Summary
Human activity has been altering many ecological cycles for decades, disturbing the natural mechanisms which are responsible for re‐establishing the normal environmental balances. Probably, the most disrupted of these cycles is the cycle of carbon. In this context, many technologies have been developed for an efficient CO 2 removal from the atmosphere. Once captured, it could be stored in large geological formations and other reservoirs like oceans. This strategy could present some environmental and economic problems. Alternately, CO 2 can be transformed into carbonates or different added‐value products, such as biofuels and bioplastics, recycling CO 2 from fossil fuel. Currently different methods are being studied in this field. We classified them into biological, inorganic and hybrid systems for CO 2 transformation. To be environmentally compatible, they should be powered by renewable energy sources. Although hybrid systems are still incipient technologies, they have made great advances in the recent years. In this scenario, biotechnology is the spearhead of ambitious strategies to capture CO 2 and reduce global warming.
The tremendous impacts of global warming are being felt all over the world due to humans’ unsustainable way of life. We have released to the atmosphere more CO2 than what nature has been capable of fixing. The best strategy requires very deep cuts in emissions, as well as the use of alternatives to fossil fuels around the world. In 2015, the United Nations Conference on Climate Change (COP21), for the first time in more than 20 years of United Nations negotiations, achieved a legally binding climate agreement, with the objective of maintaining global warming below 2°C. Meanwhile, different approaches are being implemented to diminish this environmental problem. In this study, we aimed to update the most promising biotechnological approaches to capture and exploit excess of CO2 to accomplish human beings quality of life integrated to the sustainability of our planet.
CO2 capture and storage (CCS): sweeping the dust under the carpet?
One apparently promising technology is referred to as carbon capture and storage (CCS). CCS is a process consisting of the separation of CO2 from industrial and energy‐related sources, transport to a storage location and long‐term isolation from the atmosphere (Metz et al., 2005). Because the long‐distance transportation of CO2 to available disposal sites represents a prominent part of the economic and energetic costs of CO2 capture, it should be preferentially applied to large point sources. This includes large fossil fuel or biomass energy facilities, major CO2‐emitting industries, natural gas production, synthetic fuel plants and fossil fuel‐based hydrogen production plants (Aresta and Dibenedetto, 2007). Potential storage methods are geological storage (in geological formations, such as oil and gas fields, coal beds and deep saline formations), ocean storage (direct release into the ocean water column or onto the deep seafloor) and industrial fixation of CO2 into inorganic carbonates (Metz et al., 2005) (Fig. 1).
Figure 1.
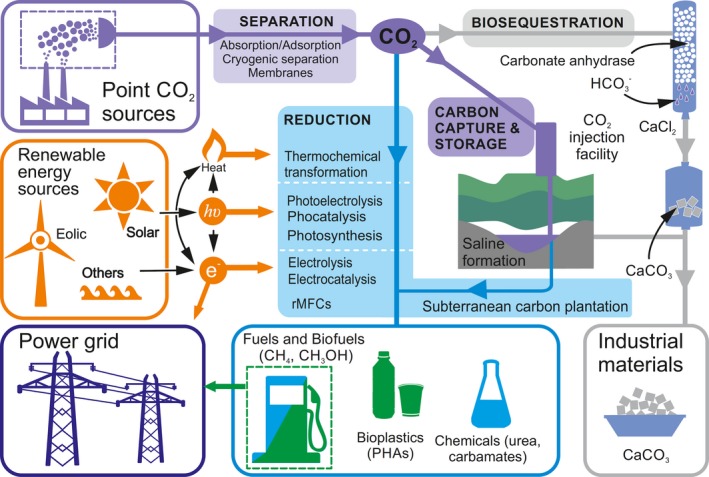
CO 2 generated in point sources is separated by any of the existing methods (absorption/adsorption, cryogenic or membrane separation). The resulting almost pure CO 2 can be captured and stored (carbon capture and storage – CCS) in geological formations (such as oil and gas fields, and saline formations) or transformed into different products. Carbonate can be produced using carbonate anhydrases from cyanobacteria and CaCl2 (biosequestration), and the resulting CaCO 3 can be stored or used as precursors for construction materials. CO 2 can also be reduced by different systems (biological, inorganic or hybrid). The energy supplied for the system to be sustainable must come from a renewable power source (wind, sun, etc.). This energy can be provided to the system as electrons (e‐), photons (hv) or heat. Subterranean carbon plantation is considered an alternative for recycling the CO 2 captured in CCS into methane. The products of CO 2 reduction can be bioplastics (PHAs), bio/fuels like methane or methanol. It can also be transformed into a wide range of chemicals (urea, carbamates, etc.). Fuels produced by CO 2 reduction are a possible solution for the intermittence of renewable energy (power grid), as they can be stored using the existing facilities from fossil fuels.
In this context, there are several commercially available technologies which can, in principle, be used for the separation of CO2 from the rest of the flue gases. However, the most employed method is a chemical absorption–desorption process, in which monoethanolamine solution is frequently used to dissolve the CO2. The almost pure CO2 is released afterwards from the liquid by heating to 100–150°C. The main drawback of this process is the requirement of intensive energy during regeneration of monoethanolamine solution. Other methods to separate CO2 include cryogenic fractionation, solid adsorption and membrane separation (Lam et al., 2012; Sreenivasulu et al., 2015).
