Abstract
Obstructive sleep apnea that characterized by chronic intermittent hypoxia (CIH) has been reported to associate with chronic liver injury. Tauroursodeoxycholic acid (TUDCA) exerts liver-protective effects in various liver diseases. The purpose of this study was to test the hypothesis that TUDCA could protect liver against CIH injury. C57BL/6 mice were subjected to intermittent hypoxia for eight weeks and applied with TUDCA by intraperitoneal injection. The effect of TUDCA on liver histological changes, liver function, oxidative stress, inflammatory response, hepatocyte apoptosis and endoplasmic reticulum (ER) stress were investigated. The results showed that administration of TUDCA attenuated liver pathological changes, reduced serum alanine aminotransferase and aspartate aminotransferase level, suppressed reactive oxygen species activity, decreased tumor necrosis factor-α and interleukin-1β level and inhibited hepatocyte apoptosis induced by CIH. TUDCA also inhibited CIH-induced ER stress in liver as evidenced by decreased expression of ER chaperone 78 kDa glucose-related protein, unfolded protein response transducers and ER proapoptotic proteins. Altogether, the present study described a liver-protective effect of TUDCA in CIH mice model, and this effect seems at least partly through the inhibition of ER stress.
Keywords: obstructive sleep apnea, chronic intermittent hypoxia, tauroursodeoxycholic acid, endoplasmic reticulum stress, apoptosis, liver
Introduction
Obstructive sleep apnea (OSA) is a common disorder occurs in 2–14% of the general adult population (1,2). In OSA, upper airway obstruction usually associated with recurrent hypoxemia interspersed with periods of reoxygenation and typically terminated by brief arousals, that result in chronic intermittent hypoxia (CIH) and sleep fragmentation (3). The major health burden reported in patients with OSA is the strong risk of cardiovascular diseases (4–7) and brain injury (8–10). There is also emerging evidence showing that OSA is associated with chronic liver injury, such as hepatic oxidative stress (11), inflammatory reactions (12), abnormal lipid metabolism (13), hepatocytes apoptosis (14) and liver function injury (15).
Accumulation studies have proved that CIH could induce endoplasmic reticulum (ER) stress (16–18), which is closely linked to oxidative stress, inflammation and apoptosis (19). ER stress has been reported to be implicated in a variety of liver diseases, including alcohol-induced liver injury, nonalcoholic fatty liver disease, hepatic insulin resistance, ischemia-reperfusion injury and other acute hepatotoxins (20–22). Tauroursodeoxycholic acid (TUDCA), a hydrophilic nontoxic bile acid, is widely known as an ER stress inhibitor (23,24). TUDCA could be synthesized in the hydrophilic conjugation pathway of ursodeoxycholic acid, which has been effectively used for improving clinical and biochemical conditions in several liver diseases without major adverse reactions (25–27). Treatment with TUDCA abolished thapsigargin induced ER stress and apoptosis of the human liver-derived cell line Huh7 (28). Moreover, administration of TUDCA suppressed carcinogen-induced ER stress-mediated hepatocytes apoptosis and hepatic inflammation in a mouse hepatocellular carcinoma model (25), suggesting that TUDCA exerts beneficial effects on ER stress-induced liver disease. However, whether TUDCA is protectively in CIH-induced liver injury has not been studied.
In the present study, C57BL/6 mice was pre-treated with TUDCA and exposed to CIH for eight weeks, the liver histopathology, activity of liver enzymes, oxidative stress, inflammatory factor, hepatocyte apoptosis and ER stress of liver were analyzed to investigate the effect of TUDCA on CIH-induced liver injury.
Materials and methods
Animal model of CIH and experimental groups
A total of 24 male 8-week-old C57BL/6 mice (18–22 g) were purchased from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China) and randomly divided into four groups, with 6 mice in each group. These were the following: Negative control (NC), TUDCA, CIH and CIH+TUDCA groups. Animals in NC group were kept in normal air (21% O2), while the CIH model was established according to Lai et al (29) with minor modification: Mice were housed in a sealed cabin, the air was controlled by an automated nitrogen/air switch pump. The concentration of O2 was gradually reduced to 7%, stabilized for 60 sec, then increased to a peak of 21%, maintained for 60 sec, this cycle was repeated over 8 h (from 8:30 to 16:30) during the animals' diurnal sleep period. Mice in CIH+TUDCA group were treated with TUDCA 100 mg/kg/day by intraperitoneal injection, start from 2 days before CIH until the end of the experiment. Mice in TUDCA group were intraperitoneal injected with 100 mg/kg/day at the same schedule but did not undergo CIH treatment. Eight weeks later, the mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg; Chengdu Xiya Chemical Technology Co., Ltd., Chengdu, China), eyeballs were removed, blood samples were collected, animals were sacrificed by exsanguination as previously reported (30), and the the livers were harvested. The care and disposal procedures of animal were strictly subject to the provisions of the Animal Ethics Committee of China Medical University (Shenyang, China).
