Abstract
Fungal biomineralization of carbonates results in metal removal from solution or immobilization within a solid matrix. Such a system provides a promising method for removal of toxic or valuable metals from solution, such as Co, Ni, and La, with some carbonates being of nanoscale dimensions. A fungal Mn carbonate biomineralization process can be applied for the synthesis of novel electrochemical materials.

Carbonate biomineralization
The term biomineralization refers to the collective processes by which organisms form minerals (Gadd, 2010). Biomineralization can be categorized into biologically‐induced mineralization (BIM) and biologically‐controlled mineralization (BCM). BIM occurs when an organism modifies its local microenvironment to create conditions for mineral precipitation, while in BCM complex cellular control mechanisms exist such as in the formation of silicaceous tests in diatoms (Gadd, 2010; Gadd and Raven, 2010; Rhee et al., 2012; Kumari et al., 2016). Most microbial biomineralization examples refer to biologically induced mineralization. Biomineralization of carbonates has received wide attention. Carbonate minerals, especially the rock‐forming minerals calcite (CaCO3) and dolomite (CaMg(CO3)2), occur in abundance on the Earth's surface as limestones (Burford et al., 2006; Ehrlich and Newman, 2008; Lippmann, 2012). Modern mineralogical methods have revealed that a significant proportion of such carbonate minerals at the Earth's surface is of biogenic origin, and many microbial species, including cyanobacteria, bacteria, microalgae and fungi, can deposit calcium carbonate extracellularly (Goudie, 1996; Verrecchia, 2000; Burford et al., 2006; Barua et al., 2012; Achal et al., 2015; Kumari et al., 2016). Carbonates of calcium and other metals are also significant substances used in a wide variety of industrial and agricultural applications. The process of microbial carbonate biomineralization has been investigated as a promising bioremediation strategy for toxic metal immobilization in soil (Kumari et al., 2016; Zhu et al., 2016a) as well as soil stabilization and the development of biocements and biogrouts for construction purposes (Achal et al., 2015; Li et al., 2015a,b). It is now known that some carbonate biominerals may be deposited in nanoscale dimensions (Li et al., 2014, 2016), providing further significant physical, chemical and biological properties of applied significance (Hochella et al., 2008). This article will describe the potential applications of fungal‐mediated metal carbonate bioprecipitation including the development of new electrochemical materials.
Carbonate biomineralization of toxic or valuable metals
Fungal biomineralization of carbonates results in metal removal from solution or immobilization within a solid matrix providing a method for detoxification as well as recovery (Table 1). Biologically‐induced mineralization (BIM) involving urea hydrolysis by urease‐positive microorganisms, which leads to metal carbonate precipitation, has been found to be effective in immobilizing several potentially toxic metals, for example Cd, Ni, Pb, Sr, and the metalloid As (Achal, 2012; Achal et al., 2012; Li et al., 2014, 2015a,b; Zhu et al., 2016a). Urease‐positive fungi, such as N. crassa, have the ability to precipitate metal carbonates in the media and around the biomass when incubated in urea‐amended media while culture supernatants also provide a biomass‐free carbonate bioprecipitation system (Li et al., 2014, 2015a,b). In a novel application of calcium carbonate biomineralization, Li et al. (2014) demonstrated that supplied cadmium could be precipitated as pure otavite (CdCO3) by culture supernatants derived from growth of Neurospora crassa in urea‐supplemented medium. A new lead hydroxycarbonate was precipitated by Paecilomyces javanicus grown in medium containing metallic lead. Other secondary lead minerals precipitated included plumbonacrite (Pb10(CO3)6O(OH)6) and hydrocerussite (Pb3(CO3)2(OH)2) (Rhee et al., 2012). The advantage of using ureolytic microorganisms for toxic metal immobilization is their ability to efficiently immobilize metals in carbonate minerals by precipitation or co‐precipitation regardless of the metal valence state and toxicity, and the redox potential (Kumari et al., 2016). It has been suggested that such a system may also provide a promising method for removal of toxic or valuable metals from solution, such as Co, Ni and La. On addition of LaCl3 to carbonate‐laden fungal culture supernatants, fusiform‐shaped lanthanum carbonate was precipitated with approximate sizes ranging from 1 to 5 μm (Fig. 1). This is the first report of lanthanum biorecovery using geoactive fungal growth supernatants. Lanthanum, as one of the rare earth elements (REE), plays an important role in advanced new materials, such as superalloys, catalysts, specialized ceramics and organic synthesis (Kanazawa and Kamitani, 2006; Das and Das, 2013). Conventional chemical methods for La extraction are based on hydrometallurgy combined with a pyrometallurgical process which are energy intensive and produce significant amounts of chemical sludge at the same time (Wang et al., 2011; Das and Das, 2013). Various biosorbents including macroalgae (Diniz and Volesky, 2005) and bacteria (Kazy et al., 2006) have also been applied for lanthanum although, despite years of research, the credibility of metal biosorption as a commercially viable technique is very limited (Gadd, 2009).
