Abstract
The aim of the present study was to examine whether single-nucleotide polymorphisms (SNPs) of β1 subunit of large-conductance Ca2+-activated K+ channel (KCNMB1) and inwardly rectifying K+ channel, subfamily J, member-11 (KCNJ11) are associated with essential hypertension (EH) in Xinjiang Kazak Chinese patients. A polymerase chain reaction-restriction fragment length polymorphism technique was applied to detect the distribution of selected alleles and genotype frequencies in a cohort of Xinjiang Kazak Chinese patients. Samples from 267 patients with EH and 259 normotensive (NT) controls were analyzed. An unconditional logistic regression analysis was used to estimate the odds ratio and 95% confidence interval of the risk factors that are associated with the development of EH. Genotype and allele frequency analyses revealed that the frequency of genotypes KCNJ11-rs2285676 and KCNMB1-rs11739136 was not significantly different between the EH and NT groups. Individuals carrying the GG genotype of KCNJ11-rs5219 had a 2.08 times higher risk of having EH than individuals carrying the GA+AA genotype of KCNJ11-rs5219. Furthermore, the G allele frequency of KCNJ11-rs5219 in the EH group was significantly higher than that of the NT group (P=0.048). Additionally, logistic regression analysis revealed that the body weight and GG genotype of KCNJ11-rs5219 were positively associated with EH in Xinjiang Kazak Chinese patients (P<0.01).
Keywords: essential hypertension, inward rectifier K+ channel, single-nucleotide polymorphism
Introduction
Essential hypertension (EH) is a complex polygenic hereditary disease caused by various genetic and environmental factors (1). In total, 25–40% of the adult population are hypertensive, and >90% of cases are of unknown origin, which are defined as EH (2). An increase of vascular tone in resistance arteries is the basic pathophysiological mechanism of EH (3–5). The large-conductance Ca2+-activated K+ (BKCa) channel, which is composed of an ion-conducting (pore forming) α-subunit and regulatory β-subunit, acts as a negative feedback in the control of vascular tone and blood pressure (6,7).
The β1-regulatory subunit of the BKCa channel is mainly expressed in vascular smooth muscle cells (VSMCs), which is encoded by the large conductance, voltage and Ca2+-sensitive K+ channel subunit β1 (KCNMB1) gene. Furthermore, association of the β1-regulatory subunit with the BKCa channel increases the Ca2+ sensitivity of the channel and decreases voltage dependence (8,9). Additionally, the single-nucleotide polymorphism (SNP) of the KCNMB1 gene, rs11739136 [which causes protein variation of Glu65Lys (KCNMB1-E65K)], increases the probability of the BK-channel opening with Ca2+ stimulation, enhances K+ efflux and membrane hyperpolarization, and reduces artery impedance (10,11).
A previous study on the Han Chinese population demonstrates that a reduced function of BKCa channels with KCNMB1-rs11739136 is associated with EH susceptibility (12). Furthermore, abnormal expression and electrical dysfunction of the adenosine 5-triphosphate (ATP)-sensitive K+ channel (KATP) is also important in the pathophysiology of cardiovascular diseases (13,14). The KATP channel is an inward rectifier K+ channel that is gated by intracellular nucleotides, ATP and adenosine 5-diphosphate (15). It is widely distributed in excitatory tissues, including skeletal cells, VSMCs and neurons, and non-excitatory tissues including renal tubular epithelial cells and oocytes (16,17). Furthermore, the KATP channel in the smooth muscle cells is composed of the inward-rectifier K+ ion channel protein Kir6.2 subunit, the high affinity sulfonylurea receptor (SUR) subunits SUR2B and additional components (15), and is important in the response to stress and changes in blood pressure (18,19).
The Kir6.2 subunit is important in the function of the KATP channel and is encoded by the K+ inwardly rectifying channel, subfamily J, member-11 (KCNJ11) gene (15). Furthermore, the human KCNJ11 gene is located on chromosome 11p15.1 with only one 1173-bp exon and no introns (20,21). Additionally, the KCNJ11 gene mutations KCNJ11-rs5219 and KCNJ11-rs2285676 are able to alter the polarity of the ATP-binding region and decrease channel sensitivity to ATP that causes dysfunction of the KATP channel (22,23). KCNJ11-rs5219 (KCNJ11-E23K) is a missense mutation located in the amino-terminal of the Kir6.2 subunit, and is associated with cardiovascular diseases and diabetes (24,25). To date, several studies have been performed that demonstrate that the KCNJ11-rs5219 gene polymorphism is significantly associated with EH in Chinese and Korean populations (26,27). However, the association between KCNJ11-rs5219 and EH is not understood, particularly in Chinese ethnic populations. Therefore, to the best of our knowledge, an association between KCNJ11-rs2285676 gene polymorphism and EH has not yet been reported in Chinese or other populations, as the distribution of genetic polymorphisms is specific to region and ethnicity. Additionally, it has been reported that the prevalence of hypertension in Kazak Chinese populations is significantly higher than that in Uygur and Han Chinese populations in Xinjiang (28).
Based on the information provided above, the aim of the present study was to genotype three SNPs, namely KCNJ11-rs5219 and KCNJ11-rs2285676 of the KCNJ11 gene, and KCNMB1-rs11739136 of the KCNMB1 gene, in a well-defined population of individuals with EH and a healthy control population. This was conducted in order to investigate whether there is an association between the EH-associated BKCa and KATP channel gene polymorphisms and the development of EH in Xinjiang Kazak Chinese populations, and to observe whether they are important genetic risk factors for EH development in the Kazak Chinese population.
