Abstract
Effects of zerumbone on chronic gastritis remain unclear. The purpose of this study was to investigate the mechanism of the protective effect of zerumbone on the treatment of chronic gastritis in rats. The animal models of chronic gastritis in rats were established, and the surface damage of gastric mucosa was observed by gross anatomy; the changes of gastric mucosal tissue and surface morphology were observed by pathological sections of gastric mucosal tissues; the expressions of heme oxygenase-1 (HO-1) and nuclear factor E2-related factor 2 (Nrf-2) proteins of gastric mucosal tissues in each group were detected by western blot analysis; the activities of superoxide dismutase (SOD) and catalase (CAT) as well as the contents of reduced glutathione (GSH) and malondialdehyde (MDA) in gastric mucosal tissues were detected by kits. The results indicated that zerumbone could significantly relieve red and swelling as well as erosion of the gastric mucosal tissues in rats with chronic gastritis; zerumbone could significantly ameliorate the loose arrangement of cells in the lamina propria of gastric mucosa, epithelial cell deformation and abscission, and inflammatory cell infiltration. The results of western blot analysis showed that compared with the model group, zerumbone could significantly upregulate the expression of HO-1 and Nrf-2 in gastric mucosal tissues. Compared with the model group, the activities of SOD and CAT as well as GSH levels in gastric mucosal tissues of rats in the zerumbone groups were obviously increased, but MDA contents were significantly decreased. Zerumbone has a protective effect on chronic gastritis in rats, which is achieved by improving the antioxidant capacity of gastric mucosal tissues through inhibiting lipid peroxidation.
Keywords: gastritis, antioxidant, lipid peroxidation, superoxide dismutase
Introduction
Gastritis is a common digestive tract disease in clinical practice, in which chronic gastritis has a higher incidence rate and various therapeutic drugs, but the treatment effect is poor and it can easily relapse (1). According to the characteristics of pathology and course of disease, gastritis is often divided into two categories, namely acute and chronic gastritis; acute gastritis, which is mainly caused by stimulating food, medicine, chemical corrosion reagent, bacterial infection and stress reaction, is divided into acute irritated gastritis, corrosive gastritis, acute infectious gastritis and acute hemorrhagic gastritis; chronic gastritis has very complex etiology and mechanisms, and it is generally caused by multiple factors (2), among which the hypothesis that gastric mucosa damaged by oxygen free radicals has been gradually accepted by people; oxygen free radical is an important pathogenic factor that can cause the injury of gastric mucosa mainly through lipid peroxidation and covalent bonding (3).
Zerumbone, as a natural compound extracted from Zingiber zerumbet which belongs to a plant in the Zingiberaceae, is mainly applied in medicine, food and spices, electronic industry and other fields at present (4). The studies have confirmed that zerumbone has anti-inflammatory and antitumor effects, which may be achieved by inhibiting inflammation and signal pathway during tumor development process (5,6). According to further studies, it has been proved that zerumbone exerts its anti-inflammatory effect mainly through inhibiting pro-inflammatory genes and antioxidant pathways (7,8). Additionally, the literature reported that zerumbone can be used in the treatment of ulcerative colitis and inflammatory bowel disease in animals, and it exerts an anti-inflammatory effect by inhibiting cyclooxygenase, prostaglandin and interleukin (9).
It has not been reported in the literature whether zerumbone has a therapeutic effect on chronic gastritis caused by multiple factors. Therefore, we established models of chronic gastritis in rats on the basis of stimulating rats by ethanol, ammonia and sodium deoxycholate in this experiment, and then investigated the protective effect of zerumbone on gastric mucosal tissues in rats with chronic gastritis as well as its possible mechanism, so as to confirm and clarify the mechanism of zerumbone on the treatment of chronic gastritis. In this experiment, we observed the protective effect of zerumbone on gastric mucosal tissues of rats by gross anatomy and pathological section, further detected the expressions of heme oxygenase-1 (HO-1) and nuclear factor E2-related factor-2 (Nrf-2) proteins in gastric mucosal tissues, and determined the activities of superoxide dismutase (SOD) and catalase (CAT) as well as the contents of reduced glutathione (GSH) and malondialdehyde (MDA) in gastric mucosal tissues by kits, thereby elucidating the mechanism of the protective effect of zerumbone on chronic gastritis in rats and providing the research basis for the treatment of chronic gastritis using zerumbone.
