138:694–698. doi: 10.1067/mpd.2001.112899. [DOI] [PubMed] [Google Scholar]
- 16.Pifferi M, Caramella D, Ragazzo V, De Marco E, Pietrobelli A, Boner AL. Montelukast and airway remodeling in children with chronic persistent asthma: An open study. Pediatr Allergy Immunol. 2004;15:472–473. doi: 10.1111/j.1399-3038.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 17.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 18.Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, Parkinson JF. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TH, Lympany P, Crea AK, Spur BW. Inhibition of leukotriene B4-induced neutrophil migration by lipoxin A4: Structure-function relationships. Biochem Biophys Res Commun. 1991;180:1416–1421. doi: 10.1016/S0006-291X(05)81354-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu SH, Liao PY, Yin PL, Zhang YM, Dong L. Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. Am J Pathol. 2009;174:115–122. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 22.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 23.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Severe Asthma Research Program, National Heart, Lung, and Blood Institute: Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planagumà A, Kazani S, Marigowda G, Haworth O, Marian TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LXA4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Wu SH, Yin PL, Zhang YM, Tao HX. Reversed changes of lipoxin A4 and leukotrienes in children with asthma in different severity degree. Pediatr Pulmonol. 2010;45:333–340. doi: 10.1002/ppul.21186. [DOI] [PubMed] [Google Scholar]
- 27.Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol. 2013;168:172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu SH, Yin PL, Zhang YM, Wu JM. Inhalation of lipoxin A4 inhibits airway inflammation and Th1/Th2 imbalance in asthmatic mice. Chin J Tubercul Respir Dis. 2009;32:386–387. [Google Scholar]
- 29.Kong X, Wu SH, Zhang L, Chen XQ. Roles of lipoxin A4 receptor activation and anti-interleukin-1β antibody on the toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-κB pathway in airway inflammation induced by ovalbumin. Mol Med Rep. 2015;12:895–904. doi: 10.3892/mmr.2015.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]