Materials and methods
Subjects
All Kazak subjects were recruited from Dongwan County of the Shawan Region in Xinjiang, China, between October 2012 and October 2013. Samples were obtained from 267 EH subjects (mean age, 48.28±9.14 years; age range, 30–75 years; 118 men and 149 women) and 259 normotensive (NT) subjects (mean age, 46.89±9.60 years; age range, 30–75 years; 99 men and 160 women), who were selected using the method described in a previous case-control study (29). All patients underwent subjective examinations in the morning following an overnight fast. Blood pressure (BP) measurement was performed in the patients dominant arm following >10 min of rest in a sitting position. A hypertensive status was determined according to blood pressure >140/90 mmHg. Furthermore, there was no familial relationship between any subjects. All patients met the criteria of World Health Organization International Society of Hypertension, where hypertension was defined as a systolic blood pressure (SBP) ≥140 mmHg and/or a diastolic blood pressure (DBP) ≥90 mmHg with repeated measurements, or receiving anti-hypertensive medication (30). Routine examination and the analysis of various other factors, including gender, obesity, smoking, duration of EH and hypersensitivity to non-steroid anti-inflammatory drugs were performed in order to establish whether subjects with high BP were primary or secondary in nature.
Subjects with secondary hypertension, endocrine diseases (diabetes), or with heart, liver and kidney diseases were excluded based on their medical history or physical examination. The present study was conducted in accordance with the Declaration of Helsinki and with approval from the Institutional Ethics Review Board (IERB) at the First Affiliated Hospital of the Shihezi University School of Medicine (Shihezi, China; IERB no. SHZ2010LL01). Furthermore, written informed consent was obtained from all subjects, prior to participation.
Source of reagents
A whole blood genomic DNA extraction reagent kit (Blood Genome DNA Extraction kit; cat. no. DP348) was acquired from Tiangen Biotech Co., Ltd. (Beijing, China). Hot Start (HS) Taq polymerase (Premix PrimeSTAR HS version; cat. no. DR040A) was obtained from Takara Biotechnology Co., Ltd., (Dalian, China). All restriction enzymes were provided by New England Biolabs, Inc. [cat. nos. R0119 for BanII; R0196 for NciI and R0102 for MbiI (BsrBI); Ipswich, MA, USA]. All primer synthesis and DNA sequencing of the polymerase chain reaction (PCR) products in the present study were performed by Sangon Biotech Co., Ltd. (Shanghai, China).
Biochemical indices
Clinical characteristics of the study subjects are presented in Table I. Peripheral venous blood was obtained from the antecubital vein of the right arm of subjects while they were sitting, and was then placed in EDTA (200 µl)-coated tubes. Blood was subsequently centrifuged at 1,500 × g for 10 min at 25°C, and then separated plasma was stored at −80°C. Triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were determined using commercially available enzymatic kits (TG kit 4657594190, TC kit 4718917190, HDL-C kit 5401488190 and LDL-C kit 5401682190; Roche Diagnostics, Basel, Switzerland) and a Roche COBAS Integra 800 automated analyzer (Roche Diagnostics).
Table I.
Clinical and biochemical characteristics of study subjects.
| Parameters analyzed | EH (n=267) | NT (n=259) | T/χ2-value | P-value |
|---|---|---|---|---|
| Age, years | 48.28±9.14 | 46.89±9.60 | 1.70 | 0.091 |
| Gender, male/female | 118/149 | 99/160 | 1.93 | 0.164 |
| SBP, mmHg | 148.75±19.46 | 117.85±11.01 | 22.34 | <0.01 |
| DBP, mmHg | 95.67±12.35 | 75.19±7.25 | 21.03 | <0.01 |
| Height, cm | 163.47±8.31 | 161.87±8.59 | 2.17 | 0.030 |
| Weight, kg | 70.18±13.88 | 65.28±12.34 | 4.33 | <0.01 |
| BMI, kg/m2 | 26.27±4.50 | 24.86±3.84 | 3.85 | <0.01 |
| TG, mmol/l | 1.41±0.99 | 1.27±0.87 | 1.77 | 0.077 |
| TC, mmol/l | 4.66±1.20 | 4.52±1.03 | 1.36 | 0.174 |
| LDL-C, mmol/l | 2.35±0.80 | 2.33±0.72 | 0.25 | 0.802 |
| HDL-C, mmol/l | 1.49±0.50 | 1.49±0.42 | 0.08 | 0.938 |
Mean ± standard deviation value for continuous variables. EH, essential hypertension; NT, normaltensive; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
DNA extraction
DNA was extracted from whole peripheral blood using the rapid whole blood genomic DNA extraction kit according to the manufacturers instructions. Genomic DNA integrity was analyzed by 1% agarose gel electrophoresis.
PCR amplification
Primers were designed using Primer Premier 5.0 Software (Premier Biosoft International, Palo Alto, CA, USA) and were synthesized by Sangon Biotech Co., Ltd. PCR amplification of the K+ channel gene was performed using the following primers: rs2285676, forward 5-CAA TTC AGG ACT GGC TCA CC-3 and reverse, 5-GTA GGC TCC ACA GCA CCA AC-3; and rs5219, forward 5-GAC TCT GCA GTG AGG CCC TA-3 and reverse 5-ACG TTG CAG TTG CCT TTC TT-3.
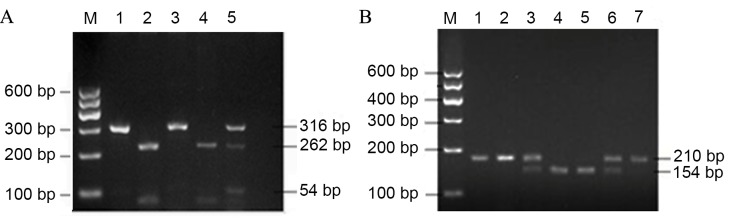
As there was no appropriate restriction endonuclease on the KCNMB1-rs11739136 gene, the present study introduced a mismatched base C on the 27th base of the forward primer according to Zhao et al (12) and to lead into the recognition site of restriction endonuclease MbiI. The forward primer of rs11739136 was 5-GGT ACT GGG GCA CCT TCT TGC ACT TCC GCT-3, and the reverse primer was 5-CGG GGA CTG TGG GGT CAT GTG CCT TT-3. Amplification was performed using an Eppendorf Mastercycler Gradient 5331 thermocycler (Eppendorf, Hamburg, Germany) in a 25 µl volume containing 50 ng DNA, 12.5 µl 2X PrimeSTAR HS [0.05 U/µl PrimeSTAR HS DNA Polymerase, 2X PrimeSTAR Buffer, 2 mM MgCl2 and 2X dNTP mixture (0.4 mM of each dNTP)], 0.1 M of each primer and double-distilled water. The PCR reaction consisted of 35 cycles of 95°C for 5 min, followed by 95°C for 30 sec, 58°C for 30 sec, 72°C for 40 sec for 35 cycles and 72°C for 8 min. The PCR products were subsequently stored at 4°C. PCR products were determined by 1% agarose gel electrophoresis. Following amplification, the lengths of PCR amplified products were 210, 316 and 256 bp for KCNJ11-rs5219, KCNJ11-rs2285676 and KCNMB1-rs11739136, respectively (Figs. 1 and 2).
