Summary
Healthcare‐associated infections (HAIs) are a global concern, affecting all western hospitals, and profoundly impairing the clinical outcome of up to 15% of all hospitalized patients. Persistent microbial contamination of hospital surfaces has been suggested to contribute to HAIs onset, representing a reservoir for hospital pathogens. On the other hand, conventional chemicals‐based sanitation do not prevent recontamination and can select drug‐resistant strains, resulting in over 50% of surfaces persistently contaminated. There is therefore an urgent need for alternative sustainable and effective ways to control pathogens contamination and transmission. Toward this goal, we recently reported that a probiotic‐based sanitation can stably decrease surface pathogens up to 90% more than conventional disinfectants, without selecting resistant species. This paper summarizes some of our most significant results.
Trying to ensure a healthy environment during hospitalization represents a vital sustainable development goal of the recent years, in the attempt to face up to the global concern of hospital acquired infections.
Such infections affect up to 15% of all hospitalized patients in high‐income countries (Allegranzi et al., 2011; Cookson et al., 2013; Suetens et al., 2013) and represent the fourth largest killer in the USA (Pharmaceutical, 2015).
Just because of their illnesses, hospital inpatients are predisposed to both contracting and spreading infections, and hospital surfaces can represent the reservoir for the associated pathogens.
In fact, the contribution of contaminated hospital surfaces is increasingly recognized as an important factor in the acquisition of infections associated with hospitalization (healthcare‐associated infections, HAIs).
So far, the control of surface contamination has been addressed almost exclusively by use of chemical compounds, which are accompanied by a non‐negligible environmental impact (Kummerer, 2001), and show important limitations.
First, although disinfectants can be effective in the immediate abatement of surface pathogens, they result ineffective in preventing recontamination phenomena, occurring as fast as 30 min, which are ultimately responsible for pathogens persistence. In agreement with this observation, several studies have shown that at least half of hospital surfaces are inadequately sanitized by use of chemical germicides (Carling et al., 2008; Goodman et al., 2008). Many pathogens are in fact persisting for long periods on high, medium or low touch nosocomial surfaces (Hota, 2004; Kramer et al., 2006; Boyce et al., 2011; Huslage et al., 2013). Staphylococci, including methicillin resistant Staphylococcus aureus (MRSA), vancomycin‐resistant enterococci (VRE), Pseudomonas spp., Acinetobacter spp. and even viruses (i.e. norovirus), are retaining their infectivity for days to months on dry inanimate surfaces (Weber and Rutala, 1997; Kramer et al., 2006; Boyce, 2007; Weber et al., 2010). Clostridium difficile spores are indeed surviving for several months on environmental surfaces and contaminate about 75% of rooms hosting infected patients (Weber et al., 2010).
Second, disinfectants can select resistant microbial strains against the disinfectant itself (Bock et al., 2016), and also, more importantly, against antibiotics, as recently reported for chlorhexidine induction of resistance against Colistin (Wand et al., 2017), an antibiotic considered till 2016 as a last‐resort drug for treatment of infections sustained by multidrug‐resistant (MDR) Gram‐negative bacteria.
The potential induction of antibiotic resistance represents a highly undesirable side‐effect of chemical cleaning, as MDR pathogens have been constantly and rapidly growing in the recent decades and a high proportion of HAIs is caused by MDR bacteria (Caini et al., 2013; Cornejo‐Juarez et al., 2015), threatening the outcome of an ever‐increasing range of infections.
Thus, taken together, these data suggest that chemical sanitation, although well‐intentioned, cannot guarantee a true healthy environment for patients, as it appears unable to maintain the environment safe and might even increase bacterial drug resistance.
Alternative methods to chemical disinfectants have been proposed to control surface contamination, including the use of ‘self‐disinfecting’ surfaces based on the use of heavy metals (silver, copper), germicide impregnated materials or light‐activated antimicrobial coatings (Carling and Bartley, 2010; Dancer, 2011; Davies et al., 2011; Rutala and Weber, 2011, 2013; Otter et al., 2013). However, these methods result very expensive and not suitable for all types of surfaces and settings, including those in low‐income countries.
Based on these observations, there is an urgent need for sustainable effective alternatives to the use of conventional germicides.
In the attempt to minimize the infectious risk for hospitalized patients, and to avoid increasing of drug resistance and environmental impact, the sanitation of hospital surfaces has been recently rethought, trying to manage the ‘health’ of the hospital environment as the health of the human body.
