Abstract
Background:
The first satellite center to offer interventional radiology procedures at Memorial Sloan Kettering Cancer Center opened in October 2014. Two of the procedures offered, fine needle aspirations and core biopsies, required rapid on-site cytologic evaluation of smears and biopsy touch imprints for cellular content and adequacy. The volume and frequency of such evaluations did not justify hiring on-site cytotechnologists, and therefore, a dynamic robotic telecytology (TC) solution was created. In this technical article, we present a detailed description of our implementation of robotic TC.
Methods:
Pathology devised the remote robotic TC solution after acknowledging that it would not be cost effective to staff cytotechnologists on-site at the satellite location. Sakura VisionTek was selected as our robotic TC solution. In addition to configuration of the dynamic robotic TC solution, pathology realized integrating the technology solution into operations would require a multidisciplinary effort and reevaluation of existing staffing and workflows.
Results:
Extensively described are the architectural framework and multidisciplinary process re-design, created to navigate the constraints of our technical, cultural, and organizational environment. Also reviewed are the benefits and challenges associated with available desktop sharing solutions, particularly accounting for information security concerns.
Conclusions:
Dynamic robotic TC is effective for immediate evaluations performed without on-site cytotechnology staff. Our goal is providing an extensive perspective of the implementation process, particularly technical, cultural, and operational constraints. Through this perspective, our template can serve as an extensible blueprint for other centers interested in implementing robotic TC without on-site cytotechnologists.
Keywords: Technical, workflow, robotic, telecytology, telepathology
INTRODUCTION
Minimally invasive biopsy procedures, such as fine-needle aspiration and core biopsies, are optimized by the use of rapid adequacy assessment. This assessment is usually performed by cytotechnologists and/or cytopathologists who are on-site. They can provide immediate feedback to the radiologist or clinician performing the procedure and inform if the biopsy is representative of the lesion if additional material is required for ancillary studies.
The establishment of an interventional radiology (IR) service capable of performing these minimally invasive procedures, located 30 miles from the cytology laboratory and in a densely populated metropolitan area, [Figure 1] required the development of alternatives to support this IR service when minimally invasive procedures are performed. Smears and biopsy touch imprints for cellular content and adequacy increase the sensitivity of the procedures. However, the low daily volume projected could not justify hiring and stationing a cytotechnologist on-site. An internal analysis that compared the cost of a robotic digital microscope and a cytotechnologist's salary demonstrated that the robotic digital microscope was more cost effective after 1 year.
Figure 1.
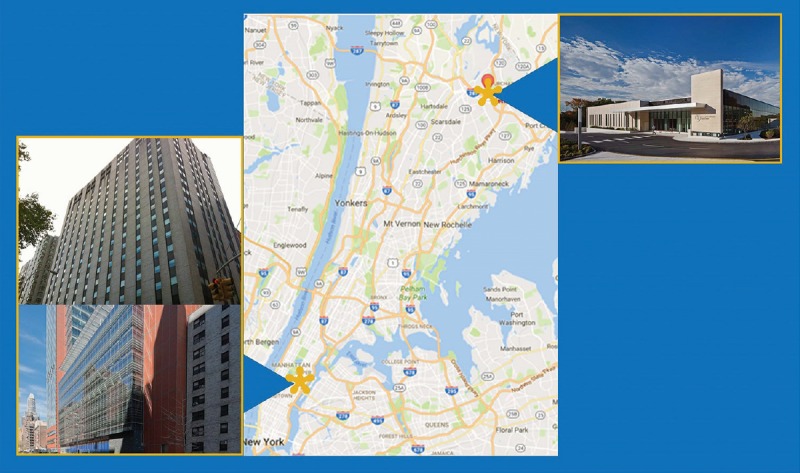
Geographic map of Memorial Sloan Kettering West Harrison (upper right) and Main Campus (lower left) Locations. This map illustrates the geography between the new satellite site at West Harrison and the main campus located on Manhattan's Upper East Side. Distance is approximately at 30 miles
Implementation of a robotic telecytology solution required a multidisciplinary process reevaluation and the configuration of a dynamic robotic telecytology solution. This technical note describes the architectural framework, multidisciplinary process re-design created to navigate the technical, cultural, and organizational environment. The challenges encountered and solutions created are discussed in detail. Such factors are likely relevant to other institutions considering dynamic robotic telecytology.
