To the editor:
Germ line–activating mutations of protein tyrosine phosphatase PTPN11 (Shp2), a positive regulator of the Ras signaling pathway,1 account for more than 50% of patients with Noonan syndrome.2 These patients have an increased risk of developing leukemias,3 especially juvenile myelomonocytic leukemia, a childhood myeloproliferative neoplasm (MPN). Previous studies have demonstrated that Ptpn11 mutations induce juvenile myelomonocytic leukemia–like MPN through cell-autonomous mechanisms in an Shp2 catalytic activity-dependent manner.4-7 We recently showed that Ptpn11-activating mutations in the mouse bone marrow (BM) microenvironment significantly promoted the development and progression of MPN through profound detrimental effects on hematopoietic stem cells (HSCs).8 Ptpn11 mutations in the BM mesenchymal stem/progenitor cells (which serve as unique sinusoidal vascular and arteriolar HSC niches9,10) and osteoprogenitors, but not differentiated osteoblasts or endothelial cells, caused excessive production of the CC chemokine CCL3 (also known as MIP-1α), which recruited monocytes to the area where HSCs also resided. Consequently, HSCs were hyperactivated by interleukin-1β and possibly other proinflammatory cytokines produced by monocytes, leading to exacerbated MPN and to donor cell–derived MPN after stem-cell transplantation therapy. Administration of the CCL3 receptor antagonists ameliorated MPN induced by the Ptpn11-mutated BM microenvironment. However, the mice were only treated and monitored for a limited time (3 weeks) with the CCL3 receptor antagonists. The role of CCL3 in mediating the pathogenic effects of Ptpn11-mutated BM stromal cells needs to be further tested. The long-term effect of CCL3 inhibition on microenvironmental Ptpn11-activating mutation–induced MPN has yet to be determined.
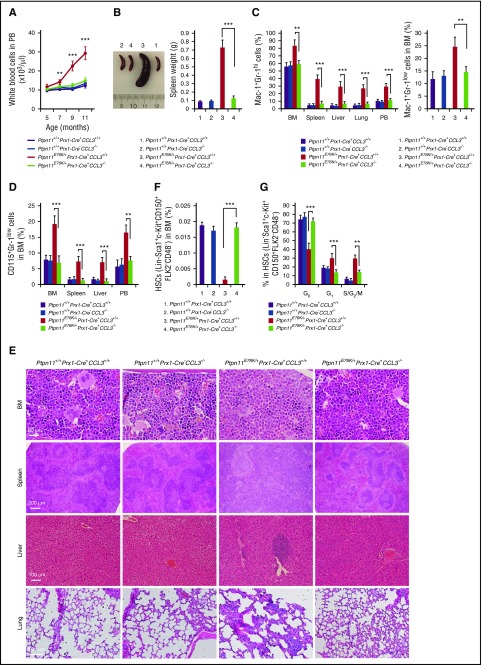
The Ptpn11E76K-activating mutation in Prx1-expressing broad mesenchymal cells in the BM, which contain/overlap with mesenchymal stem/progenitor cells, induced MPN in Ptpn11E76K/+Prx1-Cre+ mice at the age of 5 to 10 months.8 To further determine the role of CCL3 in the pathogenesis of the MPN driven by the Ptpn11E76K mutation in the stem-cell microenvironment, we took a mouse genetic approach and examined the microenvironmental effect of the Ptpn11E76K/+ mutation in the CCL3-knockout background. We generated Ptpn11E76K/+Prx1-Cre+CCL3−/− mice by crossing Ptpn11E76K conditional knockin mice5 with CCL3+/− mice11 and Prx1-Cre+ transgenic mice.12 The mice were monitored for 12 to 17 months. The phenotypes of these double mutants were compared with those of Ptpn11E76K/+Prx1-Cre+ and CCL3−/− single-mutant mice. As expected, Ptpn11E76K/+Prx1-Cre+ mice developed MPN that was derived from wild-type HSCs without Ptpn11 mutations (data not shown). These animals manifested high white blood cell counts (Figure 1A), splenomegaly (Figure 1B), elevated myeloid cells (Mac-1+Gr-1high and Mac-1+Gr-1low; Figure 1C), and monocytes (CD115+Gr-1low; Figure 1D) in the peripheral blood, BM, and spleen. Moreover, there was substantial infiltration of myeloid cells and monocytes in the liver and lung (Figure 1C-D). CCL3−/− mice seemed normal and indistinguishable from wild-type control littermates. No abnormalities in hematopoietic cell development were detected in CCL3 knockout mice (Figure 1A-D), suggesting that CCL3 is dispensable for steady-state hematopoiesis. Importantly, MPN phenotypes induced by the Ptpn11E76K/+ mutation in Prx1-expressing BM mesenchymal cells were essentially blocked in Ptpn11E76K/+Prx1-Cre+CCL3−/− double-mutant mice (Figure 1A-D). Histopathological examination verified that MPN-associated phenotypes were indeed substantially attenuated in these double mutants (Figure 1E).
Figure 1.
