Summary
Underproduction of hydrochloric acid into the stomach is frequently encountered in subjects from developing countries. We explore the hypothesis that hypochlorhydria compromises the gastric barrier and favours bacterial overgrowth in the proximal parts of the small intestine where nutrient absorption takes place. Food calories are thus deviated into bacterial metabolism. In addition to an adequate caloric supply, correcting hypochlorhydria might be needed to decrease childhood malnutrition.
Introduction
The United Nations (UN) have formulated an action plan for sustainable development, of which the two top‐ranking goals for 2030 are to end both poverty and hunger. To improve nutrition, the UN wants to achieve food security and promote sustainable agriculture. It is undisputable that eating too few calories leads over time to undernutrition. However, food security might be necessary, yet insufficient alone to correct malnutrition, as other factors are proposed to contribute. For example, malnourished children are frequently found side by side with non‐malnourished children in field studies, and nutritionists underline difficulties in correcting malnutrition by high‐calorie food alone (Ahmed et al., 2009). A landmark study demonstrated that refeeding caused better weight gain when combined with antibiotics, therefore pointing to a role of microbes in malnutrition (Trehan et al., 2013). The idea of using antibiotics in malnutrition has been and continues to be controversially discussed for now over 50 years (Alcoba et al., 2013; Brüssow, 2015), and an impact of the gut microbiota on human nutrition is not farfetched (Blanton et al., 2016). By fermenting complex polysaccharides that are inaccessible to human digestive enzymes, the gut microbiota releases short‐chain fatty acids (SCFAs) as metabolic end‐products (Sonnenburg et al., 2005), which are avidly taken up systemically and by the colon enterocytes and contribute thus to human nutrition and have important signalling functions (Brüssow and Parkinson, 2014). J. Gordon's laboratory pioneered the idea that the composition of the gut microbiota determined the extent of this extra calorie contribution and associated a particular microbial community characterized in first approximation by the ratio of Firmicutes to Bacteroidetes with obesity (Ley et al., 2006). When the microbiota–obesity connection turned out to be more complicated than initially thought, J. Gordon's laboratory teamed up with nutritionists to study the effect of gut microbiota composition on malnutrition. However, twin studies from Africa showed no remarkable differences in overall gut microbiota composition between children with kwashiorkor (i.e. protein malnutrition) and their nutritionally normal siblings. Only when their stool microbiota was transferred into axenic mice did differences with respect to energy extraction become detectable when mice were also fed local diets from African children (Smith et al., 2013). Malnourished children from Bangladesh showed a delayed gut microbiota maturation compared with age‐matched healthy children, but otherwise no gross faecal microbiota dysbiosis (Subramanian et al., 2014). Yet, the gut microbiota of the large intestine is only part of the microbiota–malnutrition equation, and it is possible that microbes colonizing the upper parts of the small intestine have a potentially larger impact on malnutrition than those colonizing the colon.
The argument runs as follows: in the upper parts of the alimentary tract, there is a conspicuous antiparallel distribution of absorptive capacities for the major three classes of caloric nutrients (proteins, carbohydrates, fats) and gut colonization with bacteria (Fig. 1; Brüssow, 2007). Digestive enzymes for carbohydrates are already produced into the oral cavity. In response to food powerful proteases (pepsin) are secreted into the acidic environment of the stomach. The exocrine pancreas then secretes the bulk of carbohydrate‐ and lipid‐digesting enzymes and a second wave of proteolytic enzymes (trypsin, chymotrypsin) into the duodenum. The absorption of nutrients is minimal from mouth to stomach, while the major share of protein, carbohydrate and lipid digestion products is absorbed in the duodenum and the upper parts of the jejunum, smaller amounts are absorbed in the lower parts of the jejunum and even less in the ileum. This proximal‐to‐distal decline in absorption function along the human gut finds a mirror image in the level of bacterial colonization (Fig. 1). The duodenum is only sparsely colonized with < 100 bacteria per ml aspirate. Bacterial titres are increasing further down to reach levels of 106 bacteria per ml aspirate in the distal ileum. The ileocaecal valve marks the transition from the small into large intestine and the transition to a high bacterial load in the colon with 1012 bacteria per gram gut content (Roland et al., 2014). From a physiological and an evolutionary viewpoint, this reciprocal distribution of absorption and bacterial colonization makes sense. The digestion of food by host enzymes transforms nutrients into absorbable forms that are then transported across the gut epithelium into lymph and blood stream. The presence of high titres of gut bacteria in the first 50 cm of the small intestine would represent undesirable food competitors stealing calories and diverting nutrients from host metabolism into bacterial biomass production. This would therefore reduce the efficiency of calorie extraction from food, which might become critical for the host if the food supply is limiting.