Materials and methods
Materials
The materials included, zerumbone and omeprazole (both from Sigma, New York, NY, USA); sodium carboxymethyl cellulose (CMC), sodium deoxycholate, ammonia and ethanol (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China); SOD, CAT, GSH and MDA assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China); HO-1 and Nrf-2 antibodies, HRP-labeled secondary antibody (Proteintech Group, Inc., Wuhan, China); BCA protein quantitation kit, tissue lysis solution (Beyotime Institute of Biotechnology, Nantong, China) and male Sprague-Dawley (SD) rats (BetterBiotechnology Co., Ltd., Nanjing, China).
Preparation of experimental rat models of chronic gastritis
Forty-eight healthy male SD rats with weight of 160–180 g were selected. All the animals were fed under conditions of 22±2°C at room temperature, and 40–60% in environmental relative humidity, and they were daily administered with artificial illumination and dark treatment, each of 12 h. Rats could drink freely, and were fed with feedstuff. The experiment started after rats were fed for 7 days. Forty rats were randomly selected to be used for establishing models. By using multifactor modeling, the specific methods were as follows: Ammonia (0.05%) could be taken freely; gastric perfusion with 2 ml sodium deoxycholate at a concentration of 20 mmol/l was administered to rats at 9 a.m. every Sunday; and, 2 ml 60% ethanol was given for gastric perfusion at 4 p.m. every Tuesday. This study was approved by the Animal Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine.
Experimental grouping, drug administration and materials
After 10 weeks of modeling, 40 rats were randomly divided into 5 groups with 8 animals in each group, namely model group, omeprazole-positive drug group, high-dose zerumbone group, middle-dose zerumbone group and low-dose zerumbone group, while 8 rats without established modeling were set as normal control group. Zerumbone was continuously administered to rats for 4 weeks, and gastric perfusion was conducted every day, with the volume of 1 ml/200 g; therein, 5% CMC was used as solvent for gastric perfusion in normal group and model group; 20 mg/kg omeprazole was taken in positive drug group, and zerumbone with doses of 20, 10 and 5 mg/kg were, respectively, adopted in high-, middle- and low-dose zerumbone groups. After 4 weeks of drug administration, rats were fasted for 12 h. Subsequently, animals were anesthetized by 10% chloral hydrate, the thoracic cavity and abdomen were opened up, and ligation was conducted at 0.5 cm below pyloric ostium. Pre-cooling phosphate-buffered saline (PBS) (2 ml) was taken for injection at cardia, and then the cardia was ligated. The stomach was taken out and the animal was sacrificed. Part of the gastric tissues was taken for fixation, and remaining gastric tissues were reserved at −20°C.
Anatomical observation of gastric mucosal injury in rats
The stomach of rat was cut open along with greater curvature and placed on a plastic whiteboard. After it was washed clean by 0.9% pre-cooling normal saline, the surface damage of gastric mucosa was observed, including bleeding, erosion and ulcer. Then images were taken by a digital camera (Canon JVC TDK; Cannon, Tokyo, Japan).
Observation of pathologic sections of gastric mucosal tissues in rats
The sample was taken from the gastric gland of rat, and fixed by 10% formalin for 24 h. Then it was dehydrated using graded ethanol, vitrification by dimethylbenzene and embedded by paraffin. The sample was cut into slices, and the section was stained with hematoxylin and eosin (H&E). Subsequently, pathological changes of the tissues were observed under a light microscope (BX-42; Olympus, Tokyo, Japan), such as deformation, hyperemia and inflammatory cell infiltration, followed by imaging.
Detecting the expressions of HO-1 and Nrf-2 proteins in gastric tissues by western blot analysis
About 50 mg tissues were respectively taken from the reserved tissues in each group, followed by adding tissue lysis solution containing protease inhibitor. They were placed on ice and fully ground. Then tissues were centrifuged at 12,000 × g for 15 min at 4°C. Thereafter, supernatant was taken for detecting protein concentration. The protein with the protein quantity as 40 µg was taken from each group, followed by electrophoresis and being transferred to membrane. Then the samples were sealed by 5% bovine serum albumin (BSA), and primary antibodies of HO-1 and Nrf-2 (diluting according to the proportion of 1:1,000) were respectively added, at 4°C overnight. Then secondary antibody was added, and incubation was performed at room temperature. After 2 h, development was conducted by electrochemiluminescence (ECL) developing solution in a dark room, followed by scanning and imaging, and then grey level analysis was performed with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal reference.