The European Emissions Trading Scheme and the International Panel on Climate Change (IPCC) recognize that geological storage could be a valid mitigation option (West et al., 2005). In addition, the International Energy Agency (IEA) Blue Map scenario envisages a 19% CO2 reductions contribution from CCS by 2050 (Scott et al., 2012). However, there are some risks associated with this technology which must be considered. Although extensive physiological research is available, the environmental impacts of elevated CO2 on terrestrial, subsurface and marine ecosystems are not fully understood (West et al., 2005). Uncontrolled leakages could have implications for the environment. In economic terms, leaks into marine and freshwater systems might affect fisheries. For terrestrial systems, leakages might damage crops, groundwater quality and/or human and animal health. Other concerns include acidification, changes in biological diversity and species composition and asphyxiation at high CO2 concentrations. In addition, biogeochemical processes may be affected as increased CO2 concentrations could change pH, microbial populations and nutrient supply (Heinrich et al., 2003; Leung et al., 2014).
For this type of storage technology to be acceptable, safe practices must be developed, the potential for leaks must be understood, and dangerous situations must be avoided or safely managed (Lee et al., 2010). Other strategies, then, should be elaborated as alternative or, at least, to complement the geological and ocean storage of CO2. We will focus on the alternatives arising from microbiological world, especially prokaryotes, and to the contribution they can make to other inorganic technologies.
Transforming greenhouse gases into added‐value products
As mentioned, final disposal of CO2 in different compartments (CCS) is a strategy to diminish its proportion in atmospheric gas composition. However, there are other strategies which are not just aiming at CO2 capture but also at its transformation and revalorization. A lot of research has been developed into this field. Mikkelsen and coworkers grouped CO2 transformations into different categories involving different chemical, physical or biological methods (Mikkelsen et al., 2010). To simplify, we will group the systems for CO2 transformation into three categories: (i) biological (which include living prokaryotes or enzymes); (ii) inorganic transformations (chemical, physical or a combination of both); and a third category (iii) hybrid systems in which the process of CO2 conversion covers biological and inorganic steps integrated into the same system.
Biological systems for CO2 transformation
In the field of biological transformations, eukaryotic microalgae and cyanobacteria have been extensively studied for biofuels production and CO2 sequestration in carbonates via carbonic anhydrases (CAs). These organisms have been proposed for almost 50 years as a source of renewable fuels to reduce global warming (Oswald and Golueke, 1960).
Photosynthetic microorganisms grow 100 times faster than terrestrial plants, and they can double their biomass in <1 day. This is due to their simple cellular structure and large surface to volume ratio that give them the ability to uptake large amount of nutrients from water sources and thus promoting their growth rate. In addition, they can convert solar energy to chemical energy with efficiency of 10–50 times greater than terrestrial plants (Lam et al., 2012). A major appeal of photosynthetic microorganism cultures in greenhouse gas mitigation is that they must use concentrated forms of CO2, such as provided by power plant flue gases (Benemann, 2003).
Although the potential of microalgae and cyanobacteria to contribute to the world energy and commodities demand is high, there is a large gap between the current available technology and the one needed to supply the potential world demand. It is still necessary to solve a large number of bottlenecks related with biological, engineering and economic aspects (Acién et al., 2012). For example, photosynthetic microorganisms suffer inefficiencies arising from suboptimal light‐harvesting properties including the low energy capture and transfer efficiency of photosynthesis that will not be probably addressed in the near term (Khunjar et al., 2012; Torella et al., 2015). Possible solutions for this issue are discussed in the next sections.
Exploiting CO2 concentrating mechanism of cyanobacteria
The decline in atmospheric CO2 levels and rising O2 were a selective pressure in the past that lead to compensate the inefficient Rubiscos from cyanobacteria, with complementary strategies for CO2 fixation. They had to develop an effective photosynthetic CO2 concentrating mechanism for improving carboxylation. This adaptation acts to raise the concentration of CO2 around Rubisco hence improving the efficiency of CO2‐fixation and providing a survival advantage in limiting CO2 environments. The main feature of the cyanobacterial CO2 concentrating mechanism is that cellular Rubisco is partitioned into a protein‐bound micro‐compartment, called the carboxysome, which allows an internal accumulation of CO2 with the contribution of carbonic anhydrase (Rae et al., 2011).
Carbonic anhydrase (CA) is an interesting option for CO2 sequestration coming from the biological world. These are found not only in cyanobacteria but in animals, plants and other microbes. CA catalyses a rapid transformation of CO2 and water to and protons. As CA has the highest catalytic efficiency for CO2 hydration (kcat ∼ 106 s−1), it is considered as prominent biocatalytic agent for CO2 sequestration technology developments. In presence of cations at modest pH in vitro, CA converts CO2 into CaCO3 (Kanth et al., 2013). CaCO3 is a common and thermodynamically stable mineral found in rocks worldwide and is the main component of shells of marine organisms, snails and eggs. If the widespread transformation of CO2 to CaCO3 is possible, it will represent a stable process for long‐term CO2 storage. The transformation of CO2 into carbonate compounds using biocatalysts in a biomimetic approach has advantages for thermodynamically stable CO2 storage as compared to other technologies. This approach does not need a monitoring system for potential leaks, as do CCS, and allows the reuse of carbonate compounds for building or industrial materials (Lee et al., 2010). There are still several problems for practical use of CA such as high cost, limited stability, narrow range of working pH, intoxication due to impurities in flue gas, various issues related to scaling up and operating conditions. So, more stable and high‐active CA should be screened or engineered for more practical CA‐catalysed CO2 sequestration system (Kanth et al., 2013).
