Summary
Biological production of hydrogen is poised to become a significant player in the future energy mix. This review highlights recent advances and bottlenecks in various approaches to biohydrogen processes, often in concert with management of organic wastes or waste CO 2. Some key bottlenecks are highlighted in terms of the overall energy balance of the process and highlighting the need for economic and environmental life cycle analyses with regard also to socio‐economic and geographical issues.
Introduction
Hydrogen provides a CO2‐free sustainable alternative to fossil fuels. A pioneering global initiative, the ‘Hydrogen Council’, comprising thirteen leading energy, transport and related industries, intends to increase investment in the hydrogen and fuel cell sectors (currently €1.4 Bn year−1) to stimulate hydrogen as a key part of the future energy mix via new policies and schemes (Anon, 2017).
Hydrogen is currently obtained mainly by steam reforming of hydrocarbons, releasing multiple greenhouse gas emissions (DOE, 2013). Hence, new H2 production methods are required such as biological production (bio‐H2; Dincer and Acar, 2015). H biotechnologies are maturing towards benchmarking against established clean energy from electrolysis of water, solar photovoltaics and wind farms. Biohydrogen can be made fermentatively from wastes, providing a simultaneous method of organic waste management (Chang et al., 2011). This short review highlights progress and bottlenecks of bio‐H2 towards a sustainable development goal to ensure access to affordable, reliable, sustainable and modern energy for all. Biohydrogen has been reviewed in comparison with other hydrogen production processes (Nikolaidis and Poullikkas, 2017).
Biohydrogen embraces any H2 production involving biological material (Mohan and Pandey, 2013). The energy source can be solar or can come from conversion of fixed carbon substrates (or both, in various combinations). An approach to CO2‐end of pipe treatment (e.g. from flue gas from fossil fuel combustion or carbon‐neutral fermentation of biomass) is to grow algae on waste CO2. Algal biohydrogen production is well‐described, but O2 from algal oxygenic photosynthesis inhibits the hydrogenase that makes H2. A key study (Kubas et al., 2017) will open the way to developing O2‐resistant hydrogenase. Emerging technology uses cyanobacteria (blue‐green algae) that make H2 via hydrogenase and also nitrogenase; their O2‐sensitivity is managed by temporal separation of photosynthetic O2 evolution and nitrogenase action, and by compartmentalization into microanaerobic heterocysts (Tiwari and Pandey, 2012). Despite a note that cyanobacterial biohydrogen is probably uneconomic (Singh et al., 2016), an environmental life cycle analysis (LCA) has shown for the first time that cyanobacterial bio‐H2 has the potential to be a competitor to desulfurized natural gas; the associated environmental impact of producing and extracting each gas, including use in a solid oxide fuel cell, was calculated and simulated respectively using the LCA software simapro (Archer et al., 2017). This research used published data from a raceway growth system (James et al., 2009). However, at latitudes above ~40°N, the generally low incident solar energy makes stand‐alone photobiological H2 systems seasonal and uneconomic without some form of process intensification. Boosting light delivery (e.g. LEDs, quantum dots) can be effective, but these may risk photopigment saturation and inhibition; this approach may be questionable economically and would be best addressed by a life cycle analysis. In sunny countries, light is plentiful, but in this case, ‘delivering cold’ is needed to extend crop product and food life; cooling is energy‐demanding and a global challenge (Strahan, 2017).
Another challenge is organic materials from agri‐food and municipal wastes, which must be managed to avoid landfilling which yields methane, a potent greenhouse gas. Current practices use anaerobic digestion (AD) with biogas – methane used for power. We review some options for combining waste treatments with bio‐H2 technology as possibly the best approach to tackling effectively these dual socio‐economic problems; stand‐alone biohydrogen is possibly uneconomic, but this awaits a life cycle analysis, currently in progress.
Biohydrogen production from waste: fermentation strategies for sustainable ‘waste to hydrogen energy’
Fermentation is the disposal of excess metabolic reductant (NADH) onto organic compounds in the absence of alternative electron acceptors such as O2 and (Guo et al., 2010). The mixed‐acid fermentation (‘dark fermentation’) pathway of the paradigm Escherichia coli (Fig. 1A) is simple, has high rates of H2 production but has limitations (Saratale et al., 2013; Fig. 1A inset). Hexose sugars can stoichiometrically deliver 12 mol H2 mol hexose−1. The mixed‐acid fermentation, while irreversible, is thermodynamically limited to 2–4 mol H2 mol hexose−1 (Hallenbeck, 2012). The ‘NADH pathway’ of some microorganisms (Hallenbeck, 2012, 2017) can deliver a higher H yield, but is reversible under a positive H2 partial pressure, which is required for with a downstream H fuel cell. Thermophilic bacteria have advantages but require input of heat energy. Hence, the focus has been mainly on mesophilic bacteria (Balachandar et al., 2013).