Figure 1.
PCR products of KCNJ11-rs2285676 and KCNJ11-rs5219 polymorphisms. (A) PCR products of KCNJ11-rs2285676 were digested by the NciI enzyme. M, marker; 1 and 3, TT genotype; 2 and 4, CC genotype; and 5, CT genotype. (B) PCR products of KCNJ11-rs5219 polymorphisms were digested by the BanII enzyme. M, marker; 4 and 5, GG genotype; 3 and 6, GA genotype; and 1, 2 and 7, AA genotype. PCR, polymerase chain reaction; KCNJ11, inwardly rectifying K+ channel, subfamily J, member-11.
Figure 2.
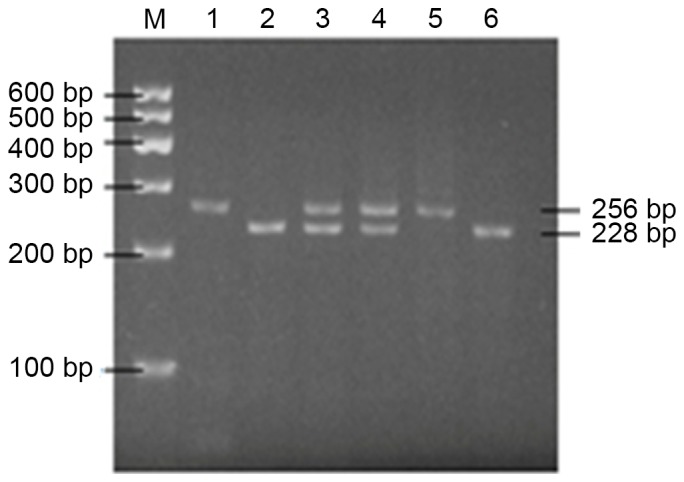
Polymerase chain reaction products of the β1 subunit of large-conductance Ca2+-activated K+ channel-rs11739136 polymorphism were digested by the MbiI enzyme. M, marker; 1 and 5, TT genotype; 2 and 6, CC genotype; and 3 and 4, CT genotype.
Restriction enzyme and screening analysis
Digestion was performed using an Eppendorf Mastercycler Gradient 5331 thermocycler (Eppendorf, Hamburg, Germany) in a 30 µl volume mixture including 10 µl PCR product, 16 µl nuclease-free water, 3 µl 10X CutSmart® Buffer (supplied with the enzyme) and 1 µl restriction enzyme (10 U/µl). The PCR products of KCNJ11-rs5219, KCNJ11-rs2285676 and KCNMB1-rs11739136 were digested with enzyme BanII, NciI and MbiI, respectively. The 30-µl mixtures were incubated at 37°C for 3 h. Restricted DNA products were then separated by 2.5% agarose gel electrophoresis and visualized under UV light. With regard to the SNP KCNJ11-rs2285676 (C/T), a single 316 bp band indicated homozygosity for the T allele (TT); two bands, 262 and 54 bp, indicated homozygosity for the C allele (CC); and the presence of three bands, 316, 262 and 54 bp, indicated heterozygosity for the T or C allele (CT) (Fig. 1A). As for the SNP KCNJ11-rs5219 (G/A), the presence of a single 210 bp band indicated homozygosity for the A allele (AA); the presence of two fragments, 154 and 56 bp, indicated homozygosity for the G allele (GG); and three fragments, 210, 154 and 56 bp indicated heterozygosity for the G and A alleles (Fig. 1B). SNP of KCNMB1-rs11739136 through PCR-restriction fragment length polymorphism (PCR-RFLP) experiments generated three types of bands (Fig. 2): One band indicates homozygosity for the T allele (TT genotype), with a fragment length of 256 bp; three bands indicate heterozygosity for the T or C allele (CT genotype), with fragment lengths of 256, 228 and 28 bp; and two bands indicates homozygosity for the C allele (CC genotype), with fragment lengths of 228 and 28 bp.
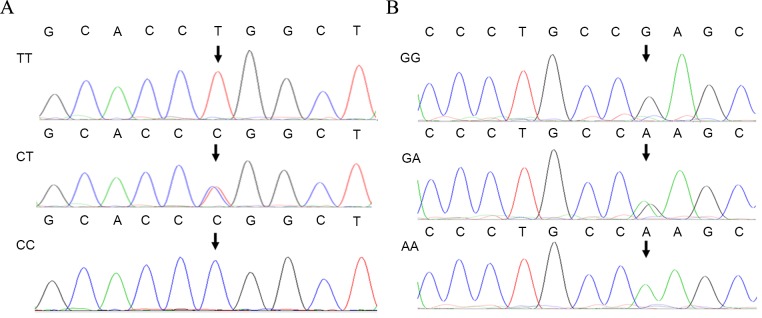
The three types of genotypes of KCNJ11-rs2285676 (TT, CT and CC), KCNJ11-rs5219 (AA, GA and GG) and KCNMB1-rs11739136 (TT, CT and CC) following digestion were consistent with the results of DNA sequencing (Figs. 3 and 4). For DNA sequencing of the PCR product, the purified product was sent for Sanger DNA sequencing at Sangon Biotech, Co., Ltd. DNA sequence alignment was analyzed against the KCNJ11 and KCNMB1 reference sequences using ClustalW2 (European Molecular Biology Laboratory, European Bioinformatics Institute, Hinxton, UKs. Additionally, the genotype polymorphism distribution of each group conformed to the Hardy-Weinberg Equilibrium (HWE).