Figure 1.
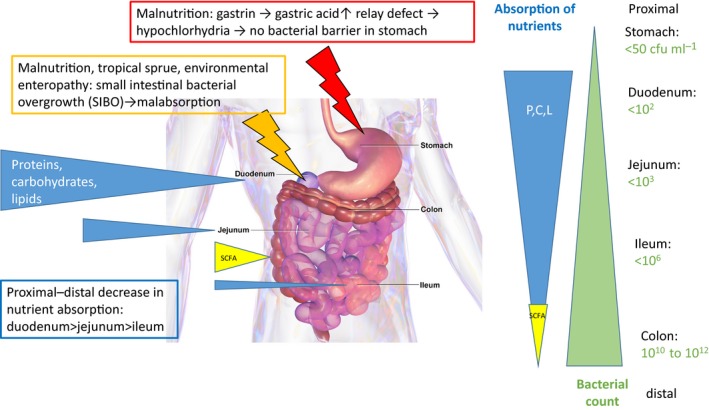
The antiparallel gradient of absorption capacities (blue wedges, size is proportional to absorption capacity) for proteins (P), carbohydrates (C) and lipids (L) compared to bacterial colonization (green wedge, size is proportional to titres indicated on right) along the proximal‐to‐distal axis of the human gastrointestinal tract, with the notable exception of short‐chain fatty acid (SCFA) absorption (yellow wedge) in the colon. Data from Gorbach et al. (1967). Broken arrows indicate two pathological conditions that interfere with the distribution of bacterial colonization along the gastrointestinal tract: low gastric acid production (red symbol) and bacterial overgrowth (orange symbol) in the upper small intestine. The background anatomical gut picture is from Blausen.com staff Wikiversity Journal of Medicine.
Keeping food competitors in check is, in energy terms, costly, but the control of undesired bacterial colonization is among other mechanisms (e.g. secretion of antimicrobial peptides by the gut) achieved by two major mechanisms that are at the same time necessary physiological requirements for food processing to gain energy and nutrients. The first such energy‐costing barrier against bacterial competitors in the absorptive sections of the gut is acid production in the stomach, which is also needed for efficient protein digestion by pepsin. Hydrochloric acid is an efficient penetration barrier for bacteria accompanying food or being swallowed with highly colonized oral secretions. When gastric acidity drops to pH 3, a rapid bactericidal effect was documented for gastric juice in vitro and in vivo, which was not observed at pH 4 (Giannella et al., 1972). Children produce less gastric acidity than adults (milk proteins are easier digested than meat), but the mean resting gastric pH remains below 3 (Maffei and Nóbrega, 1975). The second barrier function against bacterial overgrowth in the small intestine is gut peristalsis, which mixes and pushes the gut content forward for further processing, thus representing another physiologically needed energy investment. Towards the terminal ileum, peristalsis becomes less vigorous and, concomitantly, bacterial titres increase. The antibacterial efficiency of these two mechanisms has been demonstrated by clinical observations. Drugs that suppress gastric acid secretion (omeprazole) cause bacterial overgrowth in the duodenum (> 105 cfu ml−1) in half of the patients (Fried et al., 1994; Thorens et al., 1996). Bacterial overgrowth in the small intestine, as assessed by hydrogen breath tests, was also associated with disorders of peristalsis (Vantrappen et al., 1977; Roland et al., 2015).