This approach, inspired by the Microbiome Project data, considers that, rather than eradicating all pathogens, replacing pathogens by beneficial microbes might be more effective in decreasing infections (Al‐Ghalith and Knights, 2015; Pettigrew et al., 2016).
It is in fact generally accepted that beneficial microbes are important for our health and that their use can be effective in the prevention and treatment of infectious diseases (Koenigsknecht and Young, 2013). Among the microorganisms potentially useful towards this aim, probiotics appear particularly interesting, as they are defined as beneficial microbes for our health, capable to ‘fill the void’, disadvantaging the colonization by pathogens (WHO, 2001; Hill et al., 2014). Notably, probiotics have been shown effective in reducing the occurrence of different nosocomial infections, including diarrhoea, necrotizing enterocolitis (Giamarellos‐Bourboulis et al., 2009), upper respiratory infections (Banupriya et al., 2015) and infections in surgical patients (Rayes et al., 2002, 2012; Sommacal et al., 2015).
On that basis, for several years we have been working on a sanitation approach based on the addition of spores of probiotics belonging to the Bacillus genus to eco‐sustainable detergents. In this study, some of our most significant results are reviewed.
Bacteria of the Bacillus genus are apathogenic (except for two well‐recognizable species) (EFSA, 2010), ubiquitous (they are present in soil, water, vegetables, as in human gut) and have a long history of safe use in humans. Furthermore, spore former probiotics are particularly suitable for addition to detergents, as spores maintain their viability in the concentrated cleanser, originating the vegetative bacteria when diluted in water and seeded on surfaces.
The system, named Probiotic Cleaning Hygiene System (PCHS) and including three Bacillus species (B. subtilis, B. pumilus, B. megaterium), was tested in ten different hospitals, in Italy and Belgium, both by simultaneous comparison of wards treated by PCHS and conventional disinfectants, and by sequential pre‐ to post‐comparison of surface bioburden in the same wards treated sequentially with both systems. The results, collected in over 4 years, showed that PCHS stably decreased the presence of pathogens on treated surfaces, about 90% more than conventional cleansers (Vandini et al., 2014; Caselli et al., 2016a) (Fig. 1A). This effect was associated with germination of probiotic Bacillus spores, followed by an actual replacement of pathogens by PCHS Bacillus, reaching about 70% of the total surface microbiota after 1 month of application (Caselli et al., 2016a). Importantly, PCHS did not select any drug‐resistant strain, but rather it induced a general decrease in the whole antibiotic resistance genes of the residual microbiota, compared to the original pre‐existing one (Caselli et al., 2016a) (Fig. 1B).
Figure 1.
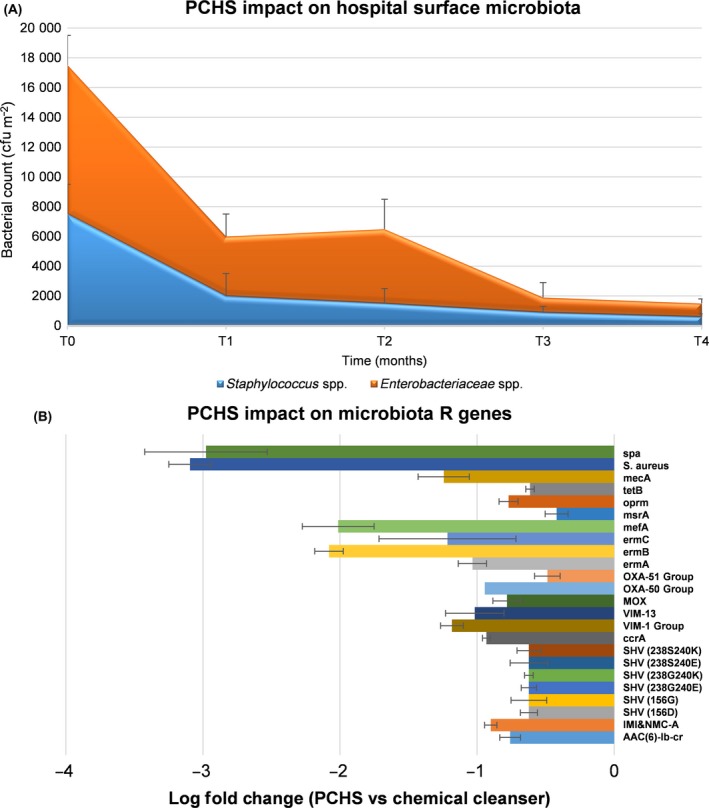
Impact of the probiotic‐based microbial cleaning on microbiota contaminating hospital surfaces.