Table 1.
Biorecovery of toxic or valuable metals by fungal carbonate biomineralization
| Metal | Fungal species | Precipitated metal carbonate | References |
|---|---|---|---|
| Ba | Verticillium sp. | BaCO3 | Rautaray et al. (2004) |
| Cd | Fusarium oxysporum, Neurospora crassa, Myrothecium gramineum, Pestalotiopsis sp. | CdCO3 | Sanyal et al. (2005); Li et al. (2014) |
| Co | N. crassa, M. gramineum, Pestalotiopsis sp., | CoCO3∙xH2O | Li, Q. and Gadd, G.M., unpublished |
| Cu | N. crassa, M. gramineum, Pestalotiopsis sp., | Cu2(OH)2CO3, Cu3(OH)2(CO3)2 | Li, Q. and Gadd, G.M., unpublished |
| La | N. crassa, M. gramineum, Pestalotiopsis sp. | La2(CO3)3·8H2O | Li, Q. and Gadd, G.M., unpublished |
| Ni | N. crassa, M. gramineum, Pestalotiopsis sp. | NiCO3∙xH2O | Li, Q. and Gadd, G.M., unpublished |
| Pb | F. oxysporum, Paecilomyces javanicus | PbCO3, Pb3(CO3)2(OH)2, Pb10(CO3)6O(OH)6), lead hydroxycarbonatea | Sanyal et al. (2005); Rhee et al. (2015) |
| Sr | F. oxysporum, N. crassa, M. gramineum, Pestalotiopsis sp. | (CaxSr1‐x)CO3), Sr(Sr, Ca)(CO3)2, SrCO3 | Li and Gadd, unpublished |
| Zn | N. crassa, M. gramineum, Pestalotiopsis sp. | (ZnCO3)2·(Zn(OH)2)3 | Li and Gadd, unpublished |
aPrecise formula not identified.
Figure 1.
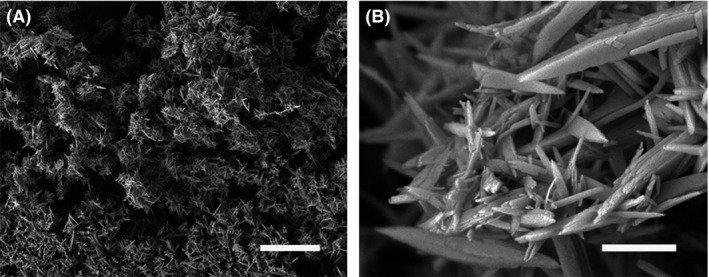
Scanning electron microscopy images of lanthanum carbonate precipitated on addition of LaCl3 to a culture supernatant derived from growth of Neurospora crassa in urea‐supplemented medium. Scale bars: (A) = 20 μm, (B) = 1 μm. Typical images are shown from many similar examples (Li, Q. and Gadd, G.M., unpublished).