Figure 1.
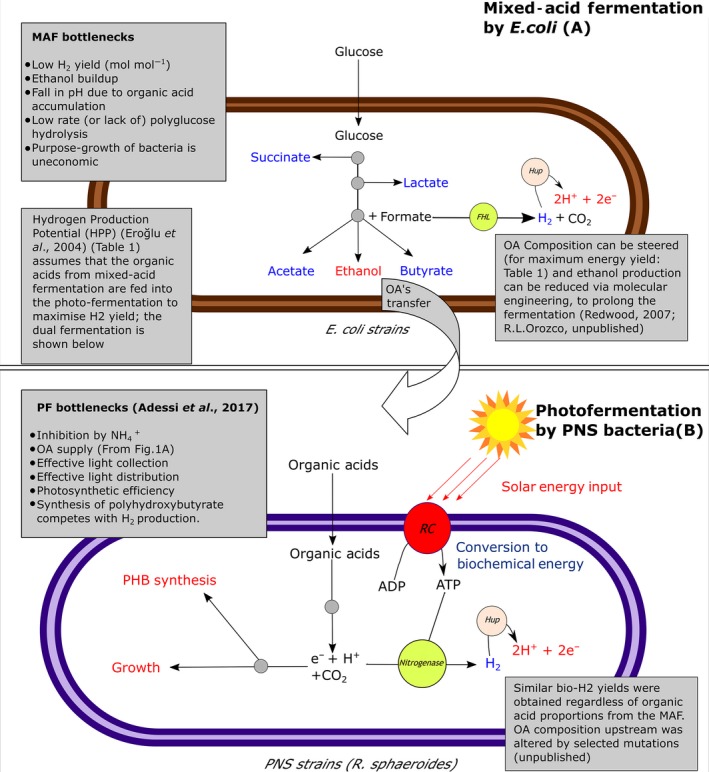
Mixed‐acid fermentation (MAF) of E. coli (A) and use of purple non‐sulfur bacteria (B) in photofermentation (PF) of organic acids (OAs) into H2. The organic acids are taken up by (e.g.) R. sphaeroides, and reducing power is generated as NADH (not shown). This reducing power can either be used for polyhydroxybutyrate synthesis or growth to maintain cellular redox or alternatively can be used for H2 production under light when growth is restricted by limitation of N or P source. Italicized bottlenecks are those overcome by use of the dual system (see text).
Most mixed‐acid fermentations follow a similar schematic: the cell forms reduced metabolic end‐products: organic acids (including toxic formate) and alcohol (Fig. 1A). Up to 2 mol H2 mol−1 hexose (Hallenbeck and Ghosh, 2009) is produced via the activity of formate hydrogen lyase (which splits formate to H2 + CO2), that is < 20% of the theoretical maximum H2. Sustained bio‐H2 production is limited by end‐product (ethanol) toxicity and acidification of the medium by accumulating organic acids (Redwood, 2007).
The organic acids provide a means to overcome the thermodynamic limitation via their use in a coupled photofermentation reactor (Redwood et al., 2012a,b; Hallenbeck, 2013, 2017) via electrodialysis (Fig 2). If organic acid mixtures are fed to purple non‐sulfur bacteria (e.g. Rhodobacter sphaeroides), the off‐gas (typically > 90% H) is suitable for direct use in fuel cells (Nakada et al., 1999). This anoxygenic photofermentative H2 process (Fig. 1B) requires input of light energy (to help overcome the thermodynamic barrier in converting organic acids into H2 (Hallenbeck, 2013)). Nitrogen‐deficient conditions are essential; in purple non‐sulfur bacteria, H2 biogenesis is a side reaction of nitrogenase, which normally fixes N2 and is downregulated in the presence of fixed nitrogen. Utilizable organic acids also feed a competing pathway to make polyhydroxybutyrate which detracts from the H2 yield (Fig. 1B). Redwood et al. (2012a,b) incorporated an electrodialysis step to concentrate the organic acids (by ~eightfold) and link the mixed‐acid and photofermentation steps (Fig. 2). Electrodialysis separates anions (negatively charged organic acids in the dark fermentation medium), removing them and also preventing the transfer of inhibitory into the photofermentation medium. This continuous dual fermentation process combines high H2 production rates and yield (Redwood et al., 2012b); the electrical energy demand of electrodialysis is counterbalanced, in part, by a third H2 stream from electrolysis of water.