Biochemical assays
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were determined by commercial kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) following the user's instructions. Liver tissues were homogenized, centrifuged and total liver lysate were collected. Reactive oxygen species (ROS) activity was measured by ROS assay kit (Nanjing Jiancheng Bioengineering Institute). The level of tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) in liver were determined by ELISA kits purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China).
Histology analysis
Liver tissues was fixed with formalin, embedded in paraffin and cut into 5-µm sections. After dehydrated with grade alcohols, the sections were stained with hematoxylin and eosin terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using a commercial kit (KeyGEN, Nanjing, China) according to the manufacturer's instruction. Briefly, the sections was incubated with 1% Triton X-100 for 8 min at room temperature, then, endogenous peroxidase activity was blocked by 3% H2O2. Followed incubation with Proteinase K solution, the sections were stained with TUNEL reaction mixture at 37°C for 60 min. Then TUNEL positive cells were labeled with streptavidin-horse radish peroxidase (HRP) and developed with 3,3′-diaminobenzidine. Finally, the sections were counterstained with hematoxylin.
Western blot analysis
The liver tissues were dissolved by radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China), total protein was isolated and the protein concentration was determined using a bicinchoninic acid Method-Protein Quantitative Reagent kit (Beyotime). After that, tan equal quantity of protein was fractionated using 8, 10 or 13% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA, USA). Then, the membranes were blocked by 5% (w/v) fat-free milk and immunoblotted with primary antibodies (dilution and suppliers were listed in Table I) 4°C overnight. After washing, the membranes was incubated with HRP-conjugated secondary antibodies (goat anti-rabbit IgG, 1:5,000; goat anti-mouse IgG, 1:5,000; Beyotime Institute of Biotechnology) at 37°C for 45 min. Next, the specific protein bands was visualized using enhanced chemiluminescence reagent (Qihai Biotec, Shanghai, China) and the gray density was analyzed using Gel-Pro Analyzer software version 3.0 (Media Cybernetics, Inc., Silver Spring, MD, USA).
Table I.
Details of antibodies.
| Antibodies | Dilution | Catalogue number | Supplier |
|---|---|---|---|
| Cleaved caspase-3 | 1:1,000 | ab2302 | Abcam (Cambridge, MA, USA) |
| Cleaved caspase-9 | 1:200 | sc-22182 | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) |
| GRP78 | 1:400 | BA4293 | Wuhan Boster Biological Technology, Ltd. (Wuhan, China) |
| PERK | 1:500 | bs-2469R | Bioss Antibodies (Beijing, China) |
| ATF6/cleaved ATF-6 | 1:400 | ab11909 | Abcam |
| p-IRE-1 | 1:500 | bs-16698R | Bioss Antibodies |
| IRE-1 | 1:500 | bs-8680R | Bioss Antibodies |
| CHOP | 1:1,000 | ab11419 | Abcam |
| Cleaved caspase-12 | 1:500 | ab18766 | Abcam |
| p-eIF2α | 1:1,000 | ab4837 | Abcam |
| eIF2α | 1:1,000 | ab26197 | Abcam |
| p-JNK | 1:500 | bs-1640R | Bioss Antibodies |
| JNK | 1:500 | bs-10562R | Bioss Antibodies |
GRP78, 78 kDa glucose-related protein; PERK, eukaryotic translation initiation factor 2 α kinase 3; ATF, activating transcription factor; JNK, janus kinase; IRE, inositol-requiring enzyme; CHOP, C/EBP homologous protein; eIF2α, eukaryotic translation initiation factor 2 subunit α.