Carboxysomes are not just interesting for microbiologists. Growth and productivity in important crop plants is limited by the inefficiencies of the C3 photosynthetic pathway. Introducing into C3 plants CO2‐concentrating mechanisms, such as carboxysomes, could overcome these limitations and lead to increased yields (Rae et al., 2017). Recently, different groups described the recombinant expression of synthetic carboxysome shells in Escherichia coli (Cai et al., 2016) and Corynebacterium glutamicum (Baumgart et al., 2017) as intermediate step before jumping onto plants for improved CO2 fixation and concomitant increased biomass production.
Rational consortia between photoautotrophs and heterotrophs
As CO2 fixation tools, cyanobacteria can be co‐cultivated with other heterotrophic microorganisms providing them of reduced compounds from CO2. In this strategy, photosynthesis would provide organic carbon to an optimized heterotrophic organism (such as E. coli) which in turn would transform it into an added‐value compound. For example, the capability of sucrose‐secreting cyanobacteria to act as a platform for the construction of a light‐driven consortia was evaluated (Hays et al., 2017). In this work, the cyanobacteria Synechococcus elongatus PCC 7942 was paired with three disparate heterotrophs: Bacillus subtilis, E. coli or Saccharomyces cerevisiae. These synthetic consortia could be stabilized over the long term (weeks to months) and persisted in the face of selected perturbations (dilution, periods of darkness and phase changes in growth media). It could also be programmed for photoproduction of target compounds and proteins, such as alpha‐amylase and the bioplastic polyhydroxybutyrate (produced by co‐culturing cyanobacteria with recombinant B. subtilis or E. coli respectively). The principal advantage of this system is its modularity, and the wide range of products that could be synthesized by means of genetically flexible organisms associated with cyanobacteria.
Natural and engineered CO2 fixation pathways
Today, six autotrophic CO2 fixation mechanisms are known: (i) the Calvin–Benson reductive pentose phosphate cycle; (ii) the reductive citric acid cycle (Arnon–Buchanan cycle); (iii) the reductive acetyl‐coA (Wood–Ljungdahl) pathway; (iv) the hydroxypropionate (Fuchs–Holo) bicycle (3‐hydroxypropionate cycle); (v) the 3‐hydroxypropionate/4‐hydroxybutyrate and (vi) the dicarboxylate/4‐hydroxybutyrate cycles (Berg, 2011). Many heterotrophs have been engineered so far with recombinant CO2 fixation enzymes and pathways (Parikh et al., 2006; Mueller‐Cajar and Whitney, 2008; Guadalupe‐Medina et al., 2013; Antonovsky et al., 2016). However, they still grow as mixotrophs, requiring an organic carbon source for the production of the starting substrates of linear CO2 fixation pathways and/or for the regeneration of ATP and/or electron donors. Converting these engineered heterotrophs into true autotrophs would require the functional transplantation of complete CO2 fixation cycles and the transplantation of, and integration with, energy‐harvesting systems (Claassens et al., 2016; Claassens, 2017).
To overcome limitations of the natural carbon fixation pathways, an extensive in silico study identified alternative pathways that combine existing metabolic building blocks from various organisms. This work suggested that some of the proposed synthetic pathways could have significant quantitative advantages over their natural counterparts, such as the overall kinetic rate (Bar‐Even et al., 2010). In an another attempt to improve CO2 fixation, Schwander et al. published the construction of a non‐natural optimized CO2 fixation cycle called the ‘crotonyl–coenzyme A (CoA)/ethylmalonyl‐CoA/hydroxybutyryl‐CoA’ (CETCH) cycle (Schwander et al., 2016). The 12 enzymes involved in the CETCH cycle come from six organisms across all three domains of life: one from a plant (Arabidopsis thaliana), one from humans (Homo sapiens) and the other 10 enzymes from microbes. Although the CETCH cycle has the fewest reactions and the lowest requirement for ATP and NADPH among the aerobic CO2 fixation pathways, it produces glyoxylate, a less reduced metabolic intermediate. As CETCH cycle functionality has only been demonstrated in vitro, it should be transplanted into a selected chassis organism to develop a whole‐cell biocatalyst (Gong and Li, 2016; Schwander et al., 2016).
Inorganic transformations
The strategies for CO2 transformations can be grouped on the basis of the type of energy supplied to the CO2 for coping with its thermodynamic and kinetic stability (ΔG°f 396 kJ mol−1). They can be classified as (i) chemical and thermochemical or as (ii) photochemical and electrochemical. They will be summarized briefly here, but for more detailed reviews see references (Aresta and Dibenedetto, 2007; Mikkelsen et al., 2010).
Chemical and thermochemical transformations
The chemical transformations include all those strategies getting the leverage over the little reactivity window offered by the CO2 structure. This transformation method is based on the electrophilicity of the central carbon and the electron‐rich behaviour of the two oxygen atoms hosted in the molecular structure. For this reason, the CO2 chemical reactions are usually associated with, for example, low‐valent metal complexes (e.g. Ni, Pa) (Sakakura et al., 2007). In addition, when the catalytic reactions are not able to promote the CO2 activation, the reactions are usually coupled with high temperature and pressure conditions (thermochemical conversion) and/or with high‐energy compounds like hydrogen (Mikkelsen et al., 2010).