Figure 2.

System for energy delivery from wastes via biohydrogen A fusion of chemical and biochemical engineering for conversion of waste into electricity via integrated biohydrogen technology. Electrodialysis (ED) separates the organic acid (OA) products from the mixed‐acid fermentation of (e.g.) E. coli (formate is converted to H2 + CO 2 via formate hydrogen lyase). OAs pass from the dark fermentation medium to the photofermentation, typically being concentrated by ~eightfold via electrodialysis for dilution into the photofermentation vessel. Alcohol is not removed by ED; this would require a catalytic oxidation stage to give the corresponding organic acid; this has been achieved via using Au(0) nanoparticle catalyst made on E. coli cells (Deplanche et al., 2007). Two bio‐H2 streams are formed from the combined dark‐ and photofermentations, with a third H2 stream from electrolysis of water. The maximum H2 yield from the mixed‐acid fermentation is 2 mol sugar−1; hence, the dark fermentation can be viewed as a generator of OAs rather than as the primary H supply. A schematic of upstream waste conversion into sugar feed is shown (see text), and downstream use of hydrogen in a fuel cell for electricity production. Note that bio‐H2 is free of catalyst poisons, which extends fuel cell life. Not all wastes (e.g. sugary fruits, bakery products) require extensive upstream treatment. The main box is the biotechnology; the grey flow sheet is the chemical engineering required to realize the positive energy balance. Both are equally important.
Redwood (2007) calculated the break‐even current efficiency to quantify the role played by specific organic acids (Table 1). Butyrate is the most attractive organic acid for electrodialysis with the lowest break‐even current efficiency at 13% (Table 1). Butyrate is a neglected organic acid product from E. coli which can predominate under some conditions (Redwood, 2007; R.L. Orozco unpublished). Using this example (Figs 1 and 2), the energy balance for bio‐H2 (via fermentation of food waste) exceeded that from anaerobic digestion, wind and solar power, even without factoring in the additional electrochemically made H2. (Redwood et al., 2012b). Although ~half of the organic acid is available (anionic) at the pH of the fermentation (according to the pK a values: Table 1), the electrodialysis chamber itself is alkaline due to OH− release.
Table 1.
Properties of organic acids relevant to their separation from spent medium by electrodialysis
| Organic acid | Carbons | Valence | pK a | HPP mol−1 | BCE (%) |
|---|---|---|---|---|---|
| Butyrate | 4 | 1 | 4.81 | 10 | 13 |
| Lactate | 3 | 1 | 3.86 | 6 | 21.6 |
| Formate | 1 | 1 | 3.75 | 2 | N/A |
| Acetate | 2 | 1 | 4.76 | 7 | 32.5 |
| Succinate | 4 | 2 | 4.19, 5.57 | 7 | 27.1 |
The break‐even current efficiency (BCE: (energy expended/energy gained) × 100)) was calculated for individual organic acids. The lower the BCE, the less energy required to transport the organic acid. The electrical energy required for organic acid transport via electrodialysis relates to the number of charges and number of carbons; butyrate (4 carbons, 1 charge) is the most favourable and also has the highest proportion of charged butyrate (c.f. butyric acid) according to the pKa. HPP is hydrogen production potential of the dual system as defined by Eroğlu et al. (2004).
Two key findings are salient. First, the role of the dark fermentation is more important as a supply of organic acids into the photofermentation than for its bio‐H2 per se. Second, recent work (R.L. Orozco and A.J. Stephen, unpublished) showed that the H2 yield in the photofermentation was largely independent of the actual organic acid proportions in the feed from the mixed‐acid fermentation and was optimal at ~40 mM organic acids. Hence, any source of organic acids could be potentially used from a dual system or, indeed, in a stand‐alone photofermentation.