Detection of SOD, CAT, GSH and MDA in gastric tissues of rats
The appropriate amount of gastric tissue was taken from each group. Tissue homogenate (10%) was prepared by physiological saline according to the proportion of 1:9 (w/v). The homogenate was centrifuged at 2,500 × g for 10 min at 4°C. Then, supernatant was taken, and the activities of SOD and CAT as well as the contents of GSH, MDA and proteins were detected according to the operating instructions in the kits.
Statistical analysis
Experimental data were expressed as mean ± standard deviation (SD). All experimental data were processed by SPSS 13.0 statistical software (IBM, Armonk, NY, USA), and one-way analysis of variance (ANOVA) was adopted. P<0.05 was considered to indicate a statistically significant difference.
Results
Gastric mucosal injury in rats
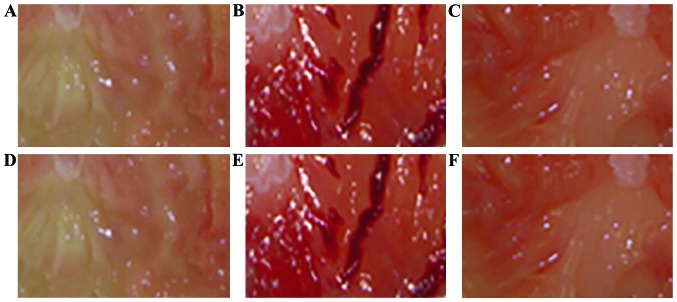
As shown in Fig. 1, the gastric mucosal surface of rat in normal group is pink, smooth and glossy; while the gastric mucosa of rat in model group is red and white, reddening and swelling, with bleeding and erosion; the injury of gastric mucosa could be obviously relieved by high-dose zerumbone and omeprazole, so the gastric mucosas of rats are light pink, with basically smooth surface and no bleeding and erosion; in addition, that the gastric mucosal injury of rats significantly reduced with the increase of the dosage of zerumbone could be found.
Figure 1.
Anatomical observation of gastric mucosa in rat of each group. (A) Normal group; (B) model group; (C) omeprazole group; (D-F) respectively represent high-dose zerumbone group, middle-dose zerumbone group and low-dose zerumbone group.
Pathological changes of gastric mucosal tissues
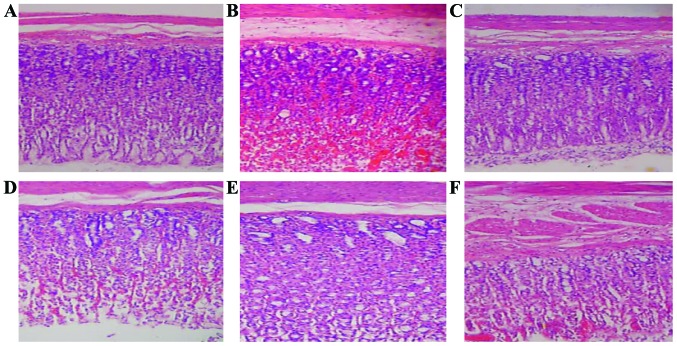
Compared with the normal group, gastric mucosa in the model group had significant pathological changes, and loose cell arrangement, pycnosis and thinning of mucosal layer, and mucosal hemorrhage and exudation in submucosa could be seen, suggesting that inflammatory cell infiltration occurred. Zerumbone and omeprazole could ameliorate the statuses of such tissue lesions, especially in the high- and middle-doses zerumbone groups, gastric mucosal pycnosis, hemorrhage, cell exudation and other statuses were significantly improved (Fig. 2).
Figure 2.
Pathological observation of gastric mucosal tissues in rats (H&E staining, ×200). (A) Normal group; (B) model group; (C) omeprazole group; (D-F) respectively represent high-dose zerumbone group, middle-dose zerumbone group and low-dose zerumbone group.