When one of these two major barrier functions break down, the body opens gates for an increased bacterial colonization of the gut. Fifty years ago, gastroenterologists reported that malnourished children showed basal gastric acid output below normal (‘hypochlorhydria’). Also, maximal acid output after stimulation by subcutaneous pentagastrin was reduced in 50% of subjects (Gracey et al., 1977). About half of malnourished children from Bangladesh and Brazil showed gastric pH above 4 and even after nutritional rehabilitation, the rates of hypochlorhydria remained unchanged (Maffei and Nóbrega, 1975; Gilman et al., 1988), therefore excluding acute energy deficiency as a cause for this condition. Hypochlorhydria is widely distributed in malnourished children and has also been described in Indonesia, Nigeria and South Africa (Sarker and Gyr, 1992). At the same time, several studies have described high titres of microbes in the upper small intestine of malnourished children, with a mean titre of 5 × 106 viable bacteria per mL jejunal fluid in Gambia (Heyworth and Brown, 1975) and Latin America (Mata et al., 1972) and mean titres of 8 × 107 ml−1 in Indonesia (Gracey et al., 1973); some children showed > 1010 bacteria per ml (Heyworth and Brown, 1975). Most of these children also showed chronic diarrhoea. Paediatricians from Nigeria found comparable bacterial counts of about 107 ml−1 duodenal aspirate in malnourished children with and without diarrhoea, while well‐nourished children with and without diarrhoea both displayed < 105 cfu ml−1 intestinal aspirate (Omoike and Abiodun, 1989). A Brazilian study showed that the gastric count of coliform bacteria in malnourished children was proportional to the gastric pH, closely linking both phenomena (Maffei and Nóbrega, 1975).
With these bacterial titres at sites critical for caloric nutrient absorption, a food drain can be anticipated, but has yet to be experimentally proven. In addition, bacterial metabolites and endotoxins released from decaying bacteria might harm the mucosa and cause inflammation, while bacterial‐mediated deconjugation of bile salts (Cassells et al., 1970) could lead to problems with fat absorption.
A number of observations suggest that the problem of low gastric acid production is widespread in developing countries (Sarker and Gyr, 1992), while the full extent is currently still unknown. More than half of better‐nourished Bangladeshi children also showed a basal gastric pH ≥ 4, but experienced a drop in pH upon betazole (histamine H2 receptor agonist) stimulation (Gilman et al., 1988). None of them showed bacterial overgrowth, pointing to the importance of pH in the digestive phase for barrier function. Forty per cent of Bangladeshi cholera patients presented with hypochlorhydria, which was identified as a risk factor and not as a consequence of cholera (Nalin et al., 1978). Stimulated acid production was also significantly lower in healthy controls from lower socio‐economic groups than from higher ones in Indian men (Sack et al., 1972). This observation concurs with epidemiology data on cholera susceptibility and points to an environmental factor that influences gastric acid production in developing countries.