Bacterial photofermentation
Purple non‐sulfur photosynthetic bacteria produce H2 from a variety of organic substrates including organic acids (Lazaro et al., 2012), sugars (Keskin and Hallenbeck, 2012) and industrial and agricultural effluents (Saratale et al., 2013), with high H2 yields from acetic, butyric and lactic acids (Hallenbeck, 2013). Bacteria used include Rhodobacter sphaeroides (Han et al., 2013), R. rubrum (Zürrer and Bachofen, 1979), R. palustris (Oh et al., 2004; Xiaobing, 2012) and R. capsulatus (Zhang et al., 2016); despite some differences, they all follow a similar general scheme (Fig. 1B), metabolizing organic acids to reduce NAD+ to the cellular reductant NADH (Oh et al., 2013). Excess reductant must be dissipated to reoxidize NADH and maintain cellular redox balance. This is achieved via cellular growth, channelling of carbon into cellular reserves (synthesis of polyhydroxybutyrate) or via H2 production under nitrogen‐deficient conditions, via nitrogenase, which produces H2 as an electron sink for excess reducing power (as with cyanobacteria: above). Nitrogenase normally fixes N2 into NH3 under light (to supply the large energy demand of N‐fixation, via ATP). Without N, the enzyme uses the reductant and ATP to produce H2 (2H+ + 2e − + 4 ATP ⇨ H2 + 4ADP + Pi). NADH is not a sufficiently strong reductant for this reaction; it is ‘upgraded’ to the stronger reductant ferredoxin via the input of energy, which is supplied by light through the action of the photosynthetic apparatus, via reverse electron transport. This apparatus also produces the ATP required for nitrogenase action (Hallenbeck, 2011). Various papers have studied the role of light (e.g. Uyar et al., 2007; Nath, 2009), showing that optimum light conversion efficiency occurs at light intensities much lower than light saturation points; e.g. Uyar et al. (2007) showed light saturation for R. sphaeroides at 270 W m−2 but similar substrate conversion efficiency could be achieved at light intensities as low 88 W m−2. Furthermore, optimum light intensities can be species specific; e.g. R. sphaeroides and R. palustris under similar conditions (Light intensity = 2500 Lux) had substrate conversion efficiencies of 60–70% and 47% respectively (Han et al., 2013; Oh et al., 2013).
Hallenbeck and Liu (2015) reviewed advances in the field, highlighting various approaches to improve substrate conversion efficiency (Table 2), while recent publications provide an up‐to‐date overview of recent developments for photobiological biohydrogen technologies (Adessi et al., 2017; Hallenbeck, 2017).
Table 2.
Some approaches to increase photofermentation H productivity (Reviewed by Adessi et al., 2017)
| Approach/Rationale | Outcomes/comments | References |
|---|---|---|
| ‘Black box’ mathematical relationships between input and output streams Box‐behnken statistical design/methods | Permits multivariable analysis: measures cause and effect; hence can be empirical SCE (glycerol) > doubled (R.palustris) | Abo‐Hashesh et al. (2013), Show and Lee (2013) and Ghosh et al. (2012a,b,c) |
| Modelling metabolic fluxes | Guided interventions: success using lactate but not malate or acetate | Golomysova et al. (2010) and Hädicke et al. (2011) |
| Deletion of polyhydroxybutyrate synthesis pathway | Increased H2 yield (by 1.5‐fold c.f. wild type) | Kim et al. (2011) |
| Reducing pigment concentration | Allows greater light penetrationa | Ma et al. (2012) |
| Use of quantum dots to ‘upgrade’ light | Doubled photosynthetic efficiency | M.D. Redwood, unpublishedb |
SCE, substrate conversion efficiency.
27% increase in H2 yield was obtained.
Collaborative study with Photon Science Institute, University of Manchester: M.D. Redwood, L.E. Macaskie and D.J. Binks, unpublished work. But note: current commercial quantum dots would be grossly uneconomic at scale.
Towards an economically competitive biohydrogen process from waste
Table 3 summarizes various options for a biohydrogen process. In the UK, food wastes at scale are generally centralized and ‘committed’ by agreements into anaerobic digestion and a ‘bolt‐on’ addition into existing anaerobic digestion and combined heat and power (CHP) processes is one option as there is insufficient waste available for a realistic stand‐alone bio‐H2 process (unpublished survey; Sustainable Resource Solutions Ltd). Agricultural wastes are currently unattractive due to high energy demands of comminution/maceration and upstream hydrolysis. A survey of wastes has indicated that vinasse (from bioethanol production) and in‐process streams from UK Utility companies contain sufficient organic acids to warrant trialling for data into a full life cycle analysis.
Table 3.