Effects of zerumbone on the expression of HO-1 and Nrf-2 proteins in gastric tissues of rats with chronic gastritis
As transcription factors regulating oxidative stress reaction in cells, HO-1 and Nrf-2 induced the expression of multiple antioxidant enzymes and detoxification enzymes, accelerate enzymatic reaction, eliminate free radical and other antioxidant substances and maintain the original intracellular redox levels. Compared with the normal group, the expression of HO-1 and Nrf-2 proteins in gastric tissues of rats in the model group were significantly decreased (p<0.01); compared with the model group, the expressions of HO-1 and Nrf-2 at each dose in zerumbone group was significantly upregulated (p<0.01) (Fig. 3).
Figure 3.
Expressions of HO-1 and Nrf-2 proteins in gastric tissues of rats with chronic gastritis. (A) Western blot results of HO-1 and Nrf-2 proteins. (B) Grey-level analysis results of HO-1 and Nrf-2 proteins; compared with the normal group, **p<0.01; compared with the model group, ##p<0.01. HO-1, heme oxygenase-1; Nrf-2, nuclear factor E2-related factor-2.
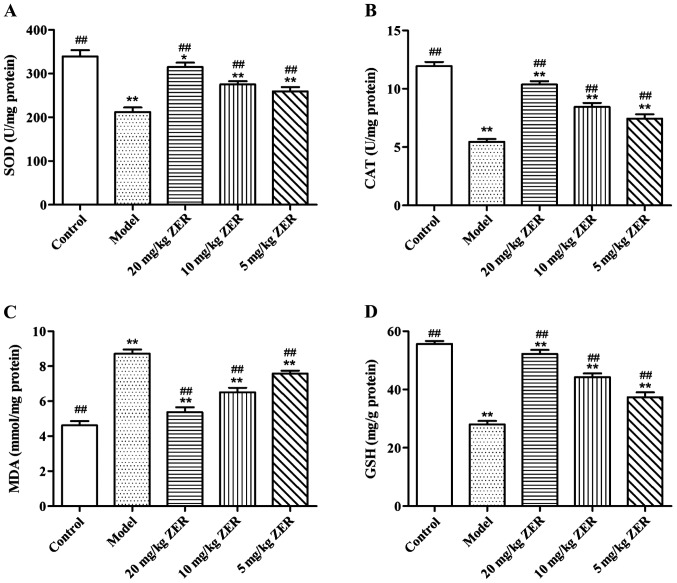
Effects of zerumbone on SOD, CAT, MDA and GSH in gastric tissues of rats with chronic gastritis
MDA could indirectly reflect the severity of tissue cell attacked by oxygen free radical, and SOD, CAT and GSH eliminated intracellular oxygen free radical, thereby protecting cells from injury. The effects of zerumbone on SOD, CAT, MDA and GSH in gastric tissues of rats with chronic gastritis are shown in Fig. 4. Compared with the model group, the activities of SOD and CAT as well as GSH levels in gastric mucosal tissues of rats in the zerumbone groups were significantly increased, but MDA contents were distinctly reduced, and the differences were statistically significant (p<0.01). The results indicated that zerumbone could improve oxygen free radical scavenging capacity through antioxidant effect, which would protect gastric mucosal cells from the attack by free radicals, thereby relieving gastric tissue injury.
Figure 4.
Effects of zerumbone on SOD, CAT, MDA and GSH in gastric tissues of rats with chronic gastritis. (A) Detection results of SOD activities in gastric tissues of rats; (B) detection results of CAT activities in gastric tissues of rats; (C) detection results of MDA contents in gastric tissues of rats; (D) detection results of GSH contents in gastric tissues of rats; compared with the normal group, *p<0.05, **p<0.01; compared with the model group, ##p<0.01. SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; GSH, glutathione.
Discussion
Gastric mucosal injury is caused because the balance between gastric mucosal defensive function and injury factor is broken, finally resulting in acute and chronic inflammatory damage of the stomach. The study confirmed that patients with chronic gastritis are more prone to suffer from gastric cancer; moreover, the active phase of inflammation in chronic gastritis is regarded as the first stage of precancerous lesions of invasive gastric cancer; therefore, in order to prevent the development of the disease, chronic gastritis should be paid enough attention and treatment obtained (10). Zerumbone is a natural product extracted from plants, which contains many unsaturated carboxyl groups in structure, and this unique chemical structure may be related to antioxidation and inhibition of inflammation (11).