Data analysis
Data are expressed as the mean ± standard deviation. Differences between two groups were analyzed by Student's t-test. P<0.05 was used to indicated a statistically significant difference. Statistical analysis was performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
TUDCA protected liver from CIH-induced pathological and function injury
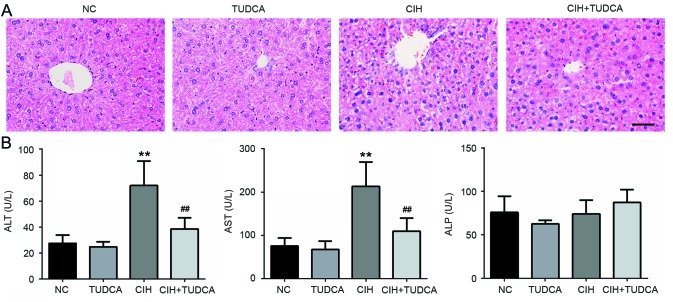
As shown in Fig. 1A, histology analysis of the liver tissues revealed normal hepatic architecture with no obvious lobular or portal inflammation, steatosis or necrosis in NC and TUDCA group. In contrast, mice exposed to CIH showed liver injury with discernible swelled, disordered hepatocytes and infiltrated inflammatory cells, however, these pathological changes were evidently ameliorated by the treatment of TUDCA. In order to evaluate the effect of TUDCA on CIH-induced liver function injury, the serum levels of liver enzymes ALT, AST and ALP were determined. As shown in Fig. 1B, the levels of ALT and AST in CIH group was significantly elevated when compare to the NC group (71.96±18.64 vs. 27.38±6.36 U/l, P<0.01; 213.06±56.24 vs. 75.18±18.97 U/l, P<0.01), but these were significantly decreased in CIH+TUDCA group when compared to CIH group (38.41±8.64 vs. 71.96±18.64 U/l, P<0.01; 109.39±30.26 vs. 213.06±56.24 U/l, P<0.01). There were no significant changes in the serum ALP level in CIH or CIH+TUDCA groups. The above results indicate that TUDCA alleviated CIH-induced liver pathological changes and protected liver function.
Figure 1.
Liver histological changes and serum biochemical indicator levels. In total, 8 weeks after CIH, (A) the histological changes of liver tissues were analyzed by hematoxylin and eosin staining and (B) the biochemical indicators of liver function were detected by commercial kits. Scale bar, 50 µm. **P<0.01 vs. NC group, ##P<0.01 vs. CIH group. CIH, chronic intermittent hypoxia; NC, negative control; TUDCA, tauroursodeoxycholic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase.
TUDCA suppressed CIH-induced liver oxidative stress and inflammatory reactions
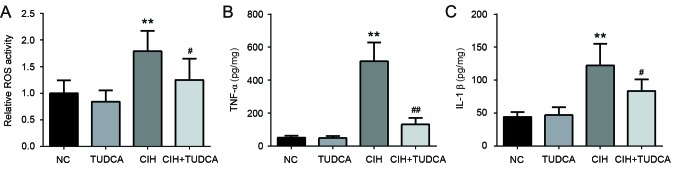
The activity of ROS in liver was detected to evaluate the role of TUDCA on CIH-induced liver oxidative stress (Fig. 2A). Exposed to CIH-induced a 1.8-fold increase in ROS activity in the liver when compared to the NC group, while treatment with TUDCA decreased by 30% of the ROS activity when compared to CIH group. We further measured the levels of proinflammatory factors in livers to detect the anti-inflammatory effect of TUDCA, as shown in Fig. 2B and C, in the CIH group, there was a significant elevation in both TNF-α (515.00±112.83 vs. 50.26±11.89 pg/mg, P<0.01) and IL-1β (122.21±32.82 vs. 44.06±7.50 pg/mg, P<0.01) level when compared to the NC group. However, these levels were strongly decreased in the CIH+TUDCA group when compared to the CIH group (TNF-α, 130.75±38.80 vs. 515.00±112.83 pg/mg, P<0.01; IL-1β, 83.60±17.58 vs. 122.21±32.82 pg/mg). Thus, treatment with TUDCA suppressed CIH-induced oxidative stress and inflammatory reactions in liver.
Figure 2.
Liver ROS activity and proinflammatory cytokine level. Relative (A) ROS activity, (B) TNF-α and (C) IL-1β level in liver tissues. **P<0.01 vs. NC group, ##P<0.01, #P<0.05 vs. CIH group. CIH, chronic intermittent hypoxia; NC, negative control; TUDCA, tauroursodeoxycholic acid; ROS, reactive oxygen species; TNF, tumor necrosis factor; IL, interleukin.