TECHNICAL BACKGROUND
The Sakura VisionTek (SkVT) (Sakura Finetek USA, Inc.; Torrance, CA 90501, USA) was selected as our robotic telecytology solution. This robotic digital microscope offers live viewing with a robotic stage that manages up to four slides simultaneously with four interchangeable objectives (×2.5, ×10, ×20, ×40). Time to view is under 17 s and the Windows-based user interface application is relatively intuitive, allowing the operator to have full control of the slide, including the location of the image, magnification, and focus. Several options to navigate the application are available to the operator. Operators at our institution are the cytotechnologists for which they use a standard institutional issue trackball mouse or keyboard controls for focusing and displaying fields of view by tile. A third method is driving the cursor on the low power thumbnail through the mouse or keyboard for magnification changes and focusing. For mouse operators, the left and right mouse click buttons are used for changes in magnification and the scroll wheel is used for fine focusing.
Domain network access and internet speed were 1GB/s at both satellite location and the main campus. Desktop sharing was performed behind the institutional firewall through both the remote desktop and Memorial Sloan-Kettering Cancer Center (MSK) dedicated WebEx applications. These two applications are the only internal remote access mechanisms approved by the MSK Information Security Office because they allow auditing of events in which potential personal health information (PHI) can be exchanged.
APPROACH
Staffing and workflow reevaluation
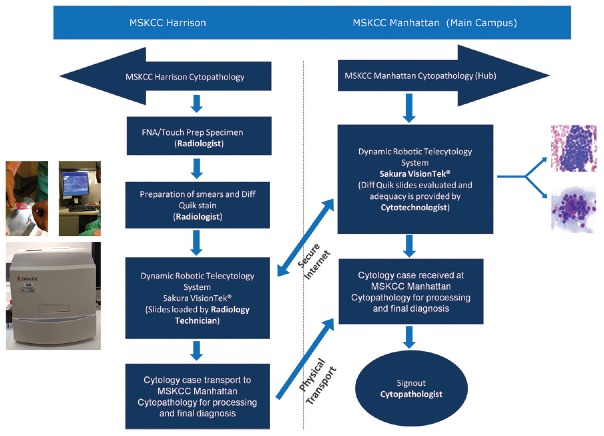
Pathology devised the remote robotic telecytology solution after acknowledging that it would not be cost effective to staff cytotechnologists on-site at the satellite location. Pathology realized that integrating the technology solution into operations would require a multidisciplinary effort and a reevaluation of existing staffing and workflows. As the cytology personnel is not present on-site, testing of the SkVT for readiness is performed by the IR staff, whereas remote network testing for readiness is performed daily by cytotechnologists at the main campus. A comprehensive in-service on preparing Diff-Quik stained slides and loading the SkVT was provided to the IR team. Management and quality control of the reagents used for the Diff-Quik solution preparation and maintenance was provided by the on-site laboratory medicine. Figure 2a is the actual Diff-Quik solution tray in the IR suite, and each morning, quality control procedures include assessment of Diff-Quik slide preparation for satisfactory staining by the Laboratory Medicine team. The SkVT [Figure 2b] was placed inside the IR procedure room to minimize slide transport [Figure 2c].
Figure 2.

Remote on-site setup (a) The Diff-Quik solution tray used in the interventional radiology suite which is maintained and quality controlled by laboratory medicine. (b) The Sakura VisionTek robotic microscope used in the interventional radiology suite. The arrow designates the slide loading door with stable tray mechanism which is simple and not prone to breakage or jamming. (c) Proximity of the Sakura VisionTek, situated adjacent to the interventional radiology procedure table
PROCEDURE
Immediately, after specimen collection by the IR staff, Diff-Quik slides are prepared by the IR team and loaded (up to 4 at one time) into the SkVT. The cytotechnologist at the main campus is notified of the procedure through a dedicated phone line when the specimen is ready. All verbal communication is performed over the phone. The cytotechnologist initiates the remote desktop application. At this point, only the cytotechnologist at main campus has access to the SkVT computer. Image viewing at the main campus is viewed on HD monitors through the high-speed intranet. To accommodate the radiologists' request to view the microscopic images in their control room, a screen sharing session through WebEx session is created. The select region of interest is provided for viewing, and once the cytology staff concludes the adequacy assessment review, the remote telepathology session is terminated. Transport of the physical glass slides to the main campus for routine processing and final microscopic evaluation is performed for concordance assessment. Figure 3 shows the overall procedure flow. Lag time of 0.2–0.5 s was experienced; however, it did not interfere with the process.
Figure 3.
Memorial Sloan-Kettering Cancer Center Harrison specimen adequacy assessment protocol diagram of the overall procedure flow from remote cytologic evaluation, through physical transport, to final cytologic evaluation on the main campus
DISCUSSION
Implementation of our remote robotic telecytology solution was a multidisciplinary effort that required participation from stakeholders in various departments, including laboratory medicine and radiology. This workflow addressed multiple logistical, operational, and stakeholder constraints. On-site resources, personnel from pathology were insufficient with pathology staff members only present on-site for routine visits to audit equipment, stain, and procedure quality and train employees on any new processes. Figure 4 summarizes the workflow transitions and stakeholder role adjustments involved in this telecytology implementation.