Compared to the simpler bacterial cell form, the fungal filamentous growth habit can provide more framework support and stability for the precipitation of carbonates or other biominerals (Kumari et al., 2016). Moreover, the physicochemical properties of formed biominerals can also be influenced by biological processes, such as their surface area‐to‐volume ratio, which can show significant differences to bulk minerals (Hochella et al., 2008). This is especially true for biominerals that are produced in nanoscale dimensions. The size variation of particles results in differences in surface and near‐surface atomic structure and crystal shape as well as surface topography, which is important in geochemical reactions and kinetics (Hochella et al., 2008). Research has demonstrated that many metal‐accumulating or transforming microbes are capable of forming nanoparticles (e.g. Te, Se, CdS, HUO2PO4) (Macaskie et al., 1992; Williams et al., 1996; Dickson, 1999; Lloyd et al., 1999; Taylor 1999; Klaus‐Joerger et al., 2001; Zhu et al., 2016b). Their production by microbial systems may allow manipulation of size, morphology, composition and crystallographic orientation, with applications in bioremediation, antimicrobial treatments (e.g. nano‐silver), solar and electrochemical energy, and microelectronics (Dameron et al., 1989; Jauho and Buzaneva, 1996; Hayashi et al., 1997; Edelstein and Cammaratra, 1998; Klaus‐Joerger et al., 2001; Zhu et al., 2016b). In a ureolytic fungal‐mediated bioprecipitation system, more than 70% of supplied Co2+, Ni2+, Cu2+ or Zn2+ was precipitated in the form of hydrated carbonates and all these minerals showed a nanoscale phase. It appears that fungal metabolites, especially extracellular protein, play an important role in the formation of such nanoscale particles (Fig. 2).
Figure 2.
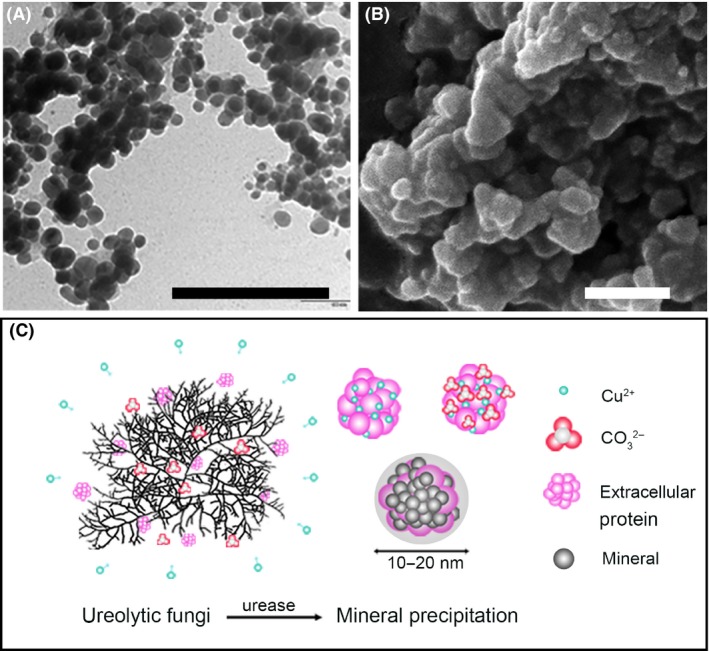
Fungal biomineralization of copper carbonate.
A. Transmission electron microscopy image of copper carbonate.
B. Scanning electron microscopy of cobalt carbonate both precipitated by addition of the metal chlorides to a culture supernatant derived from growth of Neurospora crassa in urea‐supplemented medium. Scale bars = 200 nm. Typical images are shown from many similar examples.