TUDCA protected liver from CIH-induced apoptosis
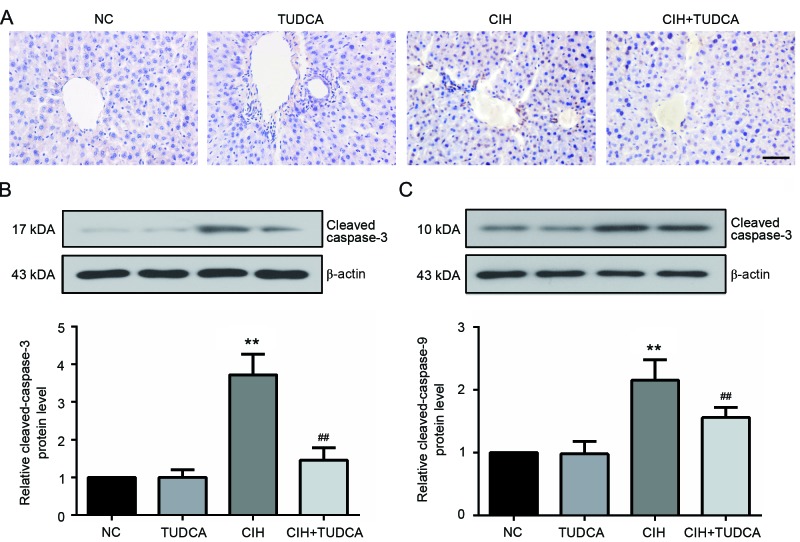
After eight weeks exposure to CIH, the presence of hepatocytes undergoing apoptosis was determined by TUNEL staining. As shown in Fig. 3A, TUNEL-positive apoptotic cells could obviously seen in the liver of CIH group, as expected, treatment with TUDCA distinctly reduced the apoptotic cell number. Additionally, the expression of two important biochemical markers of apoptosis, cleaved caspase-3 and cleaved caspase-9 were evaluated using western blot (Fig. 3B and C). Exposure to CIH significantly upregulated the protein levels of the two makers (P<0.01), while these changes were evidently restored by the treatment of TUDCA. These results suggested that TUDCA protected the liver from CIH-induced apoptosis.
Figure 3.
Apoptosis of hepatocytes. Apoptosis of hepatocytes were analyzed by (A) TUNEL, (B) the expression of cleaved caspase-3 and (C) cleaved caspase-9 in the liver were detected by western blotting. Scale bar, 50 µm. **P<0.01 vs. NC group, ##P<0.01 vs. CIH group. CIH, chronic intermittent hypoxia; NC, negative control; TUDCA, tauroursodeoxycholic acid.
TUDCA suppressed CIH-induced ER stress and related proapoptotic pathway
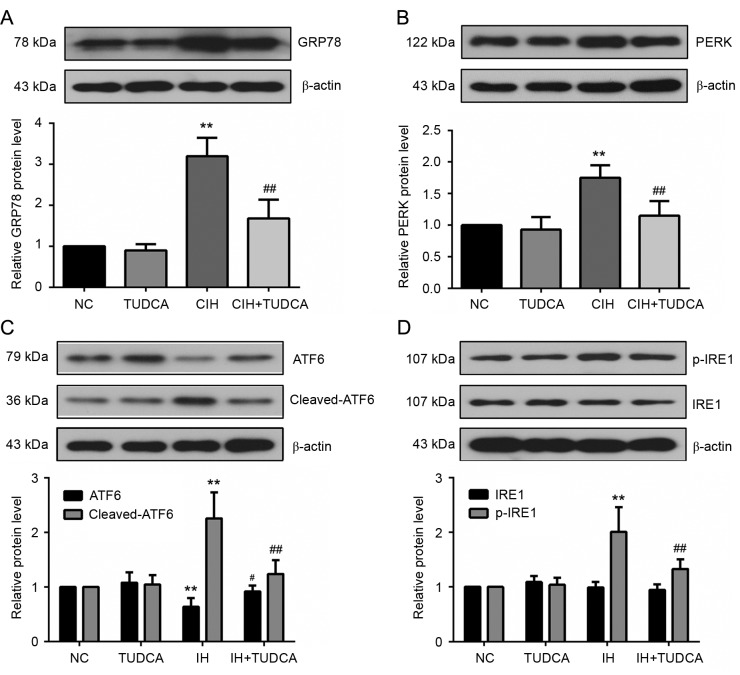
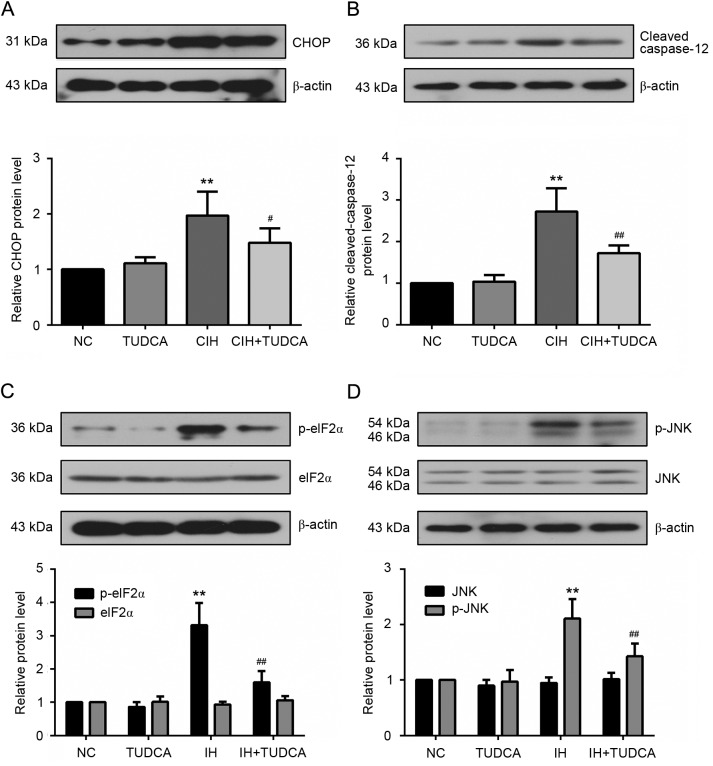
To delineate the role of TUDCA on CIH-induced ER stress, western blot was used to evaluate the protein levels of the molecular chaperone 78 kDa glucose-related protein (GRP78) and three major transducers of unfolded protein response (UPR), eukaryotic translation initiation factor 2 α kinase 3 (PERK), activating transcription factor (ATF)6 and inositol-requiring enzyme (IRE1), that are known to be specifically induced by ER stress. As shown in Fig. 4, CIH increased the protein levels of GRP78, PERK, cleaved ATF6 and p-IRE1 in liver (P<0.01); however, these proteins were significantly reduced in the CIH+TUDCA group when compared to the CIH group (P<0.05). With the changed level of GRP78 and three UPR transducers, we further investigated the signal molecules of ER stress-associated apoptotic pathways (Fig. 5). There was an evident increase of C/EBP homologous protein (CHOP), cleaved caspase-12, p-eukaryotic translation initiation factor 2 subunit α (eIF2α) and p-janus kinase (JNK) in CIH group when compared to the NC group (P<0.01). However, these changes of protein levels were all significantly reversed by the treatment of TUDCA (P<0.05). Therefore, the present findings demonstrated that TUDCA suppressed CIH-induced ER stress and ER stress-associated apoptosis of the liver.
Figure 4.
