Abstract
The present study determined the effect of dexmedetomidine (Dex) combined with flurbiprofen axetil (FA) on analgesia, immune response, and preservation of cognitive function in patients subjected to general anesthesia. We recruited 100 patients with thyroid surgery and randomly divided them into four groups: Dex (D), FA (F), Dex combined with FA (DF), and saline control (C). The extubation and recovery times for Groups D and DF were significantly longer than for Groups F and C. After extubation, the heart rate and mean arterial pressure for Groups F, D, and DF were significantly lower than for Group C, and data for Group DF was significantly lower than for Group F. The visual analog scale and Riker sedation agitation scores were significantly lower in Group DF than for the other three groups. T- and B-lymphocytes were significantly higher in Group DF than in the other three groups. Compared with Groups F and C, the levels of TNF-α and IL-6 in Group DF were significantly reduced, while IL-2 markedly increased. The combined use of Dex and FA significantly improved pain after general anesthesia thyroid surgery, reduced restlessness and postoperative cognitive dysfunction, enhanced immune function, and promoted wound repair.
Keywords: dexmedetomidine, flurbiprofen axetil, analgesia, cognitive function, immune response
Introduction
Thyroid surgery is a common procedure, relatively easy, and completed in a short time. However, the rich and complicated blood vessels and nerves around the thyroid glands, the anesthetics, and the use of analgesics require that doctors also pay attention to the effects on the immune response and postoperative cognitive function. The stress response to thyroid surgery and postoperative pain will alter the immune system. Furthermore, general anesthesia can influence the postoperative cognitive function, which should stimulate research to improve the adverse reactions after general anesthesia. Many drugs have been studied to prevent the postoperative adverse reactions, including pain and agitation after general anesthesia, but the results varied (1,2). Flurbiprofen axetil (FA) is an NSAID anti-inflammatory analgesic that can accumulate at the incision site through lipid microsphere vector targeting to reduce the conduction of nerve ending algesia, increase pain threshold, alleviate central sensitization, and play a role of preemptive analgesia (3). Studies show that FA has analgesic effect for thyroid surgery, but its effect is mild, which often requires combination with other analgesics. Dexmedetomidine (Dex) is an adrenoreceptor agonist that selectivity acts on the adrenoreceptor α2 and has powerful analgesia, sedation, antianxiety, and stress alleviation (4). Research confirms that the application of Dex during operation has a favorable postoperative analgesic effect (5,6). Here, we combined Dex with FA in the patients with thyroid surgery under general anesthesia to examine the analgesic, sedation, immune response, and postoperative cognitive function to provide experimental data for future clinical application.
Patients and methods
Clinical subjects
We recruited 100 patients that received thyroid surgery by intravenous anesthesia of Grade ASAI or II in The First People's Hospitals of Xuzhou Thyroid Breast Surgery Department. Ages ranged from 20 to 60 years. Surgery time was controlled within 3 h. Exclusion criteria: patients with difficult intubation, thyroid hyperactive adenoma, hyperthyroidism, serious diseases of lung, heart, liver or kidney, coagulation dysfunction, medical history of peptic ulcer, opiates and NSAID use history, allergic to FA and Dex. According to random number table, the patients were equally divided into four groups: FA (Group F), Dex (Group D), Dex combined with FA (Group DF), and normal saline control (Group C). The study was approved by the Medical Ethics Committee in The First People's Hospital of Xuzhou, and all patients read and signed the informed consent form.
Anesthesia methods
The patients were set up with electrocardiograph monitoring and we created a peripheral vein tunnel. Anesthesia induction: 0.05 mg/kg midazolam, 3 µg/kg fentanyl, 1–2 mg/kg propofol and 0.15 mg/kg cisatracurium. When conditions for intubation were reached, anesthesia machine was connected, with intermittent positive pressure ventilation. Maintenance of anesthesia: 10 µg∙kg-1∙h-1 continuous pump casting remifentanil of vein was applied, and the concentration of plasma propofol was adjusted to maintain the depth of anesthesia and muscle relaxation. Thirty minutes before the end of surgery, 50 mg of FA was injected into the vein of patients in Group F; 0.5 µg/kg of Dex was injected into the vein of patients in Group D; the combination of 50 mg of FA and 0.5 µg/kg of Dex was injected into the vein of patients in Group DF; equivalent normal saline was injected into the vein of patients in Group C. By the end of the surgery, pump casting remifentanil and propofol was stopped. After surgery, the patients were monitored in the Intensive Care Unit. When they recovered the autonomous respiration and the oxyhemoglobin saturation stayed at >95% as they inhaled room air, the tube was removed as they regained awareness.