Figure 4.
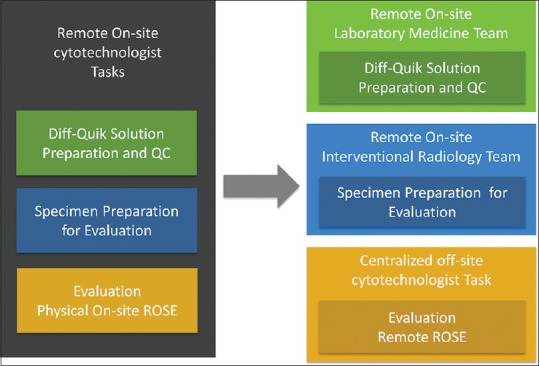
Summary of transitions with workflow and stakeholder roles
The uniquely collaborative relationship between Radiology and Pathology was crucial to the success of this implementation. Pathology simply could not provide the service without buy-in from radiology. Despite the required training and workflow changes in radiology, interventional radiologists remained very supportive of the initiative. Radiology understood pathology's inability to justify a full-time cytotechnologist on site due to low procedure volume, which likely helped facilitate cooperation between both departments. The new activities had a minimal disruptive impact and were quite straightforward. Furthermore, the effort further strengthened the relationship and teamwork between cytology and IR teams. Radiology's willingness to expand our blueprint to other emerging satellite locations is a testament to the success of our architectural template for robotic telecytology.
When considering the extensibility of our architectural template to other centers, it is important to foster similar cooperative interdepartmental relationships. There are currently no initiatives underway at our institution to implement a similar solution for endoscopic clinicians; we are therefore unable to describe experiences outside of Radiology. Moreover, endoscopic clinicians perform a high enough volume of procedures to justify hiring a full-time, on-site cytotechnologist.
From the pathology perspective, the cytotechnologist team was in-serviced on the SkVT user interface and utilization of remote desktop and WebEx sessions. All cytotechnologist team members found navigation and control of the SkVT user interface to be fairly intuitive and user-friendly. They were very happy in regard to the few steps needed to initialize the SkVT desktop application, in regard to obtaining the first image in approximately <10 s, and in regard to the fast autofocusing of the SkVT. They also found the mouse controls easy with manual focusing and with moving slides around through mouse dragging. From the Radiology perspective, minimal in-servicing was necessary. The blue arrow in Figure 2b points to the loading mechanism of the SkVT which centers on the sturdy and stable loading tray. Turning on the SkVT system and loading and unloading slides is seamless. No user application stalls during a remote session and no incidents of jamming or destruction of slides occurred in 2 years.
An important technical consideration requiring further elaboration is the use of desktop sharing applications that enable telecytology once the live image is captured on the monitor. Several desktop sharing software solutions have been shown to be effective telepathology applications. Skype has demonstrated viable desktop sharing capability to enable image sharing for telepathology.[1] Low-cost or even free mobile-mediated applications like FaceTime® have also been effective telecytology solutions.[2,3] In our opinion, however, the desktop sharing options recommended by commercial robotic microscope vendors have not been ideal for a scalable telecytology implementation from both security and operations perspectives.
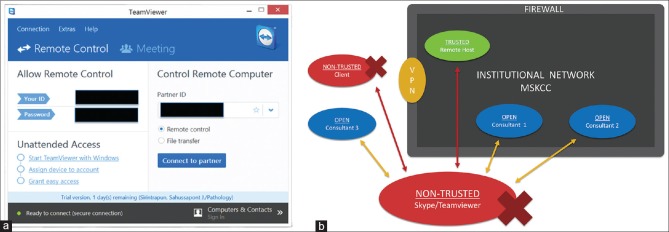
SkVT and other robotic microscopic vendors suggest desktop sharing applications such as TeamViewer. The TeamViewer user interface is intuitive and simple with only the partner ID and password (provided by the host) required for continuous remote access [Figure 5a]. Applications such as Skype and TeamViewer are potentially problematic for telecytology as most institutional information security teams have expressed concerns about these products' compliance with security procedures. Figure 5b illustrates these external server desktop sharing architectures. First, these desktop sharing applications bypass both standard login procedures and scheduled timeouts, which can potentially provide indefinite open access to workstations. Second, these desktop sharing software applications create an outbound stream to proprietary servers outside an institutional firewall. Normal VPN procedures can thus be circumvented through these desktop sharing applications. The routing of data outside the institutional firewalls creates an opportunity for security breaches and the storage of PHI beyond the institutional domain. Due to unfamiliarity with the pathology domain, most information security teams are unable to develop an appropriate risk assessment. For instance, many teams are ill-equipped to recognize whether identifiable information can be exchanged in an image stream as potential targets for a “hack.” The routing of microscopic images through an outside server without institutional security oversight and access control understandably poses compliance concerns for most institutional security teams. It is possible to configure an enterprise version of TeamViewer such that desktop sharing is internal and behind an institutional firewall. This arrangement is beyond the technical expertise of most departmental or institutional IS security teams, including ours.