Leukemogenic effects of the Ptpn11E76K/+mutation in the stem-cell microenvironment is abolished in Ptpn11E76K/+Prx1-Cre+CCL3−/− mice. (A) White blood cell counts in the peripheral blood (PB) of Ptpn11E76K/+Prx1-Cre+CCL3−/−, Ptpn11E76K/+Prx1-Cre+, CCL3−/−, and Ptpn11+/+Prx1-Cre+ mice (n = 5 mice per group) were determined at the indicated ages. (B-G) Ptpn11E76K/+Prx1-Cre+CCL3−/−, Ptpn11E76K/+Prx1-Cre+, CCL3−/−, and Ptpn11+/+Prx1-Cre+ mice were sacrificed at age 12 to 17 months. Spleen weights were documented (n = 7 mice per group) (B). Mac-1+Gr-1hi cells in the BM, spleen, liver, lung, and PB (n = 7 mice per group for BM, spleen, liver, and lung; n = 5 mice per group for PB) (C left), Mac-1+Gr-1low cells in the BM (n = 7 mice per group) (C right), and CD115+Gr-1low cells in the BM, spleen, liver, lung, and PB (n = 7 mice per group for BM, spleen, liver, and lung; n = 5 mice per group for PB) (D) were determined. Femurs, spleens, livers, and lungs were processed for histopathological examination (hematoxylin and eosin staining; n = 3 mice per group). Representative pictures are shown (E). The pool size of HSCs (Lin−Sca-1+c-Kit+CD150+CD48−Flk2−) in the BM (n = 7 mice per group) (F), and the cell-cycle distribution of HSCs in the BM (n = 7 mice per group) (G) were determined by multiparameter fluorescence-activated cell sorting. Data shown are mean ± standard deviation of all mice examined. **P < .01; ***P < .001.
To further determine whether the rescue effect of CCL3 deletion on the microenvironmental Ptpn11 mutation–induced MPN occurred at the stem-cell level, we examined HSCs in Ptpn11E76K/+Prx1-Cre+CCL3−/− double-mutant mice and compared them with those in Ptpn11E76K/+Prx1-Cre+ single mutants. The frequency of HSCs in the BM of Ptpn11E76K/+Prx1-Cre+ mice was significantly decreased because of stem-cell hyperactivation and attrition as compared with that in control animals (Figure 1F). CCL3−/− mice did not show abnormalities in HSC numbers (Figure 1F). Importantly, the size of the stem-cell pool in Ptpn11E76K/+Prx1-Cre+CCL3−/− mice was normalized (Figure 1F). Cell-cycle analyses demonstrated that the number of quiescent HSCs in the G0 phase decreased by half, whereas HSCs in the G1 or S/G2/M phase doubled in Ptpn11E76K/+Prx1-Cre+ mice (Figure 1G), consistent with what we observed in Ptpn11E76K/+Nestin-Cre+ mice,8 which also developed MPN. Moreover, aberrant HSC activation occurred before MPN was fully developed in Ptpn11E76K/+Prx1-Cre+ mice (supplemental Figure 1, available on the Blood Web site), further supporting that the Ptpn11-mutant microenvironment drives MPN development by hyperactivation of resident HSCs. Notably, the disrupted HSC cell-cycle distribution caused by the Ptpn11E76K/+ mutation in the BM stromal cells was largely corrected, and the dormancy in HSCs was restored in Ptpn11E76K/+Prx1-Cre+CCL3−/− double-mutant mice (Figure 1G).
The robust effect of CCL3 gene deletion on the MPN induced by the Ptpn11E76K/+ mutation in the BM stromal cells strongly suggests that CCL3 is a key mediator for the leukemogenic effect of the microenvironmental Ptpn11E76K/+ mutation. The fact that depletion of the CCL3 gene is more potent than pharmacological inhibition of CCL3 signaling8 in mitigating the MPN driven by the Ptpn11-mutated microenvironment further supports this notion. These mouse genetic data combined with our previous pharmacological inhibition data8 provide a clear mechanism for the detrimental effects of Ptpn11-activating mutations in the stem-cell microenvironment and suggest CCL3 as a therapeutic target for controlling leukemic progression in Noonan syndrome and for improving stem-cell transplantation therapy in Noonan syndrome–associated leukemias, especially considering that CCL3 itself is dispensable for normal hematopoiesis. Nevertheless, because both genetic and pharmacological approaches involve systemic effects, additional studies are required to determine the effects of stromal-cell type–specific deletion of CCL3 on microenvironmental Ptpn11 mutation–induced leukemogenesis.
Supplementary Material
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant HL130995 (C.-K.Q.) and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant DK092722 (C.-K.Q.).
Contribution: L.D. and H.Z. generated microenvironmental cell type–specific knockin mice and analyzed myeloproliferative neoplasm development/progression and hematopoietic stem cell phenotypes; C.-K.Q. designed the experiments and directed the entire project; and L.D. and C.-K.Q. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng-Kui Qu, Division of Hematology/Oncology/BMT, Department of Pediatrics, Aflac Cancer and Blood Disorders Center, Emory University School of Medicine, 1760 Haygood Dr NE, HSRB E302, Atlanta, GA 30322; e-mail: cheng-kui.qu@emory.edu.
References
- 1.Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Curr Opin Genet Dev. 2007;17(1):23-30. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465-468. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381(9863):333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki T, Mohi MG, Ismat FA, et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10(8):849-857. [DOI] [PubMed] [Google Scholar]
- 5.Xu D, Liu X, Yu WM, et al. Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J Exp Med. 2011;208(10):1977-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan G, Kalaitzidis D, Usenko T, et al. Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via cell-autonomous effects on multiple stages of hematopoiesis. Blood. 2009;113(18):4414-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohi MG, Williams IR, Dearolf CR, et al. Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell. 2005;7(2):179-191. [DOI] [PubMed] [Google Scholar]
- 8.Dong L, Yu WM, Zheng H, et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature. 2016;539(7628):304-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269(5230):1583-1585. [DOI] [PubMed] [Google Scholar]
- 12.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77-80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.