UPR activation in liver tissues. Expression of (A) GRP78 and three major transducers of UPR, (B) PERK, (C) ATF6 and (D) IRE1 were measured by western blotting. **P<0.01 vs. NC group, ##P<0.01, #P<0.05 vs. CIH group. CIH, chronic intermittent hypoxia; NC, negative control; TUDCA, tauroursodeoxycholic acid; GRP78, 78 kDa glucose-related protein; PERK, eukaryotic translation initiation factor 2 α kinase 3; ATF, activating transcription factor; IRE, inositol-requiring enzyme; UPR, unfolded protein response.
Figure 5.
ER stress-associated apoptosis in the liver. Expression of ER proapoptotic molecules, (A) CHOP, (B) cleaved caspase-12, (C) p-eIF2α and (D) p-JNK were detected by western blotting. **P<0.01 vs. NC group, ##P<0.01, #P<0.05 vs. CIH group. CIH, chronic intermittent hypoxia; NC, negative control; TUDCA, tauroursodeoxycholic acid; CHOP, C/EBP homologous protein; eIF2α, eukaryotic translation initiation factor 2 subunit α; JNK, janus kinase; ER, endoplasmic reticulum.
Discussion
In the present study, mice were exposed to CIH and treatment with TUDCA, and it was observed that treatment with TUDCA attenuated liver pathological injury, improved liver function, suppressed ROS and proinflammatory factor production. Moreover, CIH-induced hepatocyte apoptosis and ER stress were also inhibited by the administration of TUDCA. To the best of our knowledge, this is the first report to demonstrate that TUDCA is protective in CIH-induced liver injury.
It is widely recognized that OSA and its associated CIH is implicated in the pathogenesis of various liver diseases (31–33). Different studies have proved over the previous decades that TUDCA could recovery liver function from variety of liver diseases (34–37); however, whether TUDCA has protective effects against CIH-induced liver injury remains unknown. The present results showed that intraperitoneal administration of TUDCA significantly protected liver from CIH-induced injury, as evidenced by improved liver pathologic changes and reduced serum ALT and AST levels. A double-blind randomized trial found that TUDCA at the daily dose of 750 mg could improve liver biochemical parameters and is safe to the patients of liver cirrhosis (38). Vandewynckel et al (25) have reported that administration of 300 mg/kg/day TUDCA significantly reduced ALT and AST levels of a diethylnitrosamine-induced mouse hepatocellular carcinoma model; however, the reduction in body weight and survival was also observed. Shi et al (39) found that intraperitoneal injection of 100 mg/kg/day TUDCA inhibited CIH-induced pulmonary cell apoptosis in a mouse model without any side effects. Thus, we also chose to administer 100 mg/kg/day TUDCA by intraperitoneal injection in the present experiment, and the results showed that this dosage of TUDCA could protect liver against CIH injury markedly.