Options for delivery of bio‐H2 into power, all via electro‐photofermentation (Figs 1 and 2; M.D. Redwood, R.L.Orozco and L.E. Macaskie, unpublished work)a
| Feedstock (upstream) | Power (downstream) | Comments |
|---|---|---|
| Fermentation of food wastes | Fuel cell electricityb or combined heat and powerc | Food wastes (FW) required (tonnages). Anaerobic digestion (AD) has monopoly on FW. Bio‐H2 can power a fuel cell directly. |
| Fermentation of cellulosic wastes | Fuel cell electricity or CHP | Comminution/maceration energy demand adversely affects overall energy balancee. Upstream hydrolysis is required. |
| OAs obtained from anaerobic digestion (AD) | ‘Hythane’: mix of CH4 (AD) + bio‐H2; CHP | AD interrupted at acetogenesis stage; organic acids diverted into a bolt‐on photofermentation. Overall AD residence time is reduced. This increases process complexity but gives a higher energy output. Gas is compatible with current infrastructure. Scenario 1: 20% more powerd. Scenario 2: 70% more powerd |
| OAs used directly from wastes (e.g. wastewaters) or CHP | Fuel cell electricity | Organic acid waste streams (tonnage scale) are (e.g.) vinasse (from bioethanol production) and municipal wastewater treatment plants (see text). |
Calculations were made independently of incentivization schemes as these tend to be ephemeral and skew the longer term picture. Likewise, increasing/decreasing feed‐in tariffs would complicate economic assessments.
Fuel cell technology is still emergent at large scale, and FCs fail prematurely (see Rabis et al., 2012).
Combined heat and power (CHP: well‐established technology). In this scenario, the methane stream from anaerobic digestion can be supplemented with photofermentatively derived H2 to make ‘hythane’ for CHP.
Scenario 1: diversion of 10% of the organic acids into photofermentation and use of hythane in CHP. Scenario 2: diversion of 80% of the organic acids into photofermentation and use of AD‐methane in CHP plus use of the photofermentation H2 in a fuel cell would give 70% more power (R.L. Orozco, unpublished). The proportion of flow diverted from the acetogenesis step of anaerobic digestion (via electroseparation) could be simply ramped in response to incident light intensity to feed the photofermentation; at night the flow would pass to the methanogenic reactor as normal. By combining the two processes, the residence time in the system would also be reduced as compared to traditional anaerobic digestion due to reduced flow entering the methanogenesis reactor daily.
Using Miscanthus as an example, the energy demand of comminution to 4 mm particles is 184 kJ kg dry matter−1; energy from H is 10 kJ l−1 (at 1 atm and 125°C); that from the dark fermentation was only 110 kJ kg cellulose; hydrolysate; hence the PF (~4 times the H2 as the dark fermentation) is key to a positive energy balance from complex substrates.
The organic acid content of a typical vinasse waste is > 40 g l−1 (Ryznar‐Luty et al., 2008; Esapaňa‐Gamboa et al., 2012); the high concentration of betaine (trimethylglycine, a zwitterionic osmoprotectant; 20 g l−1) is not potentially problematic because at the low pH of vinasse (pH 3–4), it would be protonated (i.e. inaccessible to the anion transfer in electroseparation). Moreover, betaine was reported to stimulate nitrogenase activity, but it was not used as a nitrogen source (Igeňo et al., 1995).
Selected UK utility company wastewaters were trialled as potential targets for hydrogen bioenergy following filtration to remove debris but with no other modifications (Fig. 3). The energy production potential from biohydrogen via photofermentation was twice that from biogas (Fig. 3). Hence, H2 energy from organic acid wastes is a viable option for energy production by heavily populated, industrialized countries but may be limited seasonally by available natural sunlight. Stand‐alone photobiological hydrogen production has major potential in solar‐rich countries with the option to also treat wastes in areas of high population density. An environmental life cycle analysis has been developed for cyanobacterial bio‐H2 (Archer et al., 2017). The next step is to apply a similar LCA for various options with respect to geographical location, other socio‐economic factors and the global increase in demand for cooling to safeguard food supplies for expanding populations.
Figure 3.

Energy production potential (EPP) from use of real wastewater organic acids in a stand‐alone photofermentation (real test data using R. sphaeroides: R.L.Orozco, I. Mikheenko and L.E. Macaskie, unpublished). As an organic acids liquid stream is used directly, the upstream dark fermentation is not required, and there is no sacrificial energy demand for maceration.
Conflict of Interest
None declared.
Acknowledgements
AJS and SAA acknowledge with thanks studentships from the EPSRC Doctoral Training Centre ‘Fuel Cells and their Fuels’. The work was supported in part by NERC (grant NE/L014076/1) to LEM.