Figure 3.
Sequence chromatograms of KCNJ11-rs2285676 and KCNJ11-rs5219 polymorphisms. (A) Sequence chromatograms of inwardly rectifying K+ channel, subfamily J, member-11 (KCNJ11)-rs2285676 SNP. (B) Sequence chromatograms of KCNJ11-rs5219 SNP. KCNJ11, inwardly rectifying K+ channel, subfamily J, member-11.
Figure 4.
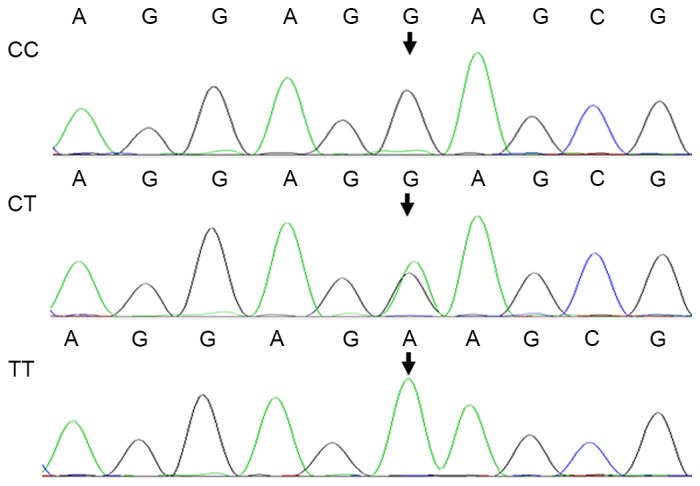
Reverse sequence chromatograms of the β1 subunit of large-conductance Ca2+-activated K+ channel-rs11739136 polymorphism.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA) and data for clinical and biochemical characteristics are presented as the mean ± standard deviation. Comparisons of continuous variables with normal distribution or two groups of mean values between EH and NT groups were performed using an independent Students t-test. If the data did not conform to a normal distribution, the data were expressed as median and quartiles.
In addition, χ2 analyses was applied to determine the difference in genotype and gene frequency. Unconditional backward stepwise logistic regression adjusted for age, gender and body mass index (BMI) was performed to determine the risk factors of EH and screen the independent variables. Additionally, hypertension was used as the dependent variable and risk factors associated with hypertension were used as the independent variables. In each model, the odds ratio (OR) for each independent variable with a 95% confidence interval (CI) was determined from unconditional logistic regression models. For the independent variable, P<0.05 was considered to indicate a statistically significant difference. The frequencies of each polymorphic site were estimated by the allele counting method and tested for HWE. Furthermore, HWE was assessed by the χ2 test to compare the observed and expected genotype frequencies among the subjects. χ2 or Fishers exact tests were used to compare the distribution of genotype and allele frequency between groups. Statistical significance was tested using χ2 and P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical and biochemical characteristics
In the present study, 267 patients with EH patients (118 males and 149 females) were diagnosed and the mean age of EH patients was 48.28±9.14 years with a mean BMI of 26.27±4.50 kg/m2. A total of 259 healthy volunteers (controls; 99 males and 160 females) were also recruited, and their mean age was 46.89±9.60 years with a mean BMI of 24.86±3.84 kg/m2 (Table I). Table I describes the clinical and biochemical characteristics of the EH patients and controls. A significant difference was observed in the SBP, DBP, height, weight and BMI between hypertensive and NT subjects (all P<0.01). The mean values of BMI (t=3.85, P<0.01), SBP (t=22.34, P<0.01) and DBP (t=21.03, P<0.01) were significantly elevated in EH patients compared with NTs. This indicates that BMI may affect the development of EH. In addition, no significant difference was detected in terms of gender, age, TG, TC, HDL-C and LDL-C between the EH control and NT groups (Table I).
Genotype and allele frequency analysis
To analyze the frequency of the genotypes for tag SNPs in the KCNMB1 and KCNJ11 genes, PCR-RFLP analysis was performed. PCR products corresponding to KCNJ11-rs2285676, KCNJ11-rs5219 and KCNMB1-rs11739136 were digested by NciI, BanII and MbiI restriction endonucleases, respectively. KCNJ11-rs2285676 polymorphisms were categorized into three genotypes: TT (316 bp), CT (316, 262 and 54 bp) and CC (262 and 54 bp) (Fig. 1A). KCNJ11-rs5219 polymorphisms were categorized into AA (210 bp), GA (210, 154 and 56 bp) and GG (154 and 56 bp) (Fig. 1B). KCNMB1-rs11739136 polymorphisms were categorized into CC (228 and 28 bp), CT (256, 228 and 28 bp) and TT (256 bp) (Fig. 2). It was noted that the 54-, 56-, and 28 bp fragments were too short to be visible. Results of restriction endonuclease analysis were compared with DNA sequencing data and lead to the corresponding results (Figs. 3 and 4).
In order to study the association between the genotype and allele frequency distributions in the KCNJ11-rs2285676, KCNJ11-rs5219 and KCNMB1-rs11739136 SNPs, and the risk of EH, the genotype and frequencies of occurrence of the polymorphic forms of KCNJ11-rs2285676, KCNJ11-rs5219 and KCNMB1-rs11739136 was analyzed. The distribution of the genotype frequencies of KCNJ11-rs5219, KCNJ11-rs2285676 and KCNMB1-rs11739136 in the EH and NT groups is in accordance with the HWE.