Observation indexes
We recorded the following indexes for the four groups: i) recovery and extubation times. The recovery time was the time between the moment the anesthesia was stopped and the patient awakening. The extubation time was the time between the moment the anesthesia was stopped and extubation. ii) Heart rate before extubation, at the time of extubation, and 5 min after extubation. iii) Change of mean arterial pressure (MAP). iv) Sedation status before extubation applying the Riker sedation-agitation scale. v) The pain visual analog scale (VAS) scores 0.5, 3, 6, and 24 h after extubation were observed according to a VAS, with the highest of 10 scores (the higher scores stood for the most severe pain). vi) Postoperative cognitive function 1 day before surgery, and 3 h, 1 day, and 3 days after surgery were conducted using the MMSE score (7). The patients responded to 30 questions (1 score for each question, with maximum score of 30). Score 21–24 for mild cognitive impairment, 11–20 for moderate cognitive impairment, <10 for severe cognitive impairment. vii) The patients received EDTA anticoagulation tube to collect 5 ml of fasting venous blood 3 days after surgery, and the serum was separated. Flow cytometry (8) was used to observe the percentage of T- and B-lymphocytes in serum, and ELISA was used to detect the levels of TNF-α, IL-2, and IL-6.
Statistical analyses
Software SPSS 19.0 was used to process data, and the measurement data were displayed as mean ± standard deviation. Comparison between two groups was analyzed by independent t-test and for comparisons among multiple groups we used one-way analysis of variance. Enumeration data were expressed by case and the comparison was detected by χ2. P<0.05 indicates a statistically significant difference.
Results
Comparison of general data among the four groups
Age, gender, weight, and surgery time were compared for the four groups and we found no statistical differences (Table I).
Table I.
General data among the four groups.
| Groups | n | Male/Female | Age (years) | Weight (kg) | Operation time (min) |
|---|---|---|---|---|---|
| F | 25 | 13/12 | 47.13±7.25 | 62.37±9.55 | 145.40±25.31 |
| D | 25 | 12/13 | 49.28±9.33 | 61.59±10.26 | 143.67±23.72 |
| DF | 25 | 11/14 | 47.95±8.36 | 63.84±11.74 | 147.16±26.59 |
| C | 25 | 13/12 | 48.07±9.19 | 62.81±9.54 | 144.82±23.07 |
F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Extubation and recovery time
The extubation and recovery time were significantly longer in Groups D and DF than in Groups F and C (Table II). We found no significant difference between Groups F and C, or between Groups D and DF (Table II).
Table II.
Extubation and recovery time among the four groups (mean ± standard deviation).
| Groups | n | Extubation time (min) | Recovery time (min) |
|---|---|---|---|
| F | 25 | 14.66±3.37 | 10.15±2.16 |
| D | 25 | 24.43±2.90a,b | 17.38±3.54a,b |
| DF | 25 | 26.05±3.74a,b | 17.92±2.71a,b |
| C | 25 | 16.22±3.45 | 9.84±3.63 |
Compared with Group C
P<0.01; compared with Group F
P<0.01. F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Heart rate and MAP
At the time of extubation and 5 min after extubation, the heart rate and MAP for Groups F and C were significantly higher than before extubation (Table III). At the time of extubation, the heart rate and MAP for Group D was significantly higher than before extubation (Table III). Before extubation, the heart rate for Group DF was significantly slower than for Group C (Table III). At the time of extubation and 5 min after extubation, the heart rate and MAP for Groups F, D, and DF were significantly lower than for Group C. The heart rate indexes for Group DF was significantly lower than for Group F (Table III).
Table III.