Microbial Biotechnology (2017) 10(5), 1120–1127
Funding Information
Natural Environment Research Council (NE/L014076/1).
References
- Abo‐Hashesh, M. , Desaunay, N. , and Hallenbeck, P.C. (2013) High yield single stage conversion of glucose to hydrogen by photofermentation with continuous cultures of Rhodobacter capsulatus JP91. Biores Technol 128: 513–517. [DOI] [PubMed] [Google Scholar]
- Adessi, A. , Corneli, E. , and Philippi, S. (2017) Photosynthetic purple nonsulfur bacteria in hydrogen production systems: new approaches in the use of well known and innovative substrates In Modern Topics in the Phototrophic Prokaryotes. Hallenbeck P.C. (ed.). Cham, Switzerland: Springer, pp. 321–350. [Google Scholar]
- Anon (2017) URL http://www.hydrogeneurope.eu/wp-content/uploads/2017/01/170113-Hydrogen-Council-International-Press-Releases.pdf.
- Archer, S.A. , Murphy, R.J. and Steinberger‐Wilckens, R. . (2017). Systematic review and life cycle analysis of biomass derived fuels for solid oxide fuel cells In Proceedings: Fuel Cell and Hydrogen Technical Conference 2017. 31st May – 1st June 2017. Birmingham, UK. [Google Scholar]
- Balachandar, G. , Khanna, N. , and Das, D. (2013) Biohydrogen production from organic wastes by dark fermentation In Biohydrogen. Pandey A., Chang J.‐S., Hallenbeck P.C., and Larroche C. (eds). Burlington, MA, USA: Elsevier, pp. 103–144. [Google Scholar]
- Chang, A.C.C. , Chang, H.‐F. , Lin, F.‐J. , Lin, K.‐H. , and Chen, C.‐H. (2011) Biomass gasification for hydrogen production. Int J Hydrogen Energy 36: 14252–14260. [Google Scholar]
- Deplanche, K. , Attard, G.A. , and Macaskie, L.E. (2007) Biorecovery of gold from jewellery waste by Escherichia coli and biomanufacture of active Au‐nanomaterial. Adv Mater Res 20–21: 647–652. [Google Scholar]
- Dincer, I. , and Acar, C. (2015) Review and evaluation of hydrogen production methods for better sustainability. Int J Hydrogen Energy 40: 11094–11111. [Google Scholar]
- DOE (2013). Report of the hydrogen production expert panel: a subcommittee of the hydrogen and fuel cell technical advisory committee. URL https://www.hydrogen.energy.gov/pdfs/hpep_report_2013.pdf.
- Eroğlu, E. , Gündüz, U. , Yücel, M. , Türker, L. , and Eroğlu, I. (2004) Photobiological hydrogen production by using olive mill wastewater as a sole substrate source. Int J Hydrogen Energy 29: 163–171. [Google Scholar]
- Esapaňa‐Gamboa, E. , Mijangos‐Cortēs, J.O. , Hernández‐Zárate, G. , Maldonado, J.A.D. , and Alzate‐Gaviria, L.M. (2012) Methane production from hydrous ethanol using a modified UASB. Biotechnol Biofuels 5: 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, D. , Sobro, I.F. , and Hallenbeck, P.C. (2012a) Optimization of the hydrogen yield from single‐stage photofermentation of glucose by Rhodobacter capsulatus JP91 using response surface methodology. Biores Technol 123: 199–206. [DOI] [PubMed] [Google Scholar]
- Ghosh, D. , Sobro, I.F. , and Hallenbeck, P.C. (2012b) Stoichiometric conversion of biodiesel derived crude glycerol to hydrogen: response surface methodology study of the effects of light intensity and crude glycerol and glutamate concentration. Biores Technol 106: 154–160. [DOI] [PubMed] [Google Scholar]
- Ghosh, D. , Tourigny, A. , and Hallenbeck, P.C. (2012c) Near stoichiometric reforming of biodiesel derived crude glycerol to hydrogen by photofermentation. Int J Hydrogen Energy 37: 2273–2277. [Google Scholar]
- Golomysova, A. , Gomelsky, M. , and Ivanov, P.S. (2010) Flux balance analysis of photoheterotrophic growth of purple nonsulfur bacteria relevant to biohydrogen production. Int J Hydrogen Energy 35: 12751–12760. [Google Scholar]