Three SNPS were genotyped in 526 individuals of the overall sample. As shown in Table II, for the KCNJ11-rs2285676 SNP, the frequency of the CC and (CT+TT) genotypes was 6.95 and 93.05% in the controls, and 4.12 and 95.88% in patients, respectively. Furthermore, there was no statistically significant difference in the genotype frequencies [CC vs. CT+TT, OR=0.58 (95% CI, 0.27–1.24); χ2=2.02; P=0.155] and allele frequencies [C vs. T, OR=1.06 (95% CI, 0.81–1.39); P=0.655] distribution between the EH and NT groups. However, for KCNJ11-rs5219, a significantly higher frequency of the rs5219 polymorphism GG genotype was shown in the EH patients group compared with the control group (34.83 vs. 20.46%, respectively; P<0.01). Furthermore, a significantly lower frequency of the KCNJ11-rs5219 polymorphism GA+AA genotype was observed in the group of EH patients in comparison with the healthy population (65.17 vs. 79.54%; P<0.01). The frequencies of KCNJ11-rs5219 G and A alleles in the EH group were 61.42 and 38.58%, respectively. Finally, the KCNJ11-rs5219 G allele distribution of the EH group was significantly higher than that of the NT group [G vs. A, OR, 1.28 (95% CI, 1.00–1.64); χ2=3.92; P=0.048]. Relative risk analysis demonstrated that subjects carrying the rs5219 GG genotype were at 2.08 times greater risk to have EH than the (GA+AA) genotype carriers (95% CI, 1.40–3.08).
Table II.
Genotype and allele frequencies of inwardly rectifying K+ channel, subfamily J, member-11 and β1 subunit of large-conductance Ca2+-activated K+ channel SNPs.
| SNP | Gene | EH (n=267) | NT (n=259) | P-value | χ2 | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| rs2285676 | Genotype | ||||||
| CC | 11 (4.12%) | 18 (6.95%) | |||||
| CT | 131 (49.06%) | 106 (40.93%) | |||||
| TT | 125 (46.82%) | 135 (52.12%) | |||||
| CT+TT | 256 (95.88%) | 241 (93.05%) | 0.155 | 2.02 | 0.58 | 0.27–1.24 | |
| Allele | |||||||
| C | 153 (28.65%) | 142 (27.41%) | |||||
| T | 381 (71.35%) | 376 (72.59%) | 0.655 | 0.20 | 1.06 | 0.81–1.39 | |
| rs5219 | Genotype | ||||||
| GG | 93 (34.83%) | 53 (20.46%) | |||||
| GA | 142 (53.18%) | 181 (69.88%) | |||||
| AA | 32 (11.99%) | 25 (9.66%) | |||||
| GA+AA | 174 (65.17%) | 206 (79.54%) | <0.01 | 13.54 | 2.08 | 1.40–3.08 | |
| Allele | |||||||
| G | 328 (61.42%) | 287 (55.41%) | |||||
| A | 206 (38.58%) | 231 (44.59%) | 0.048 | 3.92 | 1.28 | 1.00–1.64 | |
| rs11739136 | Genotype | ||||||
| CC | 187 (70.04%) | 165 (63.71%) | |||||
| CT | 67 (25.09%) | 76 (29.34%) | |||||
| TT | 13 (4.87%) | 18 (6.95%) | |||||
| CT+TT | 80 (29.96%) | 94 (36.29%) | 0.123 | 2.38 | 1.33 | 0.93–1.92 | |
| Allele | |||||||
| C | 441 (82.58%) | 406 (78.38%) | |||||
| T | 93 (17.42%) | 112 (21.62%) | 0.085 | 2.97 | 1.31 | 0.96–1.78 |
SNP, single nucleotide polymorphism; EH, essential hypertension; NT, normaltensive; OR, odds ratio; CI, confidence interval.
The KCNMB1-rs11739136 variant in EH patients of Xinjiang Kazak Chinese origin was also assessed, as shown in Table II. The genotype frequencies for CC and CT+TT in EH patients vs. NT subjects were 70.04 and 29.96% vs. 63.71 and 36.29%. Furthermore, there were no statistically significant differences in the genotypic distribution and the allelic frequency between EH and NT subjects [CC vs. CT+TT, OR=1.33 (95% CI, 0.93–1.92); P=0.123, and C vs. T, OR=1.31 (95% CI, 0.96–1.78); P=0.085].
Logistic regression analysis
As the GG genotype of the KCNJ11-rs5219 SNP in the EH group was significantly higher than that of the NT group, the present study assessed the association between the GG genotype and the risk of EH by logistic regression analysis after adjusting for BMI. This individual polymorphism combined with BMI were revealed to be significantly associated with EH (P<0.01; Table III). Therefore, in the BMI-adjusted model GG genotype [OR=2.15 (95% CI, 1.44–3.21); P<0.05] and BMI [OR=1.09 (1.04–1.1365), P<0.01] increased the risk of EH in Xinjiang Kazak Chinese individuals.
Table III.
Multivariate logistic regression analysis of hypertension in Xinjiang Kazak.
| Factors | B | SE | Walds | P-value | OR value | 95% CI |
|---|---|---|---|---|---|---|
| BMI | 0.084 | 0.022 | 14.719 | <0.01 | 1.09 | 1.04–1.1365 |
| rs5219 GG Genotype | 0.765 | 0.204 | 14.073 | <0.01 | 2.15 | 1.44–3.21 |
BMI, body mass index; SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
EH is a complex cardiovascular disease caused by the interaction of polygenic inheritance with environmental risk factors. The Kazak population from Xinjiang has a relatively unique lifestyle, which includes eating more animal fat, a high protein and high-salt diet, and lower consumption of fresh fruit and vegetables (31). Furthermore, the genetic background of the Kazak population is distinct from the Han population, and may lead to differences in genes and diseases caused by genetic susceptibility, including hypertension (31). The genetic variants studied in the present study are useful in the validation of individuals that have a high risk for EH, and this may provide useful information for disease diagnosis and prevention. In the present study, the SBP, DBP and BMI were significantly different between NT subjects and EH patients, indicating that the general clinical data were significantly different between Kazak EH patients and the Kazak NT group. In the group of EH patients, SBP and DBP were higher than that of the patients from the NT group and, therefore, may contribute to increased mortality from cardiovascular pathology (32). The mean BMI of Kazak EH patients was significantly greater than that of NT controls, which may result from the high-fat diet of the Kazak population. This result suggested that hypertension is associated with lipid metabolism.