C. Model of copper carbonate bioprecipitation in the nanoscale (Li, Q. and Gadd, G.M., unpublished).
Carbonate biomineralization for production of electrochemical materials
Increasing consumption and the decline in fossil fuel resources have driven attention to the development of other renewable and sustainable energy sources. Electrical energy storage systems (EESS) such as rechargeable lithium‐ion batteries and electrochemical supercapacitors have shown great promise in this regard (Simon and Gogotsi, 2008; Ji et al., 2011; Liu et al., 2013; Ding et al., 2015). However, performance requirements for these systems are quite critical and Li‐ion batteries have a high specific energy density (energy stored per unit mass) and act as slow and steady energy suppliers for large energy demands. In contrast, supercapacitors possess high specific power (energy transferred per unit mass per unit time) and can charge and discharge quickly for low energy demands. Thus, in the development of electrical energy storage materials, high energy density as well as high power is important (Ding et al., 2015). Many efforts have been made to improve the electrochemical performance of supercapacitors or Li‐ion batteries by design of other safe, economic and environment‐friendly electrode materials some of which have a biotic component (Ma et al., 2007; Nakayama et al., 2007; Sharma et al., 2007; Zhu et al., 2011; Falco et al., 2012; Zhang et al., 2012d; Liu et al., 2013; Sun et al., 2013; Long et al., 2015).
Fungal interactions with metals and minerals can alter their physical and chemical state and plays a significant role in environmental element biotransformations and cycling (Kolo et al., 2007; Fomina et al., 2010; Gadd, 2010; Gadd and Raven, 2010). Fungal hyphae can provide nucleation sites for the precipitation of metals following biosorption, metabolite secretion and/or oxidation or reduction of a metal or metalloid species (Gadd, 2009, 2010). Such processes appear to have potential applications in materials science which hitherto have been rather neglected. Fungal biomass represents an abundant carbon‐neutral renewable resource that can be used for the production of bioenergy and biomaterials, and research has been carried out on the application of biomass (e.g. fungi, bacteria, microalgae) as a carbonaceous electrode material for ESS (Shim et al., 2010; Zhu et al., 2011; Falco et al., 2012). A hydrothermal assisted pyrolysis procedure was applied for the preparation of activated carbon (AC) using crude biomass of an Auricularia sp. which exhibited capacitive characteristics (stability, energy density power density, surface capacitance and volumetric capacitance) in supercapacitors. This study provided a facile method for the synthesis of carbonaceous electrode materials and highlighted the potential applications of fungi in materials science (Zhu et al., 2011). Similarly, Wang and Liu (2015) used fungal biomass as carbon precursor to prepare hierarchical porous activated carbon (AC), and the fungi‐derived AC electrode showed superior cycling performance in supercapacitors (92% retention after 10 000 cycles). Furthermore, carbonaceous materials with a high porosity obtained from biological cellular structures increases the active carbon surface area which may result in superior electrical properties. They are therefore suggested to be useful electrode materials in micro‐batteries and electrochemical capacitors because of their excellent proton‐ or lithium‐conducting properties (Klaus‐Joerger et al., 2001).
Lithium‐ion batteries with high storage capacities and cycling stability are considered to be another promising power source. The performance of a Li‐ion battery is based on the diffusion of Li ions between the anode and the cathode, converting chemical energy to electrical energy which is stored within the battery. For commercial Li‐ion batteries, graphite is the most common anode material due to its low cost and long cycle life. However, some deficiencies of conventional graphite carbon, such as a high sensitivity to the electrolyte and a low charge capacity, can limit the electrochemical performance of Li‐ion batteries. In order to improve the power density and capacity of Li‐ion batteries, various other anode materials have been developed to meet high electrochemical requirements such as carbon nanotubes (CNTs) (Pol and Thackeray, 2011) and manganese oxides (MnO, MnO2, Mn2O3, Mn3O4), which have excellent electrochemical properties (Xia et al., 2013).
It is accepted that the addition of metal oxides to a carbonaceous substrate will increase the electrochemical performance of electrode materials, especially for transition metal oxides (e.g. CoxOy, VxOy, FexOy) and those in the nanoscale, with variable oxidation states, are excellent candidates for electrode materials (Poizot et al., 2000; Dillon et al., 2008; Amade et al., 2011; Wu et al., 2012; Devaraj et al., 2014). Metal carbonates can be very good precursors for preparation of metal oxides. Thus, a fungal Mn biomineralization process based on urease‐mediated manganese carbonate bioprecipitation has been applied for the synthesis of novel electrochemical materials (Li et al., 2016). Manganese carbonate encrusted mycelium of N. crassa was heat treated (300°C, 4 h) to convert the biomass/precipitated MnCO3 to a MnOx/C composite material. The electrochemical performance of this biogenic MnOx/C was investigated in a hybrid asymmetric supercapacitor as well as in a lithium‐ion battery. The carbonized fungal biomass‐mineral composite (MycMnOx/C) showed a high specific capacitance (> 350 F g−1) in a supercapacitor and excellent cycling stability (> 90% capacity was retained after 200 cycles) in a lithium‐ion battery. This was the first demonstration of the synthesis of electrode materials using a fungal biomineralization process and therefore indicates a novel method for the sustainable synthesis of electrochemical materials.