Small intestinal bacterial overgrowth (SIBO) was also reported in tropical sprue (Gorbach et al., 1970; Ghoshal et al., 2003), an enigmatic enteropathy of tropical countries with suspected, but not proven, infectious aetiology characterized by malabsorption symptoms (Ghoshal et al., 2014; Wanke, 2014). However, overgrowth was not a consistent observation in tropical sprue (Bhat et al., 1972) and the pathology seems to correlate more closely with enterotoxin levels (Klipstein et al., 1978) than the sheer amount of bacteria in the small intestine. For ethical reasons, SIBO diagnosis by gastric and intestinal intubation has been largely replaced with a non‐invasive hydrogen breath test. Notably, this breath test diagnosed SIBO in > 30% of children from urban slums in Brazil compared to 2% SIBO diagnosis in children from the same city attending private schools (dos Reis et al., 2007; Mello et al., 2012). As expected, both groups differed for anthropometric measures and environmental hygiene levels, while no difference was seen for these parameters between slum dwellers with or without SIBO diagnosis. Breath tests returned to normal after antibiotic treatment (Tahan et al., 2013). A prospective study from an impoverished Bangladeshi neighbourhood revealed 17% of children with SIBO and SIBO correlated with stunting and open sewers (Donowitz et al., 2016). This apparently rather widespread condition (Donowitz and Petri, 2015) is linked with environmental enteropathy (EE). The relationship of EE with tropical sprue is controversial, and while its pathogenesis is poorly understood, it includes chronic intestinal inflammation (Donowitz et al., 2016). Whether SIBO in EE is associated with hypochlorhydria, which also showed an association with low socio‐economic status, is not clear at present.
Environmental enteropathy is currently an active research area, and treatment modalities for EE are at the moment not clear. Its underlying pathology might explain the failure of isolated nutritional interventions in correcting malnutrition and growth delays. Controlled studies of dietary supplements with antibiotics, limited to a crucial infantile growth phase, have been proposed in the past (Gorbach, 1972) and antibiotics are indeed effective in tropical sprue. Hypochlorhydria has additionally been linked with Helicobacter pylori infection in developing countries, and gastric acid secretion ameliorated with the eradication of this pathogen (Sarker et al., 2004, 2012). However, such an intervention would require antibiotic treatment, which is difficult to envision as a mass application in view of the high prevalence of H. pylori infection in developing countries (Mahalanabis et al., 1996) in conjunction with antibiotic resistance problems. Some observations from the literature might provide hints for treatment options. Half of breastfed children from a Brazilian study showed a resting pH above 4; some showed elevated coliform counts in the stomach, while others did not (Maffei and Nóbrega, 1975). Apparently, some mothers provide breast milk that contains compounds (oligosaccharides?) that protect against bacterial overgrowth of the upper gut in the absence of a gastric barrier. Alternative pharmacological approaches should also be explored to address the fact that impaired acid production by gastric parietal cells upon gastrin and histamine H2 receptor agonist stimulation is a consistent observation in malnourished children. Some beverages are known to stimulate gastric acidity production, like coffee, alcoholic beverages and particularly digestive bitters, whose bitter ingredients also exist as a phytochemical (Tinctura amara) for hypochlorhydria treatment (Lüllmann, 2016). Finally, one could also envision direct acidification of the stomach with a pharmaceutical preparation of citric acid used in hypo‐ and achlorhydria patients (Lüllmann, 2016) or local low pH beverages.
In summary, to reach the UN sustainability goals with respect to improved nutrition in developing countries, achieving food security is not enough. The problem of low gastric acidity should also be addressed as it compromises protein digestion, leads to the production of possibly carcinogenic nitrosamine compounds via intragastric colonization (Wang et al., 2014) and limits the absorption of iron (gastric acid converts ferric iron to its absorbable ferrous form), leading to widespread iron deficiency anaemia in developing countries (Sarker et al., 2004). As early colonization with H. pylori may contribute to atrophic gastritis and thereby to low gastric acid production (El‐Omar et al., 1997), sanitation programs to decrease H. pylori transmission via drinking water (Klein et al., 1991) should also be considered. As the strength of any causal relationship for any linked observation increases from association studies to prospective studies and finally intervention studies, the link between insufficient gastric acid production and malnutrition is best tested by assessing the impact of citric acid supplementation in malnourished subjects during a refeeding trial.
Conflict of interest
None declared.
Acknowledgements
We thank Shawna McCallin, Carine Blanchard, Silas Kieser, Tiago Nunes Alves and Norbert Sprenger for critical reading of the manuscript.