- Guo, X.M. , Trably, E. , Latrille, E. , Carrère, H. and Steyer, J.P. (2010) Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrogen Energy 35, 10660–10673. [Google Scholar]
- Hädicke, O. , Grammel, H. , and Klamt, S. (2011) Metabolic network modeling of redox balancing and biohydrogen production in purple nonsulfur bacteria. BMC Syst Biol 5: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck, P.C. (2011) Microbial paths to renewable hydrogen production. Biofuels 2: 285–302. [Google Scholar]
- Hallenbeck, P.C. (2012) Fundamentals of dark hydrogen production: multiple pathways and enzymes In State of the Art and Progress in Biohydrogen. Azbar N., and Levin D. (eds). Sharjah, United Arab Emirates: Bentham Science Publishers, pp. 94–111. [Google Scholar]
- Hallenbeck, P.C. (2013) Photofermentative biohydrogen production In Biohydrogen. Pandey A., Chang J.‐S., Hallenbeck P.C., and Larroche C. (eds). Burlington, MA, USA: Elsevier, pp. 145–159. [Google Scholar]
- Hallenbeck P.C. (ed) (2017) Modern Topics in the Phototrophic Prokaryotes. Cham, Switzerland: Springer. [Google Scholar]
- Hallenbeck, P.C. , and Ghosh, D. (2009) Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol 27: 287–297. [DOI] [PubMed] [Google Scholar]
- Hallenbeck, P.C. , and Liu, Y. (2015) Recent advances in hydrogen production by photosynthetic bacteria. Int J Hydrogen Energy 41: 4446–4454. [Google Scholar]
- Han, H. , Jia, Q. , Liu, B. , Yang, H. , and Shen, J. (2013) Fermentative hydrogen production from acetate using Rhodobacter sphaeroides RV. Int J Hydrogen Energy 38: 10773–10778. [Google Scholar]
- Igeňo, M.I. , Del Moral, C.G. , Castillo, F. , and Caballero, J. (1995) Halotolerenace of the phototrophic bacterium Rhodobacter capsulatus E1F1 is dependent on the nitrogen source. Appl Environ Microbiol 61: 2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B.D. , Baum, G.N. , Perez, J. and Baum, K.N. (2009) Technoeconomic boundary analysis of biological pathways to hydrogen production. NREL Technical Monitor: Ali Jalalzadeh‐Azar, A. Subcontract No. AFH‐8‐88601‐01. Subcontract Report. NREL/SR‐560‐46674. Golden, CO, USA: NREL, pp 1–207. [Google Scholar]
- Keskin, T. , and Hallenbeck, P.C. (2012) Hydrogen production from sugar industry wastes using single‐stage photofermentation. Biores Technol 112: 131–136. [DOI] [PubMed] [Google Scholar]
- Kim, M.S. , Kim, D.H. , Son, H.N. , Ten, L.N. , and Lee, J.K. (2011) Enhancing photo‐fermentative hydrogen production by Rhodobacter sphaeroides KD131 and its PHB synthase deleted‐mutant from acetate and butyrate. Int J Hydrogen Energy 36: 13964–13971. [Google Scholar]
- Kubas, A. , Orain, C. , De Sancho, D. , Saujet, L. , Sensi, M. , Gauquelin, C. , et al (2017) Mechanism of O2 diffusion and reduction in FeFe hydrogenases. Nat Chem 9: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro, C.Z. , Vich, D.V. , Hirasawa, J.S. , and Varesche, M.B.A. (2012) Hydrogen production and consumption of organic acids by a phototrophic microbial consortium. Int J Hydrogen Energy 37: 11691–11700. [Google Scholar]
- Ma, C. , Wang, X. , Guo, L. , Wu, X. , and Yang, H. (2012) Enhanced photo‐fermentative hydrogen production by Rhodobacter capsulatus with pigment content manipulation. Biores Technol 118: 490–495. [DOI] [PubMed] [Google Scholar]
- Mohan, S.V. , and Pandey, A. (2013) Biohydrogen production: an introduction In Biohydrogen. Pandey A., Chang J.‐S., Hallenbeck P.C., and Larroche C. (eds). Burlington, MA, USA: Elsevier, pp. 1–24. [Google Scholar]
- Nakada, E. , Nishikata, S. , Asada, Y. , and Miyake, J. (1999) Photosynthetic bacterial hydrogen production combined with a fuel cell. Int J Hydrogen Energy 24: 1053–1057. [Google Scholar]
- Nath, K. (2009) Effect of light intensity and initial pH during hydrogen production by an integrated dark and photofermentation process. Int J Hydrogen Energy 34: 7497–7501. [Google Scholar]