Future prospects
With the depletion of high‐grade mineral resources and increasing energy costs, adverse environmental effects are becoming more apparent from conventional technologies. Microbial‐based biotechnologies could provide economic alternative methods for the recycling of toxic or valuable metals, and a simplified approach for the synthesis of biomaterials for bioenergy and other applications. Fungal‐mediated metal carbonate precipitation suggests that these organisms can play a role in the environmental fate, bioremediation or biorecovery of metals and radionuclides that form insoluble carbonates and also indicates novel strategies for the preparation of sustainable electrochemical materials and other biomineral products.
Conflict of interest
None declared.
Acknowledgements
The authors gratefully acknowledge the help of Dr. Yongchang Fan (Division of Physics, University of Dundee, Dundee, UK) for assistance with scanning electron microscopy and transmission electron microscopy. Financial support in the author's laboratory is received from the Natural Environment Research Council (NE/M010910/1 (TeaSe); NE/M011275/1 (COG3)), which is gratefully acknowledged. We also acknowledge financial support from the China Scholarship Council through a PhD scholarship to Q.L. (No. 201206120066) and support from the Science Foundation of the China University of Petroleum, Beijing (No. 2462017YJRC010).
Microbial Biotechnology (2017) 10(5), 1131–1136
Funding information
Financial support in the author's laboratory is received from the Natural Environment Research Council (NE/M010910/1 (TeaSe); NE/M011275/1 (COG3)), which is gratefully acknowledged. We also acknowledge financial support from the China Scholarship Council through a PhD scholarship to Q.L. (No. 201206120066) and support from the Science Foundation of the China University of Petroleum, Beijing (No. 2462017YJRC010).
References
- Achal, V. (2012) Bioremediation of Pb‐contaminated soil based on microbially induced calcite precipitation. J Microbiol Biotechnol 22: 244–247. [DOI] [PubMed] [Google Scholar]
- Achal, V. , Pan, X. , Fu, Q. , and Zhang, D. (2012) Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli . J Hazard Mater 201–202: 178–184. [DOI] [PubMed] [Google Scholar]
- Achal, V. , Mukherjee, A. , Kumari, D. , and Zhang, Q. (2015) Biomineralization for sustainable construction – a review of processes and applications. Earth‐Sci Rev 148: 1–17. [Google Scholar]
- Amade, R. , Jover, E. , Caglar, B. , Mutlu, T. , and Bertran, E. (2011) Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application. J Power Sources 196: 5779–5783. [Google Scholar]
- Barua, B.S. , Suzuki, A. , Pham, H.N.D. , and Inatomi, S. (2012) Adaptation of ammonia fungi to urea enrichment environment. J Agric Technol 8: 173–189. [Google Scholar]
- Burford, E.P. , Hillier, S. , and Gadd, G.M. (2006) Biomineralization of fungal hyphae with calcite (CaCO3) and calcium oxalate mono‐ and dihydrate in carboniferous limestone microcosms. Geomicrobiol J 23: 599–611. [Google Scholar]
- Dameron, C. , Reese, R. , Mehra, R. , Kortan, A. , Carroll, P. , Steigerwald, M. , et al (1989) Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 338: 596–597. [Google Scholar]
- Das, N. , and Das, D. (2013) Recovery of rare earth metals through biosorption: an overview. J Rare Earths 31: 933–943. [Google Scholar]