A. PCHS effect on Gram‐positive (Staphylococcus spp.) and Gram‐negative (Enterobacteriaceae spp.) pathogens amounts on treated surfaces after 1, 2, 3, 4 months of PCHS continuous sanitation (T0 values are those obtained with chemical sanitation); results are expressed as median CFU counts per m2.
B. PCHS effect on the R genes of the whole residual microbiota (resistome) after 1–4 months of PCHS sanitation; results were obtained by PCR microarray and are expressed as of log fold change in R genes compared to the values detected at T0; mean values of the 4 months ± SD are reported.
In addition, probiotic Bacilli resulted genetically very stable, as they did not acquire any new resistance gene over a period of 4 years of continuous use, despite the continuous contact with surface pathogenic and drug‐resistant neighbours (Caselli et al., 2016a).
Last, due to the enzymatic activity of probiotic Bacilli, producing esterases, lipases, glucosidases and phosphatases (unpublished personal observations), their addition to cleansers allowed to abate the need for high concentrations of chemical detergent compounds in the cleanser itself, resulting in eco‐sustainable formulations with low environmental impact and low cost.
However, as one of the major obstacles still limiting the use of probiotics for hospital sanitation regards their theoretical infectious risk, we wanted to address this point, as a few anecdotic cases of adverse events had been reported in the past (Logan, 1988), and there is a lack of systematic studies on probiotics safety (Doron and Snydman, 2015).
Thus, we implemented a microbiological surveillance for Bacillus in the healthcare structures continuously using PCHS up to 4 years, analysing for Bacillus presence all the clinical samples derived from hospitalized patients. Over 32 000 clinical specimens from subjects with and without HAIs were analysed, and no positive samples were found (Caselli et al., 2016b), even using highly sensitive molecular techniques (Caselli et al., 2016a,b), suggesting that probiotic Bacilli do not represent an infectious risk even in the particularly susceptible hospitalized patient.
Taken together, these data show that a sanitation system based on the principle of competitive antagonism between good and bad bacteria can be highly effective in reducing and maintaining stably low the level of dangerous pathogens in hospital environment. In addition, the absence of selection of resistant species can be of further help in reducing the risk of hazardous and hard‐to‐treat infections in hospitalized subjects. Last, the costs of this system are even lower compared to those of chemical disinfectants, thus rendering it suitable for a widespread application without additional costs for the healthcare structures.
At the moment, the main limitation of probiotic‐based sanitation is represented by the time needed to obtain a stable microbial balance on surfaces (2–4 weeks), that makes it ideal for a prophylactic use, rather than for instantaneous decontamination. This feature can be substantially ameliorated and will deserve future studies. Also, it will be necessary to verify whether the decrease in surface drug‐resistant pathogens corresponds to a really diminished number of developed HAIs. To this aim, we are currently performing a 18‐month multicenter study in seven Italian hospitals, involving about 19 000 patients hosted in PCHS‐treated structures (Caselli et al., 2016c), as the decrease of infections in hospitalized patients is the final goal of these researches.
We are convinced that, when using microbiological agents, a constant monitoring by microbiological and molecular analyses is mandatory, as it can guarantee both safety and effectiveness optimization of the microbial procedure.
However, based on the data collected so far, we feel that probiotic strategies may significantly advance efforts towards reducing infections in hospitals and likely in other environments (home care, breeding farms, etc.), thus deeply improving health sustainability.
Conflict of interest
The author has no conflict of interest to declare.
Acknowledgements
This article was not supported by specific funds.
Microbial Biotechnology (2017) 10(5), 1079–1083
Funding information
No funding information provided.