Heart rate and MAP among the four groups (mean ± standard deviation).
| Indexes | Groups | n | Before extubation | Moment of extubation | 5 min after extubation |
|---|---|---|---|---|---|
| Heart rate (beats/min) | F | 25 | 83.37±8.23 | 96.49±8.81a,b | 92.36±8.67c,b |
| D | 25 | 81.39±8.00 | 90.51±7.92c,b | 85.44±7.94b | |
| DF | 25 | 78.02±7.51b | 84.04±9.07b,d | 81.50±7.62b,d | |
| C | 25 | 88.55±7.69 | 107.79±8.35a | 102.06±8.19a | |
| MAP (mmHg) | F | 25 | 93.46±8.34 | 110.50±9.29a,e | 102.44±7.58a,b |
| D | 25 | 89.85±9.26 | 103.64±7.14a,b | 96.23±8.51b | |
| DF | 25 | 88.72±8.54 | 95.21±8.63b,d | 91.12±7.03b,d | |
| C | 25 | 95.22±7.69 | 123.43±8.05a | 117.40±9.36a |
Comparison of the same group, compared with before extubation
P<0.01
P<0.05; comparison of the same time, compared with Group C
P<0.01
P<0.05; compared with Group F
P<0.01. MAP, mean arterial pressure; F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Sedation
The Riker sedation agitation score for Group DF was significantly lower than for the other three groups (Fig. 1). The scores for Groups F and D were significantly lower than that for Group C (Fig. 1).
Figure 1.
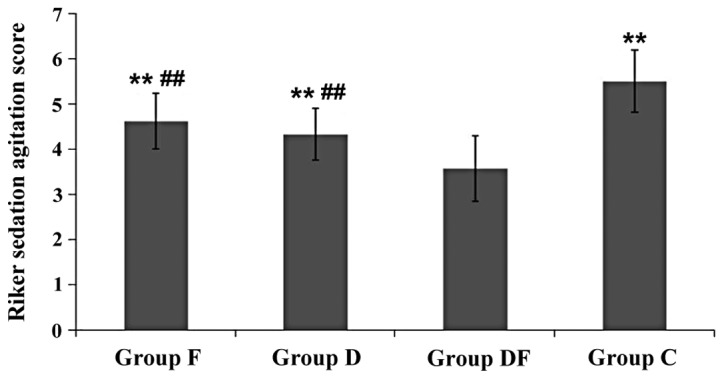
Sedation status of the four groups before extubation. Compared with Group DF, **P<0.01; compared with Group C, ##P<0.01. F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
VAS score
The pain VAS score for Group DF at each time point was significantly lower than for Groups F, D, and DF (Table IV). The VAS scores for Groups F, D, and DF were significantly lower than that for Group C (P<0.01). The VAS scores 30 min, 3 h and 6 h after surgery were significantly lower for Group DF than for Groups F and D (Table IV).
Table IV.
VAS score among the four groups (mean ± standard deviation).
| Groups | n | 30 min | 3 h | 6 h | 24 h |
|---|---|---|---|---|---|
| F | 25 | 2.25±0.63a,b | 2.73±0.66a,b | 2.39±0.50a,b | 1.27±0.54a |
| D | 25 | 1.88±0.50a,b | 2.39±0.59a,b | 2.01±0.56a,b | 1.03±0.47a |
| DF | 25 | 1.03±0.71a | 1.58±0.62a | 1.27±0.61a | 0.89±0.58a |
| C | 25 | 3.49±0.64 | 4.77±0.75 | 3.73±0.68 | 2.31±0.73 |
Compared with Group C
P<0.01; compared with Group DF
P<0.01. VAS, visual analog scale; F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Pre- and postoperative cognitive function
The MMSE score for Group C at each time period decreased significantly after surgery, and the MMSE score for Groups F and D were significantly reduced 3 h after surgery (Table V). After 3 h, 1 day, and 3 days of surgery, the MMSE scores of D and DF were significantly higher than for Group C (Table V). After 3 h of surgery, the MMSE score for Group DF was significantly higher than for Group F (Table V).
Table V.