- Devaraj, S. , Liu, H.Y. , and Balaya, P. (2014) MnCO3: a novel electrode material for supercapacitors. J Mater Chem A 2: 4276–4281. [Google Scholar]
- Dickson, D.P. (1999) Nanostructured magnetism in living systems. J Magn Magn Mater 203: 46–49. [Google Scholar]
- Dillon, A. , Mahan, A. , Deshpande, R. , Parilla, P. , Jones, K. , and Lee, S. (2008) Metal oxide nano‐particles for improved electrochromic and lithium‐ion battery technologies. Thin Solid Films 516: 794–797. [Google Scholar]
- Ding, J. , Wang, H. , Li, Z. , Cui, K. , Karpuzov, D. , Tan, X. , et al (2015) Peanut shell hybrid sodium ion capacitor with extreme energy‐power rivals lithium ion capacitors. Energy Environ Sci 8: 941–955. [Google Scholar]
- Diniz, V. , and Volesky, B. (2005) Biosorption of La, Eu and Yb using Sargassum biomass. Water Res 39: 239–247. [DOI] [PubMed] [Google Scholar]
- Edelstein, A.S. , and Cammaratra, R. (1998) Nanomaterials: Synthesis, Properties and Applications. New York: CRC Press/Taylor and Francis Group. [Google Scholar]
- Ehrlich, H.L. , and Newman, D.K. (2008) Geomicrobiology, 5th edn New York: CRC Press. [Google Scholar]
- Falco, C. , Sevilla, M. , White, R.J. , Rothe, R. , and Titirici, M.M. (2012) Renewable nitrogen‐doped hydrothermal carbons derived from microalgae. Chem Sus Chem 5: 1834–1840. [DOI] [PubMed] [Google Scholar]
- Fomina, M. , Burford, E.P. , Hillier, S. , Kierans, M. , and Gadd, G.M. (2010) Rock‐building fungi. Geomicrobiol J 27: 624–629. [Google Scholar]
- Gadd, G.M. (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84: 13–28. [Google Scholar]
- Gadd, G.M. (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiol 156: 609–643. [DOI] [PubMed] [Google Scholar]
- Gadd, G.M. , and Raven, J.A. (2010) Geomicrobiology of eukaryotic microorganisms. Geomicrobiol J 27: 491–519. [Google Scholar]
- Goudie, A. (1996) Organic agency in calcrete development. J Arid Environ 32: 103–110. [Google Scholar]
- Hayashi, C. , Uyeda, R. , and Tasaki, A. (1997) Ultra‐fine Particles: Exploratory Science and Technology. Norwich: William Andrew Publishing. [Google Scholar]
- Hochella, M.F. , Lower, S.K. , Maurice, P.A. , Penn, R.L. , Sahai, N. , Sparks, D.L. , and Twining, B.S. (2008) Nanominerals, mineral nanoparticles, and Earth systems. Science 319: 1631–1635. [DOI] [PubMed] [Google Scholar]
- Jauho, A.P. , and Buzaneva, E.V. (1996) Frontiers in Nanoscale Science of Micron/submicron Devices. Dordrecht, the Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Ji, L.W. , Lin, Z. , Alcoutlabi, M. , and Zhang, X.W. (2011) Recent developments in nanostructured anode materials for rechargeable lithium‐ion batteries. Energy Environ Sci 4: 2682–2699. [Google Scholar]
- Kanazawa, Y. , and Kamitani, M. (2006) Rare earth minerals and resources in the world. J Alloys Compd 408: 1339–1343. [Google Scholar]
- Kazy, S.K. , Das, S.K. , and Sar, P. (2006) Lanthanum biosorption by a Pseudomonas sp.: equilibrium studies and chemical characterization. J Indust Microbiol Biotechnol 33: 773–783. [DOI] [PubMed] [Google Scholar]
- Klaus‐Joerger, T. , Joerger, R. , Olsson, E. , and Granqvist, C.G. (2001) Bacteria as workers in the living factory: metal‐accumulating bacteria and their potential for materials science. Trends Biotechnol 19: 15–20. [DOI] [PubMed] [Google Scholar]
- Kolo, K. , Keppens, E. , Préat, A. , and Claeys, P. (2007) Experimental observations on fungal diagenesis of carbonate substrates. J Geophys Res: Biogeosci 112: G01007. [Google Scholar]