The range of products reached by chemical conversion technologies is wide, encompassing both chemical carbon‐based compounds with high oxidation state (e.g. carbamates, urea and polymeric materials), and energy‐rich, and consequently, more reduced molecules, such as formic acid, formaldehyde, methanol, methane and other hydrocarbons (Mikkelsen et al., 2010; Liu et al., 2015b). However, despite this vast product scenario, currently the application of CO2 as a feedstock for industrial production is limited to the production of urea, cyclic carbonates, polymers and carbamates (Mikkelsen et al., 2010). When a catalyst or a chemical activator is not available, the CO2 can be converted under extremely high temperature and pressure conditions (Lou et al., 2003; Treacy and Ross, 2004).
Photochemical and electrochemical
Leaf‐mimic system and artificial photosynthesis are alternative strategies for converting the CO2. These carbon fixation systems offer a way to avoid the high‐energy cost characterizing thermochemical conversions. They try to mimic the structural design of carbon catalyst centres or to reproduce the equivalent electron paths discovered in natural CO2 conversion systems (Berardi et al., 2014; Banerjee et al., 2016). Photochemical conversion methods are based on the exploitation of the sun‐light as energy source, a feature that renders this strategy sustainable for a further applications (Blankenship et al., 2011; Herron et al., 2015).
The artificial photosynthesis works towards a direct CO2 conversion for the synthesis of hydrocarbons or oxygenated products (Chang et al., 2016). The devices adopted for these methodologies are composed of the minimum components of natural photosynthetic systems, such as a light‐capturing antenna, a catalyst and an electron sacrificial donor compound. In this kind of devices, light promotes the electron transfer from the antenna system to the CO2, the catalyst favours a photo‐assisted multi‐electron transfer, and eventually, a sacrificial electron donor closes the electron cycle. The CO2 fixation devices are classified as photocatalytic system, if both reduction and oxidation occur in a same location, or as photo‐electrochemical cells if the redox reactions are spatially separated (Chang et al., 2016). In the latter, the redox reactions are carried out on the surface of two electrodes, which can be physically separated by a membrane (generally nafion) (Yim et al., 2015). The main products are generally represented by CO, formate and oxalate, even if also the synthesis of hydrocarbons, as methane or ethane, is attested (Jhong et al., 2013).
For electrochemical CO2 conversion, transition metals are the most used catalysts (Jhong et al., 2013). Among them, copper has the best selectivity to produce hydrocarbons and formic acid. It also offers the potentiality to catalyse the synthesis of C1‐, C2‐, C3‐based compounds and hydrocarbons with a faradic efficiency higher than 50% (Peterson and Nørskov, 2012). However, materials used for catalysing the reactions are not able to promote high values of reaction rate together with high selectivity, energetic efficiency and current density so far (Kuhl et al., 2012)(Montoya et al., 2017). Therefore, new cathode materials or modification of the catalyst surface is under investigation (Peterson and Nørskov, 2012).
Hybrid systems: Integrating different technologies for the design of more efficient CO2 transforming systems
Hybrid systems for conversion of CO2 could be the solution for two different problems emerging from the battle against climate change; on one side, photosynthetic organisms suffer inefficiencies arising from non‐optimal light‐harvesting properties (Khunjar et al., 2012; Torella et al., 2015). In a typical fermentation process of sugar cane to ethanol, the final product contains only almost 0.2% of the available solar energy. This low efficiency is mainly due to ineffective plant photosynthesis, but also to energy losses in the subsequent processing of biomass and microbial fermentation. In comparison with biological photosynthesis, the efficiency of photovoltaic solar panels is very high; solar panels that are currently available have solar to electricity efficiencies around 18%, and new innovations may enable efficiencies of more than 40% (Claassens et al., 2016). By the other side, renewable energy sources have to deal with the problem of intermittence generation of electric‐power. In particular, the amount of solar radiation incident on the earth's surface depends upon many factors such as location (latitude), time of the day, inclination of the surface, declination and weather. Storing solar energy is critical for continuous processing during these fluctuations (Herron et al., 2015). As solar and wind penetration increases in our systems, the intermittency of these two energy sources seriously compromises the stability and quality of grid power (Tuller, 2017).
One option of energy storing could come for hydrogen technology. Water splitting powered by renewable energy sources can lead to the production of hydrogen as a fuel. Hydrogen has many attractive attributes – it is clean burning and can be efficiently converted back to electricity via fuel cells. Hydrogen lacks volumetric energy density, this is the amount of energy stored per unit volume, and cannot be easily stored and distributed like hydrocarbon fuels. Its utility is much greater as an onsite fuel for converting CO2 to CH4 or for generating heat, electricity or syngas (Tuller, 2017). Liquid fuels are more appealing as a solar storage medium because of their attractive energy density and existing sophisticated distribution and storage infrastructures. However, attempts to produce liquid fuel via inorganic CO2 reduction have generally poor specificity and energy efficiency (Torella et al., 2015).