As there are substantial ethnic and regional differences in the distribution of gene polymorphisms, a Kazak Chinese population in Xinjiang was selected as the research cohort. Kazak Chinese individuals have minimal population migration and geographical environment separation, and similar genetic backgrounds; these features benefitted the study of the association between KCNJ11 and KCNMB1 gene polymorphism and EH. In the present case-control study, the association of KCNJ11 (rs5219 and rs2285676) and KCNMB1 (rs11739136) gene polymorphisms with EH was explored in a Kazak population. Previous studies have demonstrated that there is an association between different KCNJ11 SNPs and EH and diabetes in different populations (33–35). However, to the best of our knowledge, there are no studies that have been performed with these SNPs in the Kazak population. In an animal model of hypertension, knocking out the KCNJ11 gene has been demonstrated to induce heart failure and mortality (36). Therefore, the KCNJ11-encoded KATP channel is essential to prevent hypertension, with channel alteration being a molecular basis for EH.
The polymorphic loci in KCNJ11 E23K or rs5219g.67 (G>A) are associated with the risk of hypertension as well as the risk of type 2 diabetes mellitus (T2DM) in the Korean population (27). In addition, Yi et al (14) have previously reported that the EE (E:Glu) genotype of KCNJ11-E23K (rs5219) was associated with body weight, BMI, VO2 max and maximal minute ventilation in elderly females, as well as DBP in men at rest. Previously, a meta-analysis study demonstrated that the KCNJ11-rs5219 genotype is independently associated with hypertension in East Asian populations (37). Zhuang et al (38) further demonstrated that the SBP of homozygote GG individuals is higher than that of homozygous AA individuals, and the GG genotype increases the risk for hypertension in the Han Chinese population. Consistent with these previous studies, the present study also demonstrated that the frequency of the GG (rs5219) genotype and allele of the KCNJ11-rs5219 SNP site was significantly different between Kazak EH patients and NT controls. The relative risk analysis demonstrated that the individuals carrying the rs5219-GG genotype have a greater risk of developing EH than those individuals carrying the GA+AA genotype. This may be caused by a missense mutation of KCNJ11-rs5219, which would lead to glutamic acid (E) replaced by lysine (K), which would thereby reduce KATP channel sensitivity to ATP and induce KATP channel excessive activation, enhance K+ efflux and membrane hyperpolarization, and cause vasodilation (39,40). Therefore, genetic E23K variants which determine open state probability also determine vascular tone and consequently affect blood pressure. Furthermore, in a sample of this size, the single SNP and EH associations are statistically distinguishable therefore it may be determined that the signal is arising from this SNP where it sits. Therefore, the GG (rs5219) genotype may increase the risk of EH in the Kazak population.
At present, the correlation between KCNJ11-rs2285676 SNPs and hypertension has not been studied extensively. However, the KCNJ11-rs2285676 polymorphism has been demonstrated to be associated with T2DM in Japanese and Korean populations, as well as in a Chinese Han population (41). A recent study found that the T2DM patients that benefitted from dipeptidyl peptidase-4 inhibitor treatment were those with a KCNJ11 rs2285676 (genotype CC) polymorphism and DBP values within normal ranges (41). The present studys data of risk factor analysis suggests that KCNJ11-rs2285676 genotypes were not associated with EH. Whether the KCNJ11-rs2285676 SNPs are associated with EH requires further study. BKCa channel β-subunits have four different subtypes (β1-4), and the β1-subunit is predominantly expressed in the smooth muscle cell (42). It regulates the activity of the BKCa channel and increases vasodilatation (43,44). Furthermore, it has been reported that reduction or deletion of the β1 subunit causes membrane depolarization and vascular smooth muscle contraction, which results in cardiovascular disorders, including hypertension and heart failure (45,46).
Gain-of-function mutation of the β1-subunit KCNMB1 (rs11739136-Glu65Lys mutant) was caused by a single nucleotide substitution in exon-3 of KCNMB1 (47). The correlation analysis of the KCNMB1-rs11739136 gene mutation and hypertension revealed that the rs11739136 mutation increased BKCa channel sensitivity to Ca2+, which makes blood vessels easier to dilate (47). Nielsen et al (11) reported the KCNMB1 Glu65Lys (rs11739136) polymorphism is associated with reduced SBP and DBP in middle-aged Danish men. In addition, Zhao et al (12) demonstrated that the TT+CT genotype of KCNMB1-rs11739136 had a significantly decreased risk for hypertension. In the present study, the frequency analysis demonstrated that there was no significant difference in the genotype and allele frequency of KCNMB1-rs11739136 between the EH and NT groups. This demonstrates that the SNP sites of KCNMB1-rs11739136 are not associated with the development of EH in the Kazak population. Additionally, the association between KCNMB1-rs11739136 and blood pressure regulation is not affected by age, gender and anti-hypertensive therapy. Therefore, the T allele is a protective factor that decreases the prevalence of hypertension in the Chinese Han population (12).
In conclusion, the presence of the G nucleotide at position g.67 (G>A) of KCNJ11-rs5219 is associated with EH in Chinese Kazak populations in Xinjiang, therefore the higher frequency of A allele in EH cases compared with NT may be a genetic susceptibility factor for the development of EH. Therefore, polymorphisms of KCNJ11-rs5219 may be important in EH susceptibility. However, greater sample sizes are required to confirm this observation and also to identify additional populations. The results of the present study provide a basis for developing novel strategies for the diagnosis and treatment of EH in the Kazak population.
Acknowledgements
The present study was supported by grants from the National Basic Research Program of China (grant no. 2012CB52660000) and the National Natural Science Foundation of China (grant nos. 81560081, 31460264 and 81560175).