The high concentrations of ethanol, sodium deoxycholate and ammonia used in this experiment can destroy gastric mucosa, thereby inducing the occurrence of gastritis. Among them, ethanol can induce the occurrence of inflammation through direct toxic action or free radicals to activate oxidative stress; ammonia can inhibit the energy metabolism process of gastric mucosa and damage gastric mucosal epithelium; additionally, the free radicals produced by ammonia metabolism can also damage gastric mucosa; sodium deoxycholate can destroy the lipoprotein layer of gastric mucosal cells, resulting in mucosal inflammation (12). Although the injury mechanism of chronic gastritis has not been thoroughly studied, increasing research indicates that it is related to oxidative stress. The normal amount of reactive oxygen species (ROS) level can maintain various physiological functions of cells, but when the ROS level in cells exceeds the capacity of antioxidant system, it will lead to the occurrence of oxidative stress in cells (13). Organism itself can resist lipid peroxidation induced by ROS through SOD, CAT, GSH, MDA and other antioxidant barriers (14). GSH and SOD can remove superoxide, peroxide and lipid peroxidation free radicals, thereby reducing the tissue and cell damage caused by ROS; in addition, as a kind of preventive antioxidant, CAT can transform peroxide and other free radicals to water or other substances that are safe for tissues and cells (15). MDA can be used as an index to measure body's lipid peroxidation level, and its content can accurately reflect the degree of lipid peroxidation in tissues and cells (16). HO-1 protein is a kind of widely existing antioxidant enzyme, which is expressed at low levels in normal state, and its expression is regulated by Nrf-2 protein (17). Nrf-2, which is a receptor of oxidative stress, locates at upstream positions in various antioxidant enzymes; under the stimulation of oxidative stress, it can produce changes of expression levels prior to a series of antioxidant enzymes such as SOD, CAT and GSH peroxidase (18). It is found that when oxidative stress occurs, Nrf-2 can combine with the corresponding protein to form protein dipolymer, thereby activating the transcription and translation of HO-1 gene (19).
In our experiments, the chronic gastritis models in rats were established by long-term intragastric administration with ethanol, ammonia and sodium deoxycholate, followed by different doses of zerumbone for the treatment; the injury degree of gastric mucosa was investigated by anatomy, and morphological changes of gastric mucosal tissues were observed and studied by pathological sections of gastric mucosal tissues. The results indicated that zerumbone could significantly relieve gastric mucosal reddening and swelling, erosion and ulcer in rats with chronic gastritis; the observation of the pathological sections of tissues showed that zerumbone could ameliorate the loose arrangement of gastric mucosal cells, pycnosis and thinning of mucosal layer, bleeding, inflammatory cell infiltration and other phenomena. In order to further explore the mechanism of the protective effect of zerumbone on gastric mucosal tissues in rats with chronic gastritis, we investigated the effects of zerumbone on the expression of HO-1 and Nrf-2 proteins in gastric tissues of rats with chronic gastritis as well as SOD, CAT, GSH and MDA. The results indicated that zerumbone could significantly upregulate the expressions of HO-1 and Nrf-2 in gastric mucosal tissues, obviously improving the activities of SOD and CAT, increasing GSH levels, but decreasing MDA contents. Therefore, the protective effect of zerumbone on gastric mucosa may be achieved by improving scavenging ability of oxygen free radical via antioxidation.
Szabolcs et al found that zerumbone could protect rats with pancreatitis induced by cholecystokinin-8, relieve pancreatic edema, reduce the contents of interleukin-6 and tumor necrosis factor-α in pancreatic cells, and also decrease the activities of nitric oxide synthase and SOD (20). Additionally, the study showed that zerumbone could inhibit the expression of nuclear transcription factor-κB, which would cause its downstream inflammatory factors unable to activate, thereby reducing inflammatory response (21). This study further confirmed that zerumbone has an antioxidant effect, and it can treat chronic gastritis in rats via antioxidant mechanisms.
In conclusion, zerumbone can improve chronic gastritis jointly induced by multiple factors, which is achieved by increasing the activities of CAT and SOD, elevating GSH level and decreasing MDA level through upregulating the expressions of HO-1 and Nrf-2 proteins, demonstrating that zerumbone can protect gastric mucosa via antioxidant mechanisms.