- Kumari, D. , Qian, X.Y. , Pan, X. , Achal, V. , Li, Q. , and Gadd, G.M. (2016) Microbially‐induced carbonate precipitation for immobilization of toxic metals. Adv Appl Microbiol 94: 79–108. [DOI] [PubMed] [Google Scholar]
- Li, M. , Fu, Q.‐L. , Zhang, Q. , Achal, V. , and Kawasaki, S. (2015a) Bio‐grout based on microbially induced sand solidification by means of asparaginase activity. Sci Rep 5: 16128 https://doi.org/10.1038/srep16128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Csetenyi, L. , and Gadd, G.M. (2014) Biomineralization of metal carbonates by Neurospora crassa . Environ Sci Technol 48: 14409–14416. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Csetenyi, L. , Paton, G.I. , and Gadd, G.M. (2015b) CaCO3 and SrCO3 bioprecipitation by fungi isolated from calcareous soil. Environ Microbiol 17: 3082–3097. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Liu, D. , Jia, Z. , Csetenyi, L. , and Gadd, G.M. (2016) Fungal biomineralization of manganese oxides as novel source of electrochemical materials. Current Biol 26: 950–955. [DOI] [PubMed] [Google Scholar]
- Lippmann, F. (2012) Sedimentary Carbonate Minerals. Berlin: Springer Science and Business Media. [Google Scholar]
- Liu, J. , Zhang, J.G. , Yang, Z.G. , Lemmon, J.P. , Imhoff, C. , Graff, G.L. , et al (2013) Materials science and materials chemistry for large scale electrochemical energy storage: from transportation to electrical grid. Adv Func Mater 23: 929–946. [Google Scholar]
- Lloyd, J. , Ridley, J. , Khizniak, T. , Lyalikova, N. , and Macaskie, L. (1999) Reduction of technetium by Desulfovibrio desulfuricans: biocatalyst characterization and use in a flowthrough bioreactor. Appl Environ Microbiol 65: 2691–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. , Chen, X. , Jiang, L. , Zhi, L. , and Fan, Z. (2015) Porous layer‐stacking carbon derived from in‐built template in biomass for high volumetric performance supercapacitors. Nano Energy 12: 141–151. [Google Scholar]
- Ma, S.B. , Nam, K.W. , Yoon, W.S. , Yang, X.Q. , Ahn, K.Y. , Oh, K.H. , and Kim, K.B. (2007) A novel concept of hybrid capacitor based on manganese oxide materials. Electrochem Comm 9: 2807–2811. [Google Scholar]
- Macaskie, L.E. , Empson, R.M. , Cheetham, A.K. , Grey, C.P. , and Skarnulis, A.J. (1992) Uranium bioaccumulation by a Citrobacter sp. as a result of enzymically mediated growth of polycrystalline HUO2PO4 . Science 257: 782–784. [DOI] [PubMed] [Google Scholar]
- Nakayama, M. , Kanaya, T. , and Inoue, R. (2007) Anodic deposition of layered manganese oxide into a colloidal crystal template for electrochemical supercapacitor. Electrochem Comm 9: 1154–1158. [Google Scholar]
- Poizot, P. , Laruelle, S. , Grugeon, S. , Dupont, L. , and Tarascon, J. (2000) Nano‐sized transition‐metal oxides as negative‐electrode materials for lithium‐ion batteries. Nature 407: 496–499. [DOI] [PubMed] [Google Scholar]
- Pol, V.G. , and Thackeray, M.M. (2011) Spherical carbon particles and carbon nanotubes prepared by autogenic reactions: evaluation as anodes in lithium electrochemical cells. Energy Environ Sci 4: 1904–1912. [Google Scholar]
- Rautaray, D. , Ahmad, A. , and Sastry, M. (2004) Biological synthesis of metal carbonate minerals using fungi and actinomycetes. J Mater Chem 14: 2333–2340. [Google Scholar]
- Rhee, Y.J. , Hillier, S. , and Gadd, G.M. (2012) Lead transformation to pyromorphite by fungi. Current Biol 22: 237–241. [DOI] [PubMed] [Google Scholar]