References
- 1.Köhler R. Single-nucleotide polymorphisms in vascular Ca2+-activated K+-channel genes and cardiovascular disease. Pflugers Arch. 2010;460:343–351. doi: 10.1007/s00424-009-0768-6. [DOI] [PubMed] [Google Scholar]
- 2.Doris PA. Hypertension genetics, single nucleotide polymorphisms, and the common disease: Common variant hypothesis. Hypertension. 2002;39:323–331. doi: 10.1161/hy0202.104087. [DOI] [PubMed] [Google Scholar]
- 3.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Wang R, Ma KT, Li XZ, Zhang CL, Liu WD, Zhao L, Si JQ. Differential effect of calcium-activated potassium and chloride channels on rat basilar artery vasomotion. J Huazhong Univ Sci Technolog Med Sci. 2014;34:482–490. doi: 10.1007/s11596-014-1303-3. [DOI] [PubMed] [Google Scholar]
- 5.Ma KT, Li XZ, Li L, Jiang XW, Chen XY, Liu WD, Zhao L, Zhang ZS, Si JQ. Role of gap junctions in the contractile response to agonists in the mesenteric artery of spontaneously hypertensive rats. Hypertens Res. 2014;37:110–115. doi: 10.1038/hr.2013.120. [DOI] [PubMed] [Google Scholar]
- 6.Yi F, Wang H, Chai Q, Wang X, Shen WK, Willis MS, Lee HC, Lu T. Regulation of large conductance Ca2+-activated K+ (BK) channel β1 subunit expression by muscle RING finger protein 1 in diabetic vessels. J Biol Chem. 2014;289:10853–10864. doi: 10.1074/jbc.M113.520940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1-/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel beta-subunit genes: Cloning and characterization. Genomics. 1999;55:57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Garver H, Galligan JJ, Fink GD. Large-conductance Ca2+-activated K+ channel beta1-subunit knockout mice are not hypertensive. Am J Physiol Heart Circ Physiol. 2011;300:H476–H485. doi: 10.1152/ajpheart.00975.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley-Hedgepeth A, Peter I, Montefusco MC, Levy D, Benjamin EJ, Vasan RS, Mendelsohn ME, Housman D, Huggins GS, Mitchell GF. The KCNMB1 E65K variant is associated with reduced central pulse pressure in the community-based Framingham Offspring Cohort. J Hypertens. 2009;27:55–60. doi: 10.1097/HJH.0b013e328317c8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen T, Burgdorf KS, Grarup N, Borch-Johnsen K, Hansen T, Jørgensen T, Pedersen O, Andersen G. The KCNMB1 Glu65Lys polymorphism associates with reduced systolic and diastolic blood pressure in the Inter99 study of 5729 Danes. J Hypertens. 2008;26:2142–2146. doi: 10.1097/HJH.0b013e32830b894a. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q, Wang L, Yang W, Chen S, Huang J, Fan Z, Li H, Lu X, Gu D. Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics. 2008;18:459–466. doi: 10.1097/FPC.0b013e3282f97fb2. [DOI] [PubMed] [Google Scholar]
- 13.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/S0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 14.Yi Y, Dongmei L, Phares DA, Weiss EP, Brandauer J, Hagberg JM. Association between KCNJ11 E23K genotype and cardiovascular and glucose metabolism phenotypes in older men and women. Exp Physiol. 2008;93:95–103. doi: 10.1113/expphysiol.2007.038893. [DOI] [PubMed] [Google Scholar]
- 15.Haghvirdizadeh P, Mohamed Z, Abdullah NA, Haghvirdizadeh P, Haerian MS, Haerian BS. KCNJ11: Genetic Polymorphisms and Risk of Diabetes Mellitus. J Diabetes Res. 2015;2015:908152. doi: 10.1155/2015/908152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan R, Cui W, Wang H. Mutational analysis of the Kir6.1 gene in Chinese hypertensive patients treated with the novel ATP-sensitive potassium channel opener iptakalim. Exp Ther Med. 2011;2:757–760. doi: 10.3892/etm.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banas K, Clow C, Jasmin BJ, Renaud JM. The KATP channel Kir6.2 subunit content is higher in glycolytic than oxidative skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2011;301:R916–R925. doi: 10.1152/ajpregu.00663.2010. [DOI] [PubMed] [Google Scholar]
- 18.Haider S, Antcliff JF, Proks P, Sansom MS, Ashcroft FM. Focus on Kir6.2: A key component of the ATP-sensitive potassium channel. J Mol Cell Cardiol. 2005;38:927–936. doi: 10.1016/j.yjmcc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury UR, Holman BH, Fautsch MP. ATP-sensitive potassium (K(ATP)) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 2013;54:4892–4899. doi: 10.1167/iovs.13-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan YH, Chen FX. Structure, function and modulation of KATP channels. Journal of Medical Molecular Biology. 2007;4 [Google Scholar]
- 21.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 22.Xia XH, Yang AH, Hu Y. Effects of E23K polymorphism in KCNJ11 gene on membrane current. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2014;30:23–26. (In Chinese) [PubMed] [Google Scholar]
- 23.Jeron A, Hengstenberg C, Holmer S, Wollnik B, Riegger GA, Schunkert H, Erdmann J. KCNJ11 polymorphisms and sudden cardic death in patient with acute myocardial infarction. J Mol Cell Cardiol. 2004;36:287–293. doi: 10.1016/j.yjmcc.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Xi HL, Liu JF, Li L, Wan J. Relationship between dilated cardiomyopathy and the E23K and I337V polymorphisms in the Kir6.2 subunit of the KATP channel. Genet Mol Res. 2013;12:4383–4392. doi: 10.4238/2013.October.10.4. [DOI] [PubMed] [Google Scholar]
- 25.Qin LJ, Lv Y, Huang QY. Meta-analysis of association of common variants in the KCNJ11-ABCC8 region with type 2 diabetes. Genet Mol Res. 2013;12:2990–3002. doi: 10.4238/2013.August.20.1. [DOI] [PubMed] [Google Scholar]