MMSE score among the four groups (mean ± standard deviation).
| Groups | n | 1 day before surgery | 3 h after surgery | 1 day after surgery | 3 days after surgery |
|---|---|---|---|---|---|
| F | 25 | 26.85±3.04 | 22.78±2.49a,b | 24.22±3.12 | 25.53±2.73 |
| D | 25 | 26.37±3.16 | 23.89±2.55c,d | 24.86±2.48e | 25.92±2.40e |
| DF | 25 | 26.54±2.87 | 25.28±3.01c | 25.85±2.75c | 26.39±2.57c |
| C | 25 | 26.67±2.59 | 21.10±2.76b | 22.37±2.36b | 23.43±2.38b |
Compared with Group DF
P<0.01; compared with 1 day before operation
P<0.01
P<0.05; compared with Group C
P<0.01; compared with 3 h after surgery
P<0.05. F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Serum lymphocytes
The percentage of T- and B-lymphocytes in Groups F, D, and DF were significantly higher than in Group C (Table VI). The percentages of T- and B-lymphocytes in Group DF were significantly higher than in Groups F and D (Table VI).
Table IV.
Serum lymphocyte subsets among the four groups (mean ± standard deviation).
| Groups | n | T-lymphocyte | B-lymphocyte |
|---|---|---|---|
| F | 25 | 18.35±2.79a,b | 7.44±2.08a,b |
| D | 25 | 18.56±2.45a,b | 7.67±2.33a,b |
| DF | 25 | 22.17±2.53a | 16.37±2.91a |
| C | 25 | 8.75±2.34 | 4.48±1.62 |
Compared with Group C
P<0.01; compared with Group DF
P<0.01. F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Serum TNF-α, IL-2 and IL-6 levels
The levels of serum TNF-α and IL-6 for Groups F, D, and DF were significantly lower than for Group C (Table VII). The levels of serum IL-2 for Groups F, D, and DF were significantly higher than for Group C (Table III). Also, the levels of serum TNF-α and IL-6 for Group DF were significantly lower than for Group F and the levels of serum IL-2 for Group DF was significantly higher than for Group F (Table VII).
Table VII.
Serum TNF-α, IL-2 and IL-6 level among the four groups (mean ± standard deviation).
| Groups | n | TNF-α | IL-2 | IL-6 |
|---|---|---|---|---|
| F | 25 | 12.79±1.25a,b | 5.39±0.65c,b | 0.35±0.09c,b |
| D | 25 | 12.03±0.96c | 5.87±0.91c | 0.31±0.08c |
| DF | 25 | 11.22±0.93c | 6.20±0.76c | 0.22±0.09c |
| C | 25 | 14.08±1.13 | 4.54±0.72 | 0.49±0.12 |
Compared with Group C
P<0.05
P<0.01; compared with Group DF
P<0.01. F, flurbiprofen axetil; D, dexmedetomidine; DF, dexmedetomidine combined with flurbiprofen axetil; C, saline control.
Discussion
After thyroid surgery, patients often suffer incisional pain, odynophagia, and cough pain, causing discomfort. The profound state of unease (dysphoria) during awakening is a common postoperative complication after thyroid operation under general anesthesia and has a positive correlation with pain (9). Dysphoria can cause many adverse consequences, such as disruption of wound, bleeding, tachycardia, elevation of blood pressure, and heart and cerebral vessels accident. Some of the reasons for dysphoria include incisional pain, usage of doxapram, indwelling catheter, and activation of trachea cannula (10). Therefore, preventing dysphoria during awakening after general anesthesia has significant clinical consequences. Analgesic opiates were often used in the past, but caused side effects such as nausea, vomiting, and respiratory depression, limiting their application. Thus, there a critical need to identify treatments that prevent postoperative pain and dysphoria during awakening following general anesthesia.
FA is an NSAID anti-inflammatory analgesic that can treat the surgical incision through lipid microspheres vector targeting and reduce the sensitivity of sensory nerve by preventing the accumulation of inflammatory factors and prostaglandin. Application of FA before surgery can clearly alleviate postoperative pain (11). Dex is an imidazole derivative and adrenergic receptor agonist of high selectivity for α2 showing 1,620 times higher affinity than for α1 receptor (12) and 8 times higher affinity than clonidine (13). Dex also demonstrates a stronger function of analgesia, sedation, and antianxiety. We found that a single application of FA or Dex could improve postoperative pain after thyroid surgery, but the combination of FA and Dex had a stronger effect. This combined effect might be related to Dex activation of the adrenergic receptor on spinal dorsal horn and inhibition of P substance release, resulting in reduced stimulation reaction and a synergistic effect of opiates analgesic (14). Studies demonstrate that Dex can reduce the stress reaction to general anesthesia cannula, reduce the amount of anesthetic and analgesic, stabilize the hemodynamics of patients during the perioperative period, and control the stress reaction to postoperative tracheal extubation, all contributing to decrease dysphoria during awakening (15,16). We found that single application of FA or Dex inhibited angiocarpy reactions, including increasing heart rate and MAP, caused by extubation after general anesthesia and reduce dysphoria during awakening. But the benefits of combining FA and Dex were more potent. The reason might be that Dex increases the output of the central nucleus ceruleus parasympathetic nerve, stimulate the vasomotor center of the medulla oblongata system, leading to the reduction of noradrenaline, which inhibits the angiocarpy response (17). Here, we found that the recovery time and extubation time in patients that received Dex were longer than for the other groups, which was consistent with other studies (9).