Microbial Biotechnology (2017) 10(5), 1025–1030
Funding information
No funding information provided
References
- Ahmed, T. , Haque, R. , Shamsir Ahmed, A.M. , Petri, W.A. Jr , and Cravioto, A. (2009) Use of metagenomics to understand the genetic basis of malnutrition. Nutr Rev 67(Suppl 2): S201–S206. [DOI] [PubMed] [Google Scholar]
- Alcoba, G. , Kerac, M. , Breysse, S. , Salpeteur, C. , Galetto‐Lacour, A. , Briend, A. , and Gervaix, A. (2013) Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta‐analysis. PLoS ONE 8: e53184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, P. , Shantakumari, S. , Rajan, D. , Mathan, V.I. , Kapadia, C.R. , Swarnabai, C. , and Baker, S.J. (1972) Bacterial flora of the gastrointestinal tract in southern Indian control subjects and patients with tropical sprue. Gastroenterology 62: 11–21. [PubMed] [Google Scholar]
- Blanton, L.V. , Barratt, M.J. , Charbonneau, M.R. , Ahmed, T. , and Gordon, J.I. (2016) Childhood undernutrition, the gut microbiota, and microbiota‐directed therapeutics. Science 352: e1533. [DOI] [PubMed] [Google Scholar]
- Brüssow, H. (2007) The Quest for Food. A Natural History of Eating. New York, NY: Springer. [Google Scholar]
- Brüssow, H. (2015) Growth promotion and gut microbiota: insights from antibiotic use. Environ Microbiol 17: 2216–2227. [DOI] [PubMed] [Google Scholar]
- Brüssow, H. , and Parkinson, S.J. (2014) You are what you eat. Nat Biotechnol 32: 243–245. [DOI] [PubMed] [Google Scholar]
- Cassells, J.S. , Banwell, J.G. , Gorbach, S.L. , Mitra, R. , and Mazumder, D.N. (1970) Tropical sprue and malnutrition in West Bengal. IV. Bile salt deconjugation in tropical sprue. Am J Clin Nutr 23: 1579–1581. [DOI] [PubMed] [Google Scholar]
- Donowitz, J.R. , and Petri, W.A. Jr (2015) Pediatric small intestine bacterial overgrowth in low‐income countries. Trends Mol Med 21: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz, J.R. , Haque, R. , Kirkpatrick, B.D. , Alam, M. , Lu, M. , Kabir, M. , et al (2016) Small intestine bacterial overgrowth and environmental enteropathy in Bangladeshi children. MBio 7: e02102–e02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Omar, E.M. , Oien, K. , El‐Nujumi, A. , Gillen, D. , Wirz, A. , Dahill, S. , et al (1997) Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology 113: 15–24. [DOI] [PubMed] [Google Scholar]
- Fried, M. , Siegrist, H. , Frei, R. , Froehlich, F. , Duroux, P. , Thorens, J. , et al (1994) Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut 35: 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal, U.C. , Ghoshal, U. , Ayyagari, A. , Ranjan, P. , Krishnani, N. , Misra, A. , et al (2003) Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J Gastroenterol Hepatol 18: 540–547. [DOI] [PubMed] [Google Scholar]
- Ghoshal, U.C. , Srivastava, D. , Verma, A. , and Ghoshal, U. (2014) Tropical sprue in 2014: the new face of an old disease. Curr Gastroenterol Rep 16: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella, R.A. , Broitman, S.A. , and Zamcheck, N. (1972) Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman, R.H. , Partanen, R. , Brown, K.H. , Spira, W.M. , Khanam, S. , Greenberg, B. , et al (1988) Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology 94: 1308–1314. [DOI] [PubMed] [Google Scholar]