Increasing evidence suggests that oxidative stress and inflammatory is involved in the pathophysiology of several chronic diseases associated with CIH. The high level of ROS generated by oxidative stress, could initiate the lipid peroxidation process and finally leading to DNA breakage and apoptosis (40). TNF-α and IL-1β are proinflammatory cytokines that are known to be increased in livers treated with CIH (41). TUDCA has been shown to be a potent antioxidant and anti-inflammatory agent in cholestasis liver (42,43). The present data suggest that the upregulated TNF-α and IL-1β levels and ROS activity induced by CIH were all markedly decreased by the treatment of TUDCA, further suggesting that TUDCA limits liver injury by reducing oxidative stress and inflammatory reactions.
Clinical data and animal models suggest that apoptosis is a pivotal mechanism of CIH-induced tissue or organ injuries (44–46). Hepatocellular apoptosis could be observed in the majority of types of human liver disease, including hepatic ischemia reperfusion injury, fibrosis, non-alcoholic liver diseases, alcoholic liver disease and hepatocellular carcinoma (47). In particular, Zhen et al (14) reported that exposure to intermittent hypoxia significantly inhibited the proliferation and accelerated apoptosis of human liver cells. In the present study, the number of TUNEL-positive cells in the liver samples of the CIH group was obviously increased, along with the upregulation of two apoptosis markers, cleaved caspase-3 and cleaved caspase-9. In contrast, the increase in TUNEL-positive apoptotic cells and cleaved caspase-3 and cleaved caspase-9 expression was significantly attenuated by TUDCA treatment. These results are consistent with previous data showing that TUDCA could protect hepatocytes from bile-acid (48) or carcinogen (25) induced apoptosis.
ER stress may be activated by various disturbances, including CIH (18,49). In an ER stress state, the ER chaperone GRP78 was released and the transmembrane proteins, PERK, ATF6 and IRE-1 were activated to trigger three different UPR branches to protect ER from severe damage (50). When ER stress was excessive and prolonged, the downstream apoptotic proteins, CHOP, p-eIF2, p-JNK and cleaved caspase-12 were activated and turned to be executioners (51). In the present study, the activation of UPR and ER stress-associated apoptotic pathways induced by CIH were all significantly reduced by TUDCA treatment, suggesting that systemic administration of TUDCA could suppress CIH-induced ER stress in livers. In fact, TUDCA has been widely used as an ER stress inhibitor (23,24). Due to the relation between ER stress and oxidative stress and inflammatory response, we speculate that TUDCA may protect liver against CIH-induced injury at least partly by the suppression of ER stress. These were further supported by previous findings suggesting that TUDCA functions as a liver-protective agent by reducing ER stress (52).
Altogether, the present data demonstrated that TUDCA protected liver tissue from CIH-induced pathological injury, function impairment, oxidative stress, inflammatory response, hepatocyte apoptosis, as well as ER stress. These findings indicated a liver-protective effect of TUDCA in CIH-induced injury, and it is hypothesized that these effects are mediated at least partly through the inhibition of ER stress.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang AA. Obstructive sleep apnea and chronic intermittent hypoxia: A review. Chin J Physiol. 2006;49:234–243. [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Yin X, Zheng Y, Liu Q, Cai J, Cai L. Cardiac response to chronic intermittent hypoxia with a transition from adaptation to maladaptation: The role of hydrogen peroxide. Oxid Med Cell Longev. 2012;2012:569520. doi: 10.1155/2012/569520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. The Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)74229-X. [DOI] [PubMed] [Google Scholar]
- 8.Halbower AC, Degaonkar M, Barker PB, Earley CJ, Marcus CL, Smith PL, Prahme MC, Mahone EM. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3:e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Oda N, Tanaka A, Yamamoto M, Ohta S, O'Donnell CP, Adachi M. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:612–617. doi: 10.1164/rccm.200608-1141OC. [DOI] [PubMed] [Google Scholar]