Thus, the problem of energy intermittence and low light‐harvesting properties of photosynthesis could be solved by integrating into a hybrid configuration the energy coming from renewable sources with biological systems as producers of storable fuels such as methane, alcohols, alkanes and other chemicals. This innovative technology is carried out in reverse microbial fuel cells and takes advantage of a higher CO2 reduction turnover from the electrochemical side and more interesting carbon products from the bio‐side. In a standard microbial fuel cell, organisms oxidize organic fuels and transfer electrons into an electrochemical system so that fuels are converted to electrical energy. In a reverse microbial fuel cells, this process is reversed so that electrical energy is used by bacterial cells to drive CO2 fixation to high‐energy organic compounds (Khunjar et al., 2012). The origin of these electrons may be any renewable energy source (sun, wind, etc.). The process of delivering these electrons to the cell is called extracellular electron transfer (EET). This transfer can be done in an indirect or direct fashion, depending whether they require or not the diffusion of a mobile component for electron transport (Rabaey and Rozendal, 2010) (Fig. 2).
Figure 2.

Schematic representation of CO 2 reduction and extracellular electron transfer (EET) in hybrid systems. They are based on reverse microbial fuel cells and take advantage of the high CO 2 reduction turnover of electrolysis and the versatility of microbial metabolism.
Indirect EET
In a reverse microbial fuel cells, the indirect method for EET involves the production or use of so‐called electron shuttles (or electron mediators), which transport the electrons from the electrode to the cell. The use of a mediator enables the utilization of planktonic cells in the bioreactor and can also enable separate‐stage designs that afford spatial and temporal decoupling of energy capture and bioproduction. This allows both processes to be operated and optimized separately. In the mediated approach, electrons are first transferred from the electrode to a soluble mediator, and then, the mediator is oxidized by the cell. Inorganic compounds that are linked with chemoautotrophy and can be electrolytically regenerated (such as NH3, H2, , Fe2+ and H2S), are an attractive options for this platform as they can facilitate the construction of multicarbon organics from CO2 using natural carbon fixation pathways (Khunjar et al., 2012).
Ammonia can be used as an indirect EET taking advantage of its low cost, abundance, safety and solubility. For example, Nitrosomonas europaea, a chemolithoautotroph, was used as the biocatalyst due to its inherent capability to utilize ammonia as its sole energy source for growth. Calculations indicated that overall production efficiency could approach approximately 2.7% under optimal electrolysis conditions. Considering a conversion efficiency of 10% from solar energy, the biomass production efficiency of this system was 0.27 ± 0.02%. This level of efficiency is comparable to photon to biomass conversion efficiencies observed for photosynthetic systems (around 1%) (Khunjar et al., 2012), so further efforts are required to improve these results, by considering more efficient conversions from solar energy, or modifying the genetic backgrounds of the strain.
Ralstonia eutropha, as a model chemolitoautotrophus species, is a promising tool for the development of hybrid systems. This bacterium is able to grow using electrochemically generated H2 as energy source. Engineered strain of R. eutropha H16 was studied for the production of isobutanol and 3‐methyl‐1‐butanol as the target fuels. Growth of R. eutropha was inhibited by reactive oxygen and nitrogen species when electric current was introduced. To circumvent this problem, a porous ceramic cup was used to shield the anode. This shield provided a tortuous diffusion path for chemicals. Therefore, the reactive compounds produced by the anode could be quenched before reaching the cells growing outside the cup. Using this approach, healthy growth of Ralstonia strain LH74D and production of over 140 mg l−1 biofuels were achieved with the electricity and CO2 as the sole source of energy and carbon respectively (Li et al., 2012). Based on electrochemically generated H2 as energy source, R. eutropha was also engineered for the production of fatty acid‐derived, diesel‐range methyl ketones. These modifications included overexpression of a cytoplasmic version of the TesA thioesterase, the deletion of two putative β‐oxidation operons and heterologous expression of three genes (the acyl coenzyme A oxidase gene from Micrococcus luteus and fadB and fadM from E. coli). These genetic modifications led to the production of 180 mg l−1 under chemolithoautotrophic growth conditions (Muller et al., 2013).
There are other ongoing projects using hybrid systems for CO2 capture. These projects aim to bridge a cost‐effective CO2 capture and purification, with electrochemical conversion of CO2, followed by the fermentation of the CO2‐reduction intermediates (such syngas and C1 water‐soluble molecules). By selecting the appropriate microorganism, a wide range of valuable products can be synthesized, such as PHAs, isoprene, lactic acid and methane (www.celbicon.org). For example, Rhodospirillum rubrum, a purple non‐sulfur bacterium, can produce PHAs from CO as carbon and energy source (Revelles et al., 2016). This feature and its metabolical versatility make this species interesting as biological tool for CO2 fixation.
Direct EET
Direct transfer typically involves at least a series of periplasmic and outer membrane complexes. In recent years, the involvement of pili or pilus‐like appendages (called nanowires in this context) was established. It has been suggested that nanowires also establish electron transport between different microorganisms in a community (Rabaey and Rozendal, 2010). Nevin proposed the term ‘microbial electrosynthesis’ for the reduction of CO2 to multicarbon compounds with electrons donated from an electrode as the electron donor (Nevin et al., 2010).
The bioelectrochemical reduction of CO2 has been also postulated as a promising process to obtain methane. The term electromethanogenesis has been applied to the process of producing methane using CO2 as the sole carbon source, using electroactive microbes in an engineered system (biocathode) powered with electric current. CO2 can be fixed either by direct EET or indirect EET; however, we will mention examples of the former here. Electromethanogenesis‐based technologies have a great potential for storing renewable energy in the form of methane, improving waste treatment processes or upgrading gas streams containing CO2. In all cases, future studies must focus on further up‐scaling, increase process efficiencies and reduce operation costs to reach coexistence with well‐established technologies, or even a hypothetical overtaking (Blasco‐Gómez et al., 2017).