Postoperative cognitive disorder is closely related with several physiological factors, including age, and the application of anesthetics during surgery. Postoperative inflammatory reaction is an important reason for secondary cerebral injury, but the application of Dex during surgery can alleviate it (18,19). One study confirmed that Dex exerts some protection for the central nervous system (20), which can reduce the occurrence of postoperative cognition impairment. Here, we found that Dex improved cognitive function and reduced the occurrence of postoperative cognition impairment. The combination of FA and Dex has a potent effect preventing postoperative cognition impairment.
The understanding of sedation is often restricted to the central nervous system, ignoring its effects on other systems, including immune response. We verified that sedation has an important immune response component that contributes to the mechanism of serious complications. The immunomodulatory effects can control the inflammation process, including acute respiratory distress syndrome, cerebral injury, acute renal failure (21), and blood coagulation cascade reaction (22). The immune response includes cell immunity and humoral immunity, and the surgical wound can alter cellular immune function manifested in T-lymphocytes (23) and humoral immunity indicated by B-lymphocytes. Surgery- and anesthesia-induced stress can activate macrophages, monocytes, vascular endothelial cells, lymphocytes, which can induce the increase of IL-6 and TNF-α secretion directly or indirectly (24). In addition, IL-6 and TNF-α are important early markers that can indicate an organism wound (25). As an immunomodulatory factor, IL-2 has a significant immunological enhancement, and its serum level can reflect the activated state of humoral immunity. A study shows that Dex can inhibit the release of inflammatory factors (26). Zhang et al found that Dex reduces the secretion of IL-6 and TNF-α in plasma (27). Here, we found that the combination of FA and Dex reduced the postoperative inhibition effect of IL-2, restrained the release of serum IL-6 and TNF-α, and raised the levels of B- and T-lymphocytes. This response is expected to relieve wound and inflammatory responses, enhance the immune function, and promote the wound repair.
Overall, the combination of FA and Dex significantly improves pain after general anesthesia, reduces restlessness during awakening, protects cognitive dysfunction, alleviates postoperative injury and inflammatory reactions, enhances immune function, and promotes wound repair.
References
- 1.Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111:222–228. doi: 10.1093/bja/aet056. [DOI] [PubMed] [Google Scholar]
- 2.Abdulatif M, Ahmed A, Mukhtar A, Badawy S. The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia. 2013;68:1045–1052. doi: 10.1111/anae.12380. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Tan Z, Chen J, Lou F, Chen W. Intravenous flurbiprofen axetil accelerates restoration of bowel function after colorectal surgery. Can J Anaesth. 2008;55:414–422. doi: 10.1007/BF03016307. [DOI] [PubMed] [Google Scholar]
- 4.Nazarian A, Christianson CA, Hua XY, Yaksh TL. Dexmedetomidine and ST-91 analgesia in the formalin model is mediated by α2A-adrenoceptors: a mechanism of action distinct from morphine. Br J Pharmacol. 2008;155:1117–1126. doi: 10.1038/bjp.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Y, Chen GZ, Dong YL, Wang W. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9:e93114. doi: 10.1371/journal.pone.0093114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96:747–753. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 7.An J, Fang Q, Huang C, Qian X, Fan T, Lin Y, Guo Q. Deeper total intravenous anesthesia reduced the incidence of early postoperative cognitive dysfunction after microvascular decompression for facial spasm. J Neurosurg Anesthesiol. 2011;23:12–17. doi: 10.1097/ANA.0b013e3181f59db4. [DOI] [PubMed] [Google Scholar]