- 10.Algin O, Gokalp G, Ocakoglu G, Ursavas A, Taskapilioglu O, Hakyemez B. Neurochemical-structural changes evaluation of brain in patients with obstructive sleep apnea syndrome. Eur J Radiol. 2012;81:491–495. doi: 10.1016/j.ejrad.2010.12.092. [DOI] [PubMed] [Google Scholar]
- 11.Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Rosa DP, Forgiarini LF, Baronio D, Feijó CA, Martinez D, Marroni NP. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Med Inflamm. 2012;2012:879419. doi: 10.1155/2012/879419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Q, Shin MK, Jun JC, Hernandez KL, Aggarwal NR, Mock JR, Gay J, Drager LF, Polotsky VY. Effect of chronic intermittent hypoxia on triglyceride uptake in different tissues. J Lipid Res. 2013;54:1058–1065. doi: 10.1194/jlr.M034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhen YQ, Wu YM, Sang YH, Wang Y, Song QY, Yu L, Rao XJ, Dong RH. 2,3-Oxidosqualene cyclase protects liver cells from the injury of intermittent hypoxia by regulating lipid metabolism. Sleep Breath. 2015;19:1475–1481. doi: 10.1007/s11325-015-1167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng SZ, Tian JL, Zhang Q, Wang H, Sun N, Zhang Y, Chen BY. An experimental research on chronic intermittent hypoxia leading to liver injury. Sleep Breath. 2011;15:493–502. doi: 10.1007/s11325-010-0370-3. [DOI] [PubMed] [Google Scholar]
- 16.Ding W, Zhang X, Huang H, Ding N, Zhang S, Hutchinson SZ, Zhang X. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One. 2014;9:e94545. doi: 10.1371/journal.pone.0094545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai XH, Li XC, Jin SW, Liang DS, Wen ZW, Cao HC, Mei HF, Wu Y, Lin ZD, Wang LX. Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp Neurol. 2014;257:148–156. doi: 10.1016/j.expneurol.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Yin X, Zheng Y, Miao X, Feng W, Cai J, Cai L. Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol Lett. 2014;227:113–123. doi: 10.1016/j.toxlet.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 21.Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21:S7–S9. doi: 10.1111/j.1440-1746.2006.04581.x. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 22.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23:S16–S24. doi: 10.1111/j.1440-1746.2007.05276.x. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belaidi E, Thomas A, Bourdier G, Moulin S, Lemarié E, Levy P, Pépin JL, Korichneva I, Godin-Ribuot D, Arnaud C. Endoplasmic reticulum stress as a novel inducer of hypoxia inducible factor-1 activity: Its role in the susceptibility to myocardial ischemia-reperfusion induced by chronic intermittent hypoxia. Int J Cardiol. 2016;210:45–53. doi: 10.1016/j.ijcard.2016.02.096. [DOI] [PubMed] [Google Scholar]
- 24.Shi S, Tan P, Yan B, Gao R, Zhao J, Wang J, Guo J, Li N, Ma Z. ER stress and autophagy are involved in the apoptosis induced by cisplatin in human lung cancer cells. Oncol Rep. 2016;35:2606–2614. doi: 10.3892/or.2016.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandewynckel YP, Laukens D, Devisscher L, Paridaens A, Bogaerts E, Verhelst X, Van den Bussche A, Raevens S, Van Steenkiste C, Van Troys M, et al. Tauroursodeoxycholic acid dampens oncogenic apoptosis induced by endoplasmic reticulum stress during hepatocarcinogen exposure. Oncotarget. 2015;6:28011–28025. doi: 10.18632/oncotarget.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebensztejn DM. Application of ursodeoxycholic acid (UDCA) in the therapy of liver and biliary duct diseases in children. Med Sci Monit. 2000;6:632–636. [PubMed] [Google Scholar]
- 27.Nousia-Arvanitakis S, Fotoulaki M, Economou H, Xefteri M, Galli-Tsinopoulou A. Long-term prospective study of the effect of ursodeoxycholic acid on cystic fibrosis-related liver disease. J Clin Gastroenterol. 2001;32:324–328. doi: 10.1097/00004836-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 29.Lai MC, Lin JG, Pai PY, Lai MH, Lin YM, Yeh YL, Cheng SM, Liu YF, Huang CY, Lee SD. Protective effect of salidroside on cardiac apoptosis in mice with chronic intermittent hypoxia. Int J Cardiol. 2014;174:565–573. doi: 10.1016/j.ijcard.2014.04.132. [DOI] [PubMed] [Google Scholar]