Electromethanogenesis can also add value to CO2 stored in geological formations by means of subterranean carbon plantation. This concept proposed by Sato and coworkers, is based on CCS technologies, in situ biological conversion of the stored CO2 to methane and harvest of the biogenic methane as a recycled energy source (Sato et al., 2013). When supplied with CO2 through CCS operations, such reservoirs could function as natural bioreactors that prompt methanogens to convert CO2 to methane. Intermittent electrical energy provided by, for instance, wind turbines and photovoltaic cells can be stored in a stable form as methane. The current limitation of the system is the relatively slow rate of electromethanogenesis, and further studies are required on electrochemical reduction of CO2 under more realistic conditions: at higher (hydrostatic) pressures, in presence of solid (rock) surfaces and in microbial symbioses (Kuramochi et al., 2013; Sato et al., 2013).
Methane may not be the only product of electrosynthesis. Using consortia of cathodophilic microorganisms from brewery wastewater, it is possible to obtain a mixture of products (acetate, methane and hydrogen) through electrosynthesis in the same process, with CO2 as the only carbon source. The electrochemical evidence suggests that the electron transfer between the electrode and microbes in a biofilm operates in the absence of soluble redox active components in the medium (Marshall et al., 2012). Liu and coworkers created a two‐step strategy that mimics natural photosynthesis, where light capture by a biocompatible nanowire array interfaced and directly provided reducing equivalents to living organisms. The high‐surface‐area silicon nanowire array harvests light energy to provide reducing equivalents to the anaerobic bacterium, Sporomusa ovata, for the photo‐electrochemical production of acetic acid under aerobic conditions (21% O2). The resulting acetate (∼6 g l−1) fed genetically engineered E. coli to produce a variety of value‐added chemicals. The yield of target molecules was as high as 26% for n‐butanol, 25% for one of the isoprenoid compounds (amorphadiene) and up to 52% for PHAs, comparable with literature values (Liu et al., 2015a).
Concluding remarks
Global warming is an ongoing threat for the maintenance of ecological systems, for economy and human life quality. Solving the problem implies the convergence of different approaches to achieve a net CO2 removal from the atmosphere. CCS may account for part of the solution, but they must be complemented with other green technologies with lower environmental risks and higher economical sustainability. Microbial biotechnology can lend a great hand to this aim by providing recombinant microorganisms able to transform the low reactive molecule of CO2 into a wide variety of compounds, such as biofuels, bioplastics and chemicals. New hybrid technologies should also be explored to make the whole process more efficient. Electrochemical cells, as inorganic systems for CO2 fixation, have the potentiality to sequestrate high amounts of CO2, but their low selectivity and capacity for generating added‐value products are limitations in a commercial scenario. In contrast, biological systems are much more versatile for the production of C‐based molecules than electrochemical cells, but they are quite inefficient in harnessing solar energy. A promising strategy for sequestrate and fix CO2 is definitively based on the combination of bio and electrochemical systems.
Conflict of interest
None declared.
Microbial Biotechnology (2017) 10(5), 1216–1225
Funding Information
Research on polymer biotechnology at the laboratory of Auxiliadora Prieto is supported by grants from the European Union's Horizon 2020 Research and Innovation Programme under grant agreements, no 633962, no. 679050, no. 745791 and no. 745737. We also acknowledge the Community of Madrid (P2013/MIT2807) and the Spanish Ministry of Economy (BIO201344878R, BIO2014‐61515‐EXP).
References
- Acién, F.G. , Fernández, J.M. , Magán, J.J. , and Molina, E. (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30: 1344–1353. [DOI] [PubMed] [Google Scholar]
- Antonovsky, N. , Gleizer, S. , Noor, E. , Zohar, Y. , Herz, E. , Barenholz, U. , et al (2016) Sugar synthesis from CO2 in Escherichia coli . Cell 166: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aresta, M. , and Dibenedetto, A. (2007) Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Trans 0: 2975–2992. [DOI] [PubMed] [Google Scholar]
- Banerjee, A. , Dick, G.R. , Yoshino, T. , and Kanan, M.W. (2016) Carbon dioxide utilization via carbonate‐promoted C‐H carboxylation. Nature 531: 215–219. [DOI] [PubMed] [Google Scholar]
- Bar‐Even, A. , Noor, E. , Lewis, N.E. , and Milo, R. (2010) Design and analysis of synthetic carbon fixation pathways. Proc Natl Acad Sci 107: 8889–8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart, M. , Huber, I. , Abdollahzadeh, I. , Gensch, T. , and Frunzke, J. (2017) Heterologous expression of the Halothiobacillus neapolitanus carboxysomzral gene cluster in Corynebacterium glutamicum. J Biotechnol [Epub ahead of print] doi: 10.1016/j.jbiotec.2017.03.01. [DOI] [PubMed] [Google Scholar]