References
- 1.Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50:657–667. doi: 10.3109/00365521.2015.1019918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, Lu ML, Wang MR, Guo CH, Yu SZ, Kurtz RC, et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int J Cancer. 2001;92:600–604. doi: 10.1002/ijc.1231. [DOI] [PubMed] [Google Scholar]
- 3.Zhu HW, Ruan CX, Cao SF, Wu HG, Li J. Review on clinical and mechanism studies of moxibustion therapy for chronic gastritis. J Acupunct Tuina Sci. 2014;12:203–210. doi: 10.1007/s11726-014-0774-x. [DOI] [Google Scholar]
- 4.Kitayama T, Awata M, Kawai Y, Tsuji A, Yoshida Y. Asymmetric synthesis of versatile monoepoxyzerumbone and monoepoxyzerumbol. Tetrahedron Asymmetry. 2008;19:2367–2373. doi: 10.1016/j.tetasy.2008.10.002. [DOI] [Google Scholar]
- 5.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–6969. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 6.Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The alpha, beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23:795–802. doi: 10.1093/carcin/23.5.795. [DOI] [PubMed] [Google Scholar]
- 7.Deng WH, Yu J, Wang WX, et al. Zerumbone attenuates the severity of acute necrotizing pancreatitis and pancreatitisinduced hepatic injury. Mediators Inflamm. 2012;2012:156507. doi: 10.1155/2012/156507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JW, Ohnishi K, Murakami A, Lee JS, Kundu JK, Na HK, Ohigashi H, Surh YJ. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev Res (Phila) 2011;4:860–870. doi: 10.1158/1940-6207.CAPR-10-0354. [DOI] [PubMed] [Google Scholar]
- 9.Murakami A, Hayashi R, Tanaka T, Kwon KH, Ohigashi H, Safitri R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: Separately and in combination. Biochem Pharmacol. 2003;66:1253–1261. doi: 10.1016/S0006-2952(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 10.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci Biotechnol Biochem. 1999;63:1811–1812. doi: 10.1271/bbb.63.1811. [DOI] [PubMed] [Google Scholar]
- 12.Pan JS, He SZ, Xu HZ, Zhan XJ, Yang XN, Xiao HM, Shi HX, Ren JL. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008;14:5857–5867. doi: 10.3748/wjg.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A, Li H, Li H, Szeto IM, Shi Y, et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic Biol Med. 2014;67:396–407. doi: 10.1016/j.freeradbiomed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Matés JM, Pérez-Gómez C, de Núñez Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 15.Wong JY, Abdulla MA, Raman J, Phan CW, Kuppusamy UR, Golbabapour S, Sabaratnam V. Gastroprotective effects of Lion's mane mushroom Hericium erinaceus (Bull.:Fr.) Pers. (Aphyllophoromycetideae) extract against ethanol-induced ulcer in rats. Evid Based Complement Alternat Med. 2013;2013:492976. doi: 10.1155/2013/492976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 17.Ye Q, Dalavanga Y, Poulakis N, Sixt SU, Guzman J, Costabel U. Decreased expression of haem oxygenase-1 by alveolar macrophages in idiopathic pulmonary fibrosis. Eur Respir J. 2008;31:1030–1036. doi: 10.1183/09031936.00125407. [DOI] [PubMed] [Google Scholar]
- 18.Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13:43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazaki T, Kirino Y, Takeno M, Samukawa S, Hama M, Tanaka M, Yamaji S, Ueda A, Tomita N, Fujita H, et al. Expression of heme oxygenase-1 in human leukemic cells and its regulation by transcriptional repressor Bach1. Cancer Sci. 2010;101:1409–1416. doi: 10.1111/j.1349-7006.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabolcs A, Tiszlavicz L, Kaszaki J, Pósa A, Berkó A, Varga IS, Boros I, Szüts V, Lonovics J, Takács T. Zerumbone exerts a beneficial effect on inflammatory parameters of cholecystokinin octapeptide-induced experimental pancreatitis but fails to improve histology. Pancreas. 2007;35:249–255. doi: 10.1097/mpa.0b013e318070d791. [DOI] [PubMed] [Google Scholar]
- 21.Sung B, Murakami A, Oyajobi BO, Aggarwal BB. Zerumbone abolishes RANKL-induced NF-kappaB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009;69:1477–1484. doi: 10.1158/0008-5472.CAN-08-3249. [DOI] [PubMed] [Google Scholar]