- 26.Li JY, Li ZB, Zhu M, Liu YQ, Li Y, Wang SW, Zhu QL. Mutational analysis of KCNJ11 in Chinese elderly essential hypertensive patients. J Geriatr Cardiol. 2012;9:153–157. doi: 10.3724/SP.J.1263.2011.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo BK, Cho YM, Park BL, Cheong HS, Shin HD, Jang HC, Kim SY, Lee HK, Park KS. Polymorphisms of KCNJ11 (Kir6.2 gene) are associated with Type 2 diabetes and hypertension in the Korean population. Diabet Med. 2007;24:178–186. doi: 10.1111/j.1464-5491.2006.02050.x. [DOI] [PubMed] [Google Scholar]
- 28.Ma XJ, Zhang M, Guo SX, Ma RL, Ding YS, Guo H, Zhang JQ, Niu Q, Liu JM, Li SG, et al. Prevalence of hypertension in Uygur, Kazakhs, and Han people in rural areas of Xinjiang. Chin J Hypertension. 2013;21:1164–1168. [Google Scholar]
- 29.He F, Zhao D, Deng F, Zhong H, Shi X, Yang J, Guo S, Cheng J, Huang G, Tang B, et al. Association of TGF-beta1 gene polymorphisms in exon1 and blood levels with essential hypertension. Blood Press. 2010;19:225–233. doi: 10.3109/08037051003768254. [DOI] [PubMed] [Google Scholar]
- 30.Whitworth JA. World Health Organization, International Society of Hypertension Writing Group: 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Wang H, Yan Z, Yao X, Hong J, Zhou L. Ethnic disparities in the clustering of risk factors for cardiovascular disease among the Kazakh, Uygur, Mongolian and Han populations of Xinjiang: A cross-sectional study. BMC Public Health. 2012;12:499. doi: 10.1186/1471-2458-12-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grygiel-Górniak B, Kaczmarek E, Mosor M, Przysławski J, Nowak J. Association of PPAR-γ2 and β3-AR Polymorphisms With Postmenopausal Hypertension. J Clin Hypertens (Greenwich) 2015;17:549–556. doi: 10.1111/jch.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhou XO, Zhang Y, Gao PJ, Zhu DL. Association of KCNJ11 with impaired glucose regulation in essential hypertension. Genet Mol Res. 2011;10:1111–1119. doi: 10.4238/vol10-2gmr1127. [DOI] [PubMed] [Google Scholar]
- 34.Khan IA, Vattam KK, Jahan P, Mukkavli KK, Hasan Q, Rao P. Correlation between KCNQ1 and KCNJ11, gene polymorphisms and type 2 and post-transplant diabetes mellitus in the Asian Indian population. Genes Diseases. 2015;2:276–282. doi: 10.1016/j.gendis.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamaluddin JL, Huri HZ, Vethakkan SR. Clinical and genetic predictors of dipeptidyl peptidase-4 inhibitor treatment response in Type 2 diabetes mellitus. Pharmacogenomics. 2016;17:867–881. doi: 10.2217/pgs-2016-0010. [DOI] [PubMed] [Google Scholar]
- 36.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto Y, Inoue H, Keshavarz P, Miyawaki K, Yamaguchi Y, Moritani M, Kunika K, Nakamura N, Yoshikawa T, Yasui N, et al. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. J Hum Genet. 2007;52:781–793. doi: 10.1007/s10038-007-0190-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang L, Zhao Y, Zhao W, Li M, Yu M, Lu M, Zhang R, Ge X, Zheng T, Li C, et al. The E23K and A190A variations of the KCNJ11 gene are associated with early-onset type 2 diabetes and blood pressure in the Chinese population. Mol Cell Biochem. 2015;404:133–141. doi: 10.1007/s11010-015-2373-7. [DOI] [PubMed] [Google Scholar]
- 39.Kawano N, Emoto M, Mori K, Yamazaki Y, Urata H, Tsuchikura S, Motoyama K, Morioka T, Fukumoto S, Shoji T, et al. Association of endothelial and vascular smooth muscle dysfunction with cardiovascular risk factors, vascular complications and subclinical carotid atherosclerosis in type 2 diabetic patients. J Atheroscler Thromb. 2012;19:276–284. doi: 10.5551/jat.10629. [DOI] [PubMed] [Google Scholar]
- 40.Schwanstecher C, Meyer U, Schwanstecher M. K(IR)6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic beta-cell ATP-sensitive K(+) channels. Diabetes. 2002;51:875–879. doi: 10.2337/diabetes.51.3.875. [DOI] [PubMed] [Google Scholar]
- 41.Jamaluddin JL, Huri HZ, Vethakkan SR. Clinical and genetic predictors of dipeptidyl peptidase-4 inhibitor treatment response in Type 2 diabetes mellitus. Pharmacogenomics. 2016;17:867–881. doi: 10.2217/pgs-2016-0010. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constitutents of maxi KCa channels in human coronary smooth muscle: Predominat alpha + beta subunit complexes. J Physiol. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruslova A, Semenov I, Wang B. An extracellular domain of the accessory β1 subunit is required for modulating BK channel voltage sensor and gate. J Gen Physiol. 2012;139:57–67. doi: 10.1085/jgp.201110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweet TB, Cox DH. Measuring the influence of the BKCa {beta}1 subunit on Ca2+ binding to the BKCa channel. J Gen Physiol. 2009;133:139–150. doi: 10.1085/jgp.200810129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amberg GC, Santana LF. Down regulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 46.Wan E, Kushner JS, Zakharov S, Nui XW, Chudasama N, Kelly C, Waase M, Doshi D, Liu G, Iwata S, et al. Reduced vascular smooth muscle BK channel current underlies heart failure-induced vasoconstriction in mice. FASEB J. 2013;27:1859–1867. doi: 10.1096/fj.12-223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-Fernández JM, Tomás M, Vázquez E, Orio P, Latorre R, Sentí M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113:1032–1039. doi: 10.1172/JCI200420347. [DOI] [PMC free article] [PubMed] [Google Scholar]