- Benemann, J.R. (2003) Biofixation of CO2 and greenhouse gas abatement with microalgae – Technology roadmap. Final Rep US Dep Energy Natl Energy Technol Lab 1–29. [Google Scholar]
- Berardi, S. , Drouet, S. , Francàs, L. , Gimbert‐Suriñach, C. , Guttentag, M. , Richmond, C. , et al (2014) Molecular artificial photosynthesis. Chem Soc Rev 43: 7501–7519. [DOI] [PubMed] [Google Scholar]
- Berg, I.A. (2011) Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77: 1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship, R.E. , Tiede, D.M. , Barber, J. , Brudvig, G.W. , Fleming, G. , Ghirardi, M. , et al (2011) Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332: 805–809. [DOI] [PubMed] [Google Scholar]
- Blasco‐Gómez, R. , Batlle‐Vilanova, P. , Villano, M. , Balaguer, M. , Colprim, J. , and Puig, S. (2017) On the edge of research and technological application: a critical review of electromethanogenesis. Int J Mol Sci 18: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, F. , Bernstein, S.L. , Wilson, S.C. , and Kerfeld, C.A. (2016) Production and characterization of synthetic carboxysome shells with incorporated luminal proteins. Plant Physiol 170: 1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, X. , Wang, T. , and Gong, J. (2016) CO2 photo‐reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9: 2177–2196. [Google Scholar]
- Claassens, N.J. , Sousa, D.Z. , dos Santos, V.A.P.M. , de Vos, W.M. , and van der Oost, J. (2016) Harnessing the power of microbial autotrophy. Nat Rev Microbiol 14: 692–706. [DOI] [PubMed] [Google Scholar]
- Claassens, N. J. (2017). A warm welcome for alternative CO2 fixation pathways in microbial biotechnology. Microb. Biotechnol., 10, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, F. , and Li, Y. (2016) Fixing carbon, unnaturally. Science 354: 830–831. [DOI] [PubMed] [Google Scholar]
- Guadalupe‐Medina, V. , Wisselink, H. , Luttik, M.A. , de Hulster, E. , Daran, J.‐M. , Pronk, J.T. , and van Maris, A.J. (2013) Carbon dioxide fixation by Calvin‐Cycle enzymes improves ethanol yield in yeast. Biotechnol Biofuels 6: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays, S.G. , Yan, L.L.W. , Silver, P.A. , and Ducat, D.C. (2017) Synthetic photosynthetic consortia define interactions leading to robustness and photoproduction. J Biol Eng 11: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, J.J. , Herzog, H.J. and Reiner, D.M. (2003) Environmental assessment of geologic storage of CO2 . In Second National Conference on Carbon Sequestration Citeseer, pp. 5–8.
- Herron, J.A. , Kim, J. , Upadhye, A.A. , Huber, G.W. , and Maravelias, C.T. (2015) A general framework for the assessment of solar fuel technologies. Energy Environ Sci 8: 126–157. [Google Scholar]
- Jhong, H.M. , Ma, S. , and Kenis, P.J.A. (2013) Electrochemical conversion of CO2 to useful chemicals : current status, remaining challenges, and future opportunities. Curr Opin Chem Eng 2: 191–199. [Google Scholar]
- Kanth, B.K. , Lee, J. , and Pack, S.P. (2013) Carbonic anhydrase : Its biocatalytic mechanisms and functional properties for efficient CO2 capture process development. Eng Life Sci 1: 422–431. [Google Scholar]
- Khunjar, W.O. , Sahin, A. , West, A.C. , Chandran, K. , and Banta, S. (2012) Biomass production from electricity using ammonia as an electron carrier in a reverse microbial fuel cell. PLoS ONE 7: e44846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl, K.P. , Cave, E.R. , Abram, D.N. , and Jaramillo, T.F. (2012) New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ Sci 5: 7050–7059. [Google Scholar]
- Kuramochi, Y. , Fu, Q. , Kobayashi, H. , Ikarashi, M. , Wakayama, T. , Kawaguchi, H. , et al (2013) Electromethanogenic CO2 conversion by subsurface‐reservoir microorganisms. Energy Procedia 37: 7014–7020. [Google Scholar]
- Lam, M.K. , Lee, K.T. , and Mohamed, A.R. (2012) Current status and challenges on microalgae‐based carbon capture. Int J Greenhouse Gas Control 10: 456–469. [Google Scholar]
- Lee, S.W. , Park, S.B. , Jeong, S.K. , Lim, K.S. , Lee, S.H. , and Trachtenberg, M.C. (2010) On carbon dioxide storage based on biomineralization strategies. Micron 41: 273–282. [DOI] [PubMed] [Google Scholar]
- Leung, D.Y.C. , Caramanna, G. , and Maroto‐Valer, M.M. (2014) An overview of current status of carbon dioxide capture and storage technologies. Renew Sustain Energy Rev 39: 426–443. [Google Scholar]
- Li, H. , Opgenorth, P.H. , Wernick, D.G. , Rogers, S. , Wu, T.‐Y. , Higashide, W. , et al (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335: 1596. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Gallagher, J.J. , Sakimoto, K.K. , Nichols, E.M. , Chang, C.J. , Chang, M.C.Y. , and Yang, P. (2015a) Nanowire‐bacteria hybrids for unassisted solar carbon dioxide fixation to value‐added chemicals. Nano Lett 15: 3634–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Wu, L. , Jackstell, R. , and Beller, M. (2015b) Using carbon dioxide as a building block in organic synthesis. Nat Commun 6: 5933. [DOI] [PubMed] [Google Scholar]