- Gorbach, S.L. (1972) Microflora of the gastrointestinal tract in tropical enteritis: a current appraisal. Am J Clin Nutr 25: 1127–1133. [DOI] [PubMed] [Google Scholar]
- Gorbach, S.L. , Plaut, A.G. , Nahas, L. , Weinstein, L. , Spanknebel, G. , and Levitan, R. (1967) Studies of intestinal microflora. II. Microorganisms of the small intestine and their relations to oral and fecal flora. Gastroenterology 53: 856–867. [PubMed] [Google Scholar]
- Gorbach, S.L. , Banwell, J.G. , Jacobs, B. , Chatterjee, B.D. , Mitra, R. , Mazumder, D.N. , and Sen, N.N. (1970) Tropical sprue and malnutrition in West Bengal. I. Intestinal microflora and absorption. Am J Clin Nutr 23: 1545–1558. [DOI] [PubMed] [Google Scholar]
- Gracey, M. , Suharjono, Sunoto, and Stone, D.E. (1973) Microbial contamination of the gut: another feature of malnutrition. Am J Clin Nutr 26: 1170–1174. [DOI] [PubMed] [Google Scholar]
- Gracey, M. , Cullity, G.J. , Suharjono., and Sunoto. (1977) The stomach in malnutrition. Arch Dis Child 52: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth, B. , and Brown, J. (1975) Jejunal microflora in malnourished Gambian children. Arch Dis Child 50: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, P.D. , Graham, D.Y. , Gaillour, A. , Opekun, A.R. , and Smith, E.O. (1991) Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet 337: 1503–1506. [DOI] [PubMed] [Google Scholar]
- Klipstein, F.A. , Engert, R.F. and Short, H.B. (1978) Enterotoxigenicity of colonising coliform bacteria in tropical sprue and blind‐loop syndrome. Lancet ii, 342–344. [DOI] [PubMed] [Google Scholar]
- Ley, R.E. , Turnbaugh, P.J. , Klein, S. , and Gordon, J.I. (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Lüllmann, H. (2016) Pharmakologie und Toxikologie. Stuttgart, Germany: Thieme Verlag. [Google Scholar]
- Maffei, H.V. , and Nóbrega, F.J. (1975) Gastric pH and microflora of normal and diarrhoeic infants. Gut 16: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalanabis, D. , Rahman, M.M. , Sarker, S.A. , Bardhan, P.K. , Hildebrand, P. , Beglinger, C. , and Gyr, K. (1996) Helicobacter pylori infection in the young in Bangladesh: prevalence, socioeconomic and nutritional aspects. Int J Epidemiol 25: 894–898. [DOI] [PubMed] [Google Scholar]
- Mata, L.J. , Jiménez, F. , Cordón, M. , Rosales, R. , Prera, E. , Schneider, R.E. , and Viteri, F. (1972) Gastrointestinal flora of children with protein‐calorie malnutrition. Am J Clin Nutr 25: 118–126. [DOI] [PubMed] [Google Scholar]
- Mello, C.S. , Tahan, S. , Melli, L.C. , Rodrigues, M.S. , de Mello, R.M. , Scaletsky, I.C. , and de Morais, M.B. (2012) Methane production and small intestinal bacterial overgrowth in children living in a slum. World J Gastroenterol 18: 5932–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin, D.R. , Levine, R.J. , Levine, M.M. , Hoover, D. , Bergquist, E. , McLaughlin, J. , et al (1978) Cholera, non‐vibrio cholera, and stomach acid. Lancet ii: 856–859. [DOI] [PubMed] [Google Scholar]
- Omoike, I.U. , and Abiodun, P.O. (1989) Upper small intestinal microflora in diarrhea and malnutrition in Nigerian children. J Pediatr Gastroenterol Nutr 9: 314–321. [DOI] [PubMed] [Google Scholar]
- dos Reis, J.C. , de Morais, M.B. , Oliva, C.A. , and Fagundes‐Neto, U. (2007) Breath hydrogen test in the diagnosis of environmental enteropathy in children living in an urban slum. Dig Dis Sci 52: 1253–1258. [DOI] [PubMed] [Google Scholar]