- Nikolaidis, P. , and Poullikkas, A. (2017) A comparative overview of hydrogen production processes. Renew Sustain Energy Rev 67: 597–611. [Google Scholar]
- Oh, Y.‐K. , Seol, E.‐H. , Kim, M.‐S. , and Park, S. (2004) Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int J Hydrogen Energy 29: 1115–1121. [Google Scholar]
- Oh, Y.K. , Raj, S.M. , Jung, G.Y. , and Park, S. (2013) Metabolic engineering of microorganisms for biohydrogen production In Biohydrogen. Pandey A., Chang J.‐S., Hallenbeck P.C., and Larroche C. (eds). Burlington, MA, USA: Elsevier, pp. 45–65. [Google Scholar]
- Rabis, A. , Rodriguez, P. , and Schmidt, J.J. (2012) Electrocatalysis for polymer electrolyte fuel cells. Recent achievements and future challenges. ACS Catal 2: 864–890. [Google Scholar]
- Redwood, M.D. . (2007) Bio‐hydrogen production and biomass‐supported palladium catalyst for energy production and waste minimization. PhD Thesis. Burlington, MA, UK: University of Birmingham. [Google Scholar]
- Redwood, M.D. , Orozco, R.L. , Majewski, A.J. , and Macaskie, L.E. (2012a) Electro‐extractive fermentation for efficient biohydrogen production. Biores Technol 107: 166–174. [DOI] [PubMed] [Google Scholar]
- Redwood, M.D. , Orozco, R.L. , Majewski, A.J. , and Macaskie, L.E. (2012b) An integrated biohydrogen refinery: synergy of photofermentation, extractive fermentation and hydrothermal hydrolysis of food wastes. Biores Technol 119: 384–392. [DOI] [PubMed] [Google Scholar]
- Ryznar‐Luty, A. , Krzywonos, M. , Obis, E. , and Miskiewicz, T. (2008) Aerobic biodegradation of vinasse by a mixed culture of bacteria of the genus Bacillus. Optimization of temperature, pH and oxygenation state. Pol J Environ Stud 17: 101–112. [Google Scholar]
- Saratale, G.D. , Saratale, R.G. , and Chang, J.‐S. (2013) Biohydrogen from renewable resources In Biohydrogen. Pandey A., Chang J.‐S., Hallenbeck P.C., and Larroche C. (eds). Burlington, MA, USA: Elsevier, pp. 185–221. [Google Scholar]
- Show, K.Y. , and Lee, D.J. (2013) Biioreactor and bioprocess design for biohydrogen production In Biohydrogen. Pandey A., Chang J.‐S., Hallenbeck P.C., and Larroche C. (eds.). Burlington, MA, USA: Elsevier, pp. 317–337. [Google Scholar]
- Singh, J.S. , Kumar, A. , Rai, A.N. , and Singh, D.P. (2016) Cyanobacteria: a precious bio‐resource in agriculture, ecosystem and environmental sustainability. Front Microbiol 7: 1–19. article 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahan, D. . (ed.) (2017) Clean cold and the global goals. URL www.birmingham.ac.uk/Documents/college-eps/energy/Publications/Clean-Cold-and-the-Global-Goals.pdf.
- Tiwari, A. , and Pandey, A. (2012) Cyanobacterial hydrogen‐ a step towards clean environment. Int J Hydrogen Energy 37: 139–150. [Google Scholar]
- Uyar, B. , Eroglu, I. , Yücel, M. , Gündüz, U. , and Türker, L. (2007) Effect of light intensity, wavelength and illumination protocol on hydrogen production in photobioreactors. Int J Hydrogen Energy 32: 4670–4677. [Google Scholar]
- Xiaobing, W. (2012) Enhanced photo‐fermentative hydrogen production from different organic substrate using hupL inactivated Rhodopseudomonas palustris . Afr J Microbiol Res 6: 5362–5370. [Google Scholar]
- Zhang, Y. , Yang, H. , and Guo, L. (2016) Enhancing photo‐fermentative hydrogen production performance of Rhodobacter capsulatus by disrupting methylmalonate‐semialdehyde dehydrogenase gene. Int J Hydrogen Energy 41: 190–197. [Google Scholar]
- Zürrer, H. , and Bachofen, R. (1979) Hydrogen production by the photosynthetic bacterium Rhodospirillum rubrum . Appl Environ Microbiol 37: 789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]