- Lou, Z. , Chen, Q. , Zhang, Y. , Wang, W. , and Qian, Y. (2003) Diamond Formation by Reduction of Carbon Dioxide at Low Temperatures. J. Am. Chem. Soc 125: 9302–9303. [DOI] [PubMed] [Google Scholar]
- Marshall, C.W. , Ross, D.E. , Fichot, E.B. , Norman, R.S. , and May, H.D. (2012) Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl Environ Microbiol 78: 8412–8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz, B. , Davidson, O. , de Coninck, H. , Loos, M. and Meyer, L.M. (eds.) (2005) IPCC special report on carbon dioxide capture and storage. New York, NY: Cambridge University Press. [Google Scholar]
- Mikkelsen, M. , Jørgensen, M. , and Krebs, F.C. (2010) The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ Sci 3: 43–81. [Google Scholar]
- Montoya, J.H. , Seitz, L.C. , Chakthranont, P. , Vojvodic, A. , Jaramillo, T.F. , and Nørskov, J.K. (2017) Materials for solar fuels and chemicals. Nat Mater 16: 70–81. [DOI] [PubMed] [Google Scholar]
- Mueller‐Cajar, O. , and Whitney, S. (2008) Evolving improved Synechococcus Rubisco functional expression in Escherichia coli . Biochem J 414: 205–214. [DOI] [PubMed] [Google Scholar]
- Muller, J. , MacEachran, D. , Burd, H. , Sathitsuksanoh, N. , Bi, C. , Yeh, Y.‐C. , et al (2013) Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl Environ Microbiol 79: 4433–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin, K.P. , Woodard, T.L. , and Franks, A.E. (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 1: e00103–e00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald, W.J. , and Golueke, C.G. (1960) Biological transformation of solar energy. Adv Appl Microbiol 2: 223–262. [DOI] [PubMed] [Google Scholar]
- Parikh, M.R. , Greene, D.N. , Woods, K.K. , and Matsumura, I. (2006) Directed evolution of RuBisCO hypermorphs through genetic selection in engineered E.coli. Protein Eng Des Sel 19: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, A. , and Nørskov, J. (2012) Activity descriptors for CO2 electroreduction to methane on transition metal catalysts. J Phys Chem Lett 3: 251–258. [Google Scholar]
- Rabaey, K. , and Rozendal, R.A. (2010) Microbial electrosynthesis – revisiting the electrical route for microbial production. Nat Rev Microbiol 8: 706–716. [DOI] [PubMed] [Google Scholar]
- Rae, B.D. , Förster, B. , Badger, M.R. , and Price, G.D. (2011) The CO2‐concentrating mechanism of Synechococcus WH5701 is composed of native and horizontally‐acquired components. Photosynth Res 109: 59–72. [DOI] [PubMed] [Google Scholar]
- Rae, B.D. , Long, B.M. , Förster, B. , Nguyen, N.D. , Velanis, C.N. , Atkinson, N. , et al (2017) Progress and challenges of engineering a biophysical carbon dioxide‐concentrating mechanism into higher plants. J Exp Bot 1–21. [DOI] [PubMed] [Google Scholar]
- Revelles, O. , Tarazona, N. , García, J.L. , and Prieto, M.A. (2016) Carbon roadmap from syngas to polyhydroxyalkanoates in Rhodospirillum rubrum . Environ Microbiol 18: 708–720. [DOI] [PubMed] [Google Scholar]
- Sakakura, T. , Choi, J. , and Yasuda, H. (2007) Transformation of carbon dioxide. Chem Rev 107: 2365–2387. [DOI] [PubMed] [Google Scholar]
- Sato, K. , Kawaguchi, H. , and Kobayashi, H. (2013) Bio‐electrochemical conversion of carbon dioxide to methane in geological storage reservoirs. Energy Convers Manag 66: 343–350. [Google Scholar]
- Schwander, T. , Schada von Borzyskowski, L. , Burgener, S. , Cortina, N.S. , and Erb, T.J. (2016) A synthetic pathway for the fixation of carbon dioxide in vitro . Science 354: 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, V. , Gilfillan, S. , Markusson, N. , Chalmers, H. , and Haszeldine, R.S. (2012) Last chance for carbon capture and storage. Nat Clim Chang 3: 105–111. [Google Scholar]
- Sreenivasulu, B. , Gayatri, D.V. , Sreedhar, I. , and Raghavan, K.V. (2015) A journey into the process and engineering aspects of carbon capture technologies. Renew Sustain Energy Rev 41: 1324–1350. [Google Scholar]
- Torella, J.P. , Gagliardi, C.J. , Chen, J.S. , Bediako, D.K. , Colón, B. , Way, J.C. , et al (2015) Efficient solar‐to‐fuels production from a hybrid microbial–water‐splitting catalyst system. Proc Natl Acad Sci 112: 201503606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treacy, D. , and Ross, J.R.H. (2004) The potential of the CO2 reforming of CH4 as a method of CO2 mitigation. A thermodynamic study. Am Chem Soc Div Fuel Chem 49: 126–127. [Google Scholar]
- Tuller, H.L. (2017) Solar to fuels conversion technologies: a perspective. Mater Renew Sustain Energy 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, J.M. , Pearce, J. , Bentham, M. , and Maul, P. (2005) Environmental issues and the geological storage of CO2 . Eur Environ 15: 250–259. [Google Scholar]
- Yim, K.‐J. , Song, D.‐K. , Kim, C.‐S. , Kim, N.‐G. , Iwaki, T. , Ogi, T. , et al (2015) Selective, high efficiency reduction of CO2 in a non‐diaphragm‐based electrochemical system at low applied voltage. RSC Adv 5: 9278–9282. [Google Scholar]