- Roland, B.C. , Ciarleglio, M.M. , Clarke, J.O. , Semler, J.R. , Tomakin, E. , Mullin, G.E. , and Pasricha, P.J. (2014) Low ileocecal valve pressure is significantly associated with small intestinal bacterial overgrowth (SIBO). Dig Dis Sci 59: 1269–1277. [DOI] [PubMed] [Google Scholar]
- Roland, B.C. , Ciarleglio, M.M. , Clarke, J.O. , Semler, J.R. , Tomakin, E. , Mullin, G.E. , and Pasricha, P.J. (2015) Small intestinal transit time is delayed in small intestinal bacterial overgrowth. J Clin Gastroenterol 49: 571–576. [DOI] [PubMed] [Google Scholar]
- Sack, G.H. Jr , Pierce, N.F. , Hennessey, K.N. , Mitra, R.C. , Sack, R.B. , and Mazumder, D.N. (1972) Gastric acidity in cholera and noncholera diarrhoea. Bull World Health Organ 47: 31–36. [PMC free article] [PubMed] [Google Scholar]
- Sarker, S.A. , and Gyr, K. (1992) Non‐immunological defence mechanisms of the gut. Gut 33: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker, S.A. , Davidsson, L. , Mahmud, H. , Walczyk, T. , Hurrell, R.F. , Gyr, N. , and Fuchs, G.J. (2004) Helicobacter pylori infection, iron absorption, and gastric acid secretion in Bangladeshi children. Am J Clin Nutr 80: 149–153. [DOI] [PubMed] [Google Scholar]
- Sarker, S.A. , Sultana, S. , Sattar, S. , Ahmed, T. , Beglinger, C. , Gyr, N. , and Fuchs, G.J. (2012) Influence of Helicobacter pylori infection on gastric acid secretion in pre‐school Bangladeshi children. Helicobacter 17: 333–339. [DOI] [PubMed] [Google Scholar]
- Smith, M.I. , Yatsunenko, T. , Manary, M.J. , Trehan, I. , Mkakosya, R. , Cheng, J. , et al (2013) Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg, J.L. , Xu, J. , Leip, D.D. , Chen, C.H. , Westover, B.P. , Weatherford, J. , et al (2005) Glycan foraging in vivo by an intestine‐adapted bacterial symbiont. Science 307: 1955–1959. [DOI] [PubMed] [Google Scholar]
- Subramanian, S. , Huq, S. , Yatsunenko, T. , Haque, R. , Mahfuz, M. , Alam, M.A. , et al (2014) Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahan, S. , Melli, L.C. , Mello, C.S. , Rodrigues, M.S. , Bezerra Filho, H. , and de Morais, M.B. (2013) Effectiveness of trimethoprim‐sulfamethoxazole and metronidazole in the treatment of small intestinal bacterial overgrowth in children living in a slum. J Pediatr Gastroenterol Nutr 57: 316–318. [DOI] [PubMed] [Google Scholar]
- Thorens, J. , Froehlich, F. , Schwizer, W. , Saraga, E. , Bille, J. , Gyr, K. , et al (1996) Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut 39: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehan, I. , Goldbach, H.S. , LaGrone, L.N. , Meuli, G.J. , Wang, R.J. , Maleta, K.M. , and Manary, M.J. (2013) Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 368: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantrappen, G. , Janssens, J. , Hellemans, J. , and Ghoos, Y. (1977) The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest 59: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.L. , Yu, X.J. , Zhan, S.H. , Jia, S.J. , Tian, Z.B. , and Dong, Q.J. (2014) Participation of microbiota in the development of gastric cancer. World J Gastroenterol 20: 4948–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke, C.A. (2014) Tropical sprue: enteropathy In Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 8th edn Bennett J.E., Dolin R. & Blaser M.J. (ed). Elsevier Inc.: New York, pp. 1297–1301. [Google Scholar]