References
- Al‐Ghalith, G.A. , and Knights, D. (2015) Bygiene: the New Paradigm of Bidirectional Hygiene. Yale J Biol Med 88: 359–365. [PMC free article] [PubMed] [Google Scholar]
- Allegranzi, B. , Bagheri Nejad, S. , Combescure, C. , Graafmans, W. , Attar, H. , Donaldson, L. , and Pittet, D. (2011) Burden of endemic health‐care‐associated infection in developing countries: systematic review and meta‐analysis. Lancet 377: 228–241. [DOI] [PubMed] [Google Scholar]
- Banupriya, B. , Biswal, N. , Srinivasaraghavan, R. , Narayanan, P. , and Mandal, J. (2015) Probiotic prophylaxis to prevent ventilator associated pneumonia (VAP) in children on mechanical ventilation: an open‐label randomized controlled trial. Intensive Care Med 41: 677–685. [DOI] [PubMed] [Google Scholar]
- Bock, L.J. , Wand, M.E. , and Sutton, J.M. (2016) Varying activity of chlorhexidine‐based disinfectants against Klebsiella pneumoniae clinical isolates and adapted strains. J Hosp Infect 93: 42–48. [DOI] [PubMed] [Google Scholar]
- Boyce, J.M. (2007) Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 65(Suppl 2): 50–54. [DOI] [PubMed] [Google Scholar]
- Boyce, J.M. , Havill, N.L. , Havill, H.L. , Mangione, E. , Dumigan, D.G. , and Moore, B.A. (2011) Comparison of fluorescent marker systems with 2 quantitative methods of assessing terminal cleaning practices. Infect Control Hosp Epidemiol 32: 1187–1193. [DOI] [PubMed] [Google Scholar]
- Caini, S. , Hajdu, A. , Kurcz, A. and Borocz, K. (2013) Hospital‐acquired infections due to multidrug‐resistant organisms in Hungary, 2005‐2010. Euro Surveill 18: 1–9. [PubMed] [Google Scholar]
- Carling, P.C. , and Bartley, J.M. (2010) Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. Am J Infect Control 38: S41–S50. [DOI] [PubMed] [Google Scholar]
- Carling, P.C. , Parry, M.F. , and Von Beheren, S.M. (2008) Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol 29: 1–7. [DOI] [PubMed] [Google Scholar]
- Caselli, E. , D'Accolti, M. , Vandini, A. , Lanzoni, L. , Camerada, M.T. , Coccagna, M. , et al (2016a) Impact of a probiotic‐based cleaning intervention on the microbiota ecosystem of the hospital surfaces: focus on the resistome remodulation. PLoS ONE 11: e0148857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli, E. , Antonioli, P. , and Mazzacane, S. (2016b) Safety of probiotics used for hospital environmental sanitation. J Hosp Infect 94: 193–194. [DOI] [PubMed] [Google Scholar]
- Caselli, E. , Berloco, F. , Tognon, L. , Villone, G. , La Fauci, V. , Nola, S. , et al (2016c) Influence of sanitizing methods on healthcare‐associated infections onset: a multicentre, randomized, controlled pre‐post interventional study. J Clin Trials 6: 6. [Google Scholar]
- Cookson, B. , Mackenzie, D. , Kafatos, G. , Jans, B. , Latour, K. , Moro, M.L. , et al (2013) Development and assessment of national performance indicators for infection prevention and control and antimicrobial stewardship in European long‐term care facilities. J Hosp Infect 85: 45–53. [DOI] [PubMed] [Google Scholar]
- Cornejo‐Juarez, P. , Vilar‐Compte, D. , Perez‐Jimenez, C. , Namendys‐Silva, S.A. , Sandoval‐Hernandez, S. , and Volkow‐Fernandez, P. (2015) The impact of hospital‐acquired infections with multidrug‐resistant bacteria in an oncology intensive care unit. Int J Infect Dis 31: 31–34. [DOI] [PubMed] [Google Scholar]
- Dancer, S.J. (2011) Hospital cleaning in the 21st century. Eur J Clin Microbiol Infect Dis 30: 1473–1481. [DOI] [PubMed] [Google Scholar]
- Davies, A. , Pottage, T. , Bennett, A. , and Walker, J. (2011) Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J Hosp Infect 77: 199–203. [DOI] [PubMed] [Google Scholar]
- Doron, S. , and Snydman, D.R. (2015) Risk and safety of probiotics. Clin Infect Dis 60(Suppl 2): S129–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2010) Panel on Biological Hazards (BIOHAZ). Scientific opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2010 update). EFSA J 8: 1–89. [Google Scholar]
- Giamarellos‐Bourboulis, E.J. , Bengmark, S. , Kanellakopoulou, K. , and Kotzampassi, K. (2009) Pro‐ and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma 67: 815–821. [DOI] [PubMed] [Google Scholar]