- 8.Piyasena ME, Graves SW. The intersection of flow cytometry with microfluidics and microfabrication. Lab Chip. 2014;14:1044–1059. doi: 10.1039/C3LC51152A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15:762–766. doi: 10.1111/j.1460-9592.2004.01541.x. [DOI] [PubMed] [Google Scholar]
- 10.Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth. 2010;57:843–848. doi: 10.1007/s12630-010-9338-9. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita K, Fukusaki M, Ando Y, Fujinaga A, Tanabe T, Terao Y, Sumikawa K. Preoperative administration of intravenous flurbiprofen axetil reduces postoperative pain for spinal fusion surgery. J Anesth. 2006;20:92–95. doi: 10.1007/s00540-006-0389-6. [DOI] [PubMed] [Google Scholar]
- 12.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 13.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–461. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 14.Kumpulainen E, Välitalo P, Kokki M, Lehtonen M, Hooker A, Ranta VP, Kokki H. Plasma and cerebrospinal fluid pharmacokinetics of flurbiprofen in children. Br J Clin Pharmacol. 2010;70:557–566. doi: 10.1111/j.1365-2125.2010.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunisawa T, Nagata O, Nagashima M, Mitamura S, Ueno M, Suzuki A, Takahata O, Iwasaki H. Dexmedetomidine suppresses the decrease in blood pressure during anesthetic induction and blunts the cardiovascular response to tracheal intubation. J Clin Anesth. 2009;21:194–199. doi: 10.1016/j.jclinane.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth Analg. 2008;107:1871–1874. doi: 10.1213/ane.0b013e3181887fcc. [DOI] [PubMed] [Google Scholar]
- 17.Kim JE, Kim NY, Lee HS, Kil HK. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull. 2013;36:959–965. doi: 10.1248/bpb.b12-01067. [DOI] [PubMed] [Google Scholar]
- 18.Can M, Gul S, Bektas S, Hanci V, Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53:1068–1072. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 19.Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C, Martin E, Graf BM, Bierhaus A, Weigand MA. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13:R11. doi: 10.1186/cc7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma D, Rajakumaraswamy N, Maze M. alpha2-adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull. 2005;71:77–92. doi: 10.1093/bmb/ldh036. [DOI] [PubMed] [Google Scholar]
- 21.Sun BZ, Chen L, Wu Q, Wang HL, Wei XB, Xiang YX, Zhang XM. Suppression of inflammatory response by flurbiprofen following focal cerebral ischemia involves the NF-κB signaling pathway. Int J Clin Exp Med. 2014;7:3087–3095. [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25(ix):551–570. doi: 10.1016/j.ccc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Hu JK, Zhou ZG, Chen ZX, Wang LL, Yu YY, Liu J, Zhang B, Li L, Shu Y, Chen JP. Comparative evaluation of immune response after laparoscopical and open total mesorectal excisions with anal sphincter preservation in patients with rectal cancer. World J Gastroenterol. 2003;9:2690–2694. doi: 10.3748/wjg.v9.i12.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schietroma M, Rossi M, Fraioli F, Liakos C, Carloni A, Mattucci S, Carlei F, Pistoia MA. Inflammatory markers after laparoscopy versus laparotomy cholecystectomy. Ann Ital Chir. 2001;72:477–482. discussion 482–483. (In Italian) [PubMed] [Google Scholar]
- 25.Zhang N, Liu H, Zhang Z, Wang S, Guo S. The difference of the impacts of surgical approaches on cellular immunity in patients with uterine malignancies: a comparative study of laparoscopy and laparotomy surgery. Gynecol Obstet Invest. 2011;71:177–182. doi: 10.1159/000317255. [DOI] [PubMed] [Google Scholar]
- 26.Xianbao L, Hong Z, Xu Z, Chunfang Z, Dunjin C. Dexmedetomidine reduced cytokine release during postpartum bleeding-induced multiple organ dysfunction syndrome in rats. Mediators Inflamm. 2013;2013:627831. doi: 10.1155/2013/627831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Wang Z, Wang Y, Zhou G, Li H. The effect of dexmedetomidine on inflammatory response of septic rats. BMC Anesthesiol. 2015;15:68. doi: 10.1186/s12871-015-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