- 30.Li B, Zhang XY, Yang JZ, Zhang YJ, Li WX, Fan CH, Huang Q. Influence of polyethylene glycol coating on biodistribution and toxicity of nanoscale graphene oxide in mice after intravenous injection. Int J Nanomedicine. 2014;9:4697–4707. doi: 10.2147/IJN.S66591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, Gerhard G, Still C. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring) 2016;24:871–877. doi: 10.1002/oby.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Lin X, Chen LD, Lin QC, Chen GP, Yu YH, Huang JC, Zhao JM. Obstructive sleep apnea is associated with fatty liver index, the index of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2016;28:650–655. doi: 10.1097/MEG.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 33.Cakmak E, Duksal F, Altinkaya E, Acibucu F, Dogan OT, Yonem O, Yilmaz A. Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat Mon. 2015;15:e32655. doi: 10.5812/hepatmon.32655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiménez-Castro MB, Elias-Miro M, Mendes-Braz M, Lemoine A, Rimola A, Rodés J, Casillas-Ramírez A, Peralta C. Tauroursodeoxycholic acid affects PPARγ and TLR4 in Steatotic liver transplantation. Am J Transplant. 2012;12:3257–3271. doi: 10.1111/j.1600-6143.2012.04261.x. [DOI] [PubMed] [Google Scholar]
- 36.Dombrowski F, Stieger B, Beuers U. Tauroursodeoxycholic acid inserts the bile salt export pump into canalicular membranes of cholestatic rat liver. Lab Invest. 2006;86:166–174. doi: 10.1038/labinvest.3700371. [DOI] [PubMed] [Google Scholar]
- 37.Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- 38.Pan XL, Zhao L, Li L, Li AH, Ye J, Yang L, Xu KS, Hou XH. Efficacy and safety of tauroursodeoxycholic acid in the treatment of liver cirrhosis: A double-blind randomized controlled trial. J Huazhong Univ Sci Technolog Med Sci. 2013;33:189–194. doi: 10.1007/s11596-013-1095-x. [DOI] [PubMed] [Google Scholar]
- 39.Shi Z, Xu L, Zhou R. Tauroursodeoxycholic acid suppresses endoplasmic reticulum stress in pulmonary tissues of intermittent hypoxia mice. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40:1165–1172. doi: 10.11817/j.issn.1672-7347.2015.11.001. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Wang W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim Sci J. 2016;87:1490–1500. doi: 10.1111/asj.12550. [DOI] [PubMed] [Google Scholar]
- 41.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–G877. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 42.Portincasa P, Grattagliano I, Testini M, Caruso ML, Wang DQ, Moschetta A, Calamita G, Vacca M, Valentini AM, Renna G, et al. Parallel intestinal and liver injury during early cholestasis in the rat: Modulation by bile salts and antioxidants. Free Radic Biol Med. 2007;42:1381–1391. doi: 10.1016/j.freeradbiomed.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 43.Ueno Y, Francis H, Glaser S, Demorrow S, Venter J, Benedetti A, Fava G, Marzioni M, Alpini G. Taurocholic acid feeding prevents tumor necrosis factor-alpha-induced damage of cholangiocytes by a PI3K-mediated pathway. Exp Biol Med (Maywood) 2007;232:942–949. [PubMed] [Google Scholar]
- 44.Ding W, Cai Y, Wang W, Ji L, Dong Y, Zhang X, Su M, Liu J, Lu G, Zhang X. Adiponectin protects the kidney against chronic intermittent hypoxia-induced injury through inhibiting endoplasmic reticulum stress. Sleep Breath. 2016;20:1069–1074. doi: 10.1007/s11325-016-1321-4. [DOI] [PubMed] [Google Scholar]
- 45.Pai P, Lai CJ, Lin CY, Liou YF, Huang CY, Lee SD. Effect of superoxide anion scavenger on rat hearts with chronic intermittent hypoxia. J Appl Physiol (1985) 2016;120:982–990. doi: 10.1152/japplphysiol.01109.2014. [DOI] [PubMed] [Google Scholar]
- 46.Chen HL, Lu CH, Lin HC, Chen PC, Chou KH, Lin WM, Tsai NW, Su YJ, Friedman M, Lin CP, Lin WC. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38:361–370. doi: 10.5665/sleep.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783. doi: 10.1053/j.gastro.2014.07.018. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benz C, Angermüller S, Töx U, Klöters-Plachky P, Riedel HD, Sauer P, Stremmel W, Stiehl A. Effect of tauroursodeoxycholic acid on bile-acid-induced apoptosis and cytolysis in rat hepatocytes. J Hepatol. 1998;28:99–106. doi: 10.1016/S0168-8278(98)80208-0. [DOI] [PubMed] [Google Scholar]
- 49.Xu LH, Xie H, Shi ZH, Du LD, Wing YK, Li AM, Ke Y, Yung WH. Critical role of endoplasmic reticulum stress in chronic intermittent hypoxia-induced deficits in synaptic plasticity and long-term memory. Antioxid Redox Signal. 2015;23:695–710. doi: 10.1089/ars.2014.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 51.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: An overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36:S3–S12. doi: 10.1016/S2210-7401(12)70015-3. (Suppl 1) [DOI] [PubMed] [Google Scholar]