- Goodman, E.R. , Platt, R. , Bass, R. , Onderdonk, A.B. , Yokoe, D.S. , and Huang, S.S. (2008) Impact of an environmental cleaning intervention on the presence of methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 29: 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G.R. , Merenstein, D.J. , Pot, B. , et al (2014) Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11: 506–514. [DOI] [PubMed] [Google Scholar]
- Hota, B. (2004) Contamination, disinfection, and cross‐colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis 39: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huslage, K. , Rutala, W.A. , Gergen, M.F. , Sickbert‐Bennett, E.E. , and Weber, D.J. (2013) Microbial assessment of high‐, medium‐, and low‐touch hospital room surfaces. Infect Control Hosp Epidemiol 34: 211–212. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht, M.J. , and Young, V.B. (2013) Faecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection: current promise and future needs. Curr Opin Gastroenterol 29: 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, A. , Schwebke, I. , and Kampf, G. (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerer, K. (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources–a review. Chemosphere 45: 957–969. [DOI] [PubMed] [Google Scholar]
- Logan, N.A. (1988) Bacillus species of medical and veterinary importance. J Med Microbiol 25: 157–165. [DOI] [PubMed] [Google Scholar]
- Otter, J.A. , Yezli, S. , Perl, T.M. , Barbut, F. , and French, G.L. (2013) The role of ‘no‐touch’ automated room disinfection systems in infection prevention and control. J Hosp Infect 83: 1–13. [DOI] [PubMed] [Google Scholar]
- Pettigrew, M.M. , Johnson, J.K. , and Harris, A.D. (2016) The human microbiota: novel targets for hospital‐acquired infections and antibiotic resistance. Ann Epidemiol 26: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceutical, I.M. (2015) RID Study: infection caught in the US hospitals is the 4th biggest killer.
- Rayes, N. , Seehofer, D. , Hansen, S. , Boucsein, K. , Muller, A.R. , Serke, S. , et al (2002) Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 74: 123–127. [DOI] [PubMed] [Google Scholar]
- Rayes, N. , Pilarski, T. , Stockmann, M. , Bengmark, S. , Neuhaus, P. , and Seehofer, D. (2012) Effect of pre‐ and probiotics on liver regeneration after resection: a randomised, double‐blind pilot study. Benef Microbes 3: 237–244. [DOI] [PubMed] [Google Scholar]
- Rutala, W.A. , and Weber, D.J. (2011) Are room decontamination units needed to prevent transmission of environmental pathogens? Infect Control Hosp Epidemiol 32: 743–747. [DOI] [PubMed] [Google Scholar]
- Rutala, W.A. , and Weber, D.J. (2013) Disinfectants used for environmental disinfection and new room decontamination technology. Am J Infect Control 41: S36–S41. [DOI] [PubMed] [Google Scholar]
- Sommacal, H.M. , Bersch, V.P. , Vitola, S.P. , and Osvaldt, A.B. (2015) Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: a randomized, double‐blind clinical trial. Nutr Cancer 67: 457–462. [DOI] [PubMed] [Google Scholar]
- Suetens, C. , Hopkins, S. , Kolman, J. , and Diaz Högberg, L. (2013) Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Stockholm, Sweden: European Centre for Disease Prevention and Control. [Google Scholar]
- Vandini, A. , Temmerman, R. , Frabetti, A. , Caselli, E. , Antonioli, P. , Balboni, P.G. , et al (2014) Hard surface biocontrol in hospitals using microbial‐based cleaning products. PLoS ONE 9: e108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand, M.E. , Bock, L.J. , Bonney, L.C. and Sutton, J.M. (2017) Mechanisms of increased resistance to chlorhexidine and cross‐resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 61: e01162–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, D.J. , and Rutala, W.A. (1997) Role of environmental contamination in the transmission of vancomycin‐resistant enterococci. Infect Control Hosp Epidemiol 18: 306–309. [DOI] [PubMed] [Google Scholar]
- Weber, D.J. , Rutala, W.A. , Miller, M.B. , Huslage, K. , and Sickbert‐Bennett, E. (2010) Role of hospital surfaces in the transmission of emerging health care‐associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control 38: S25–S33. [DOI] [PubMed] [Google Scholar]
- WHO, F. (2001) Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. http://www.fao.org/3/a-a0512e.pdf: World Health Organization [online]. [Google Scholar]