- Rhee, Y.J. , Hiller, S. , and Gadd, G.M. (2015) A new lead hydroxycarbonate produced during transformation of lead metal by the soil fungus Paecilomyces javanicus . Geomicrobiol J 33: 1–11. [Google Scholar]
- Sanyal, A. , Rautaray, D. , Bansal, V. , Ahmad, A. , and Sastry, M. (2005) Heavy‐metal remediation by a fungus as a means of production of lead and cadmium carbonate crystals. Langmuir 21: 7220–7224. [DOI] [PubMed] [Google Scholar]
- Sharma, R.K. , Oh, H.S. , Shul, Y.G. , and Kim, H. (2007) Carbon‐supported, nano‐structured, manganese oxide composite electrode for electrochemical supercapacitor. J Power Sources 173: 1024–1028. [Google Scholar]
- Shim, H.W. , Jin, Y.H. , Seo, S.D. , Lee, S.H. , and Kim, D.W. (2010) Highly reversible lithium storage in Bacillus subtilis‐directed porous Co3O4 nanostructures. ACS Nano 5: 443–449. [DOI] [PubMed] [Google Scholar]
- Simon, P. , and Gogotsi, Y. (2008) Materials for electrochemical capacitors. Nature Mater 7: 845–854. [DOI] [PubMed] [Google Scholar]
- Sun, H.M. , He, W.H. , Zong, C.H. , and Lu, L.H. (2013) Template‐free synthesis of renewable macroporous carbon via yeast cells for high‐performance supercapacitor electrode materials. ACS Appl Mater Interfaces 5: 2261–2268. [DOI] [PubMed] [Google Scholar]
- Taylor, D.E. (1999) Bacterial tellurite resistance. Trends Microbiol 7: 111–115. [DOI] [PubMed] [Google Scholar]
- Verrecchia, E.P. (2000) Fungi and sediments In: Microbial Sediments. Robert E.R. and Stanley M.A. (Eds). Heidelberg: Springer Berlin Heidelberg, pp. 68–75. [Google Scholar]
- Wang, J.C. , and Liu, Q. (2015) Fungi‐derived hierarchically porous carbons for high‐performance supercapacitors. RSC Adv 5: 4396–4403. [Google Scholar]
- Wang, W. , Pranolo, Y. and Cheng, C.Y. (2011) Metallurgical processes for scandium recovery from various resources: a review. Hydrometall 108, 100–108. [Google Scholar]
- Williams, P. , Keshavarz‐Moore, E. , and Dunnill, P. (1996) Production of cadmium sulphide microcrystallites in batch cultivation by Schizosaccharomyces pombe . J Biotechnol 48: 259–267. [DOI] [PubMed] [Google Scholar]
- Wu, Z.S. , Zhou, G. , Yin, L.C. , Ren, W. , Li, F. , and Cheng, H.M. (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1: 107–131. [Google Scholar]
- Xia, Y. , Xiao, Z. , Dou, X. , Huang, H. , Lu, X. , Yan, R. , et al (2013) Green and facile fabrication of hollow porous MnO/C microspheres from microalgae for lithium‐ion batteries. ACS Nano 7: 7083–7092. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Zhang, X. , He, W. , Yue, Y. , Liu, H. , and Ma, J. (2012d) Biocarbon‐coated LiFePO4 nucleus nanoparticles enhancing electrochemical performances. Chem Comm 48: 10093–10095. [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Wang, X.L. , Yang, F. , and Yang, X.R. (2011) Promising carbons for supercapacitors derived from fungi. Adv Mater 23: 2745–2748. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Li, W. , Zhan, L. , Huang, M. , Zhang, Q. , and Achal, V. (2016a) The large‐scale process of microbial carbonate precipitation for nickel remediation from an industrial soil. Environ Poll 219: 149–155. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Kumari, D. , Huang, M. , and Achal, V. (2016b) Biosynthesis of CdS nanoparticles through microbial induced calcite precipitation. Mater Design 98: 209–214. [Google Scholar]