Figure 5.
The Teamviewer desktop sharing solution (a) screenshot of Teamviewer's desktop sharing interface which is simple and requires only knowledge of the partner ID and password. Security mechanisms like two factors and VPNs are obviated, despite the simplicity of the workflow. (b) overall schematic of Teamviewer server desktop sharing architecture
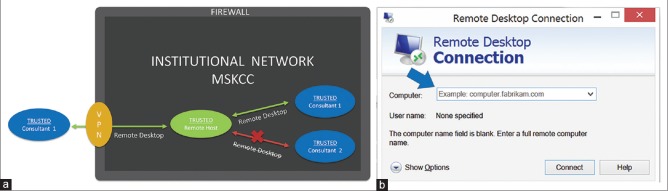
In our architecture, remote access to the SkVT desktop is first obtained through the remote desktop application. Figure 6a illustrates the remote desktop architecture. All remote access occurs behind the institutional firewall on our network domain or through VPN connections for outside access. Figure 6b shows a screenshot which demonstrates the simple workflow of just providing the workstation ID or IP address. The workflow requires a minimal number of entries and clicks. The remote desktop application also forces users to abide by normal login and authentication procedures to access the image. Multiple views are not possible under the remote desktop application since only one-to-one remote access is provided. Once the SkVT desktop is accessed remotely through the application, the screen goes blank locally.
Figure 6.
The Remote desktop sharing solution (a) overall schematic of the remote desktop architecture which provides more direct access to remote workstations. With normal VPN procedures, the remote desktop solution can be used to access internal workstations behind the firewall from external sources. (b) screenshot of the remote desktop interface which demonstrates the simple workflow requiring only a workstation ID or IP address, followed by standard workstation login procedures
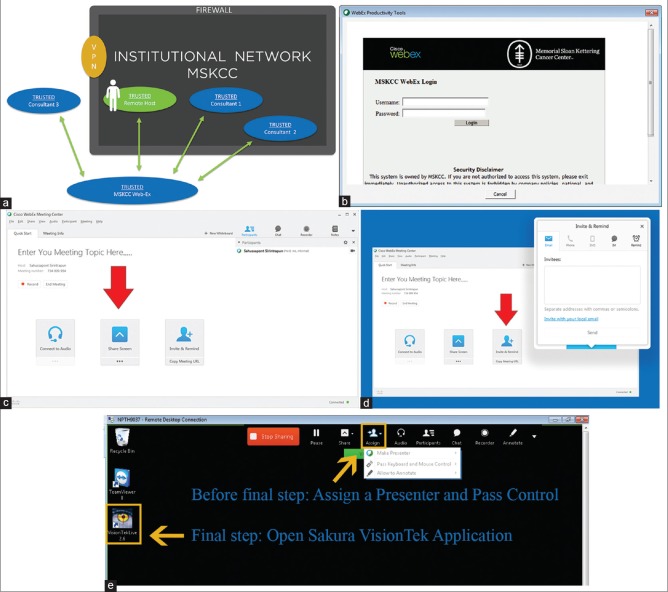
To satisfy the interventional radiologists' request to view the cytologic images during the adequacy assessment process, we utilized the desktop sharing functionality of the MSK dedicated WebEx application. Figure 7a illustrates the use of the MSK dedicated WebEx application sharing architecture. The exchange of images with the IR team is not a required component of remote telecytology rapid on-site evaluation procedure. However, the cytotechnology team willingly incorporated this into the workflow as radiology's support was an imperative for successful telecytology implementation.
Figure 7.
The WebEx external desktop sharing solution (a) overall schematic of the Memorial Sloan-Kettering dedicated WebEx architecture. WebEx can bypass the normal VPN procedures to access internal workstations behind the firewall, from external sources. Images from the Sakura VisionTek desktop application can then be shared with multiple parties both internal and external to our institutional firewall along with desktop control. The human figure represents the need for a person at the remote satellite site to initiate the WebEx session or another person off-site using remote desktop to login the workstation and then initiate the WebEx session by performing the WebEx login and password procedure. (b) screenshot of the Web-Ex login with the input of username and password. For first time logins, a Web-Ex plug-in is required per workstation that can cause further delays. (c) screenshot of host setup with screen share, once logged in. Each workstation must be manually configured for Voice over Internet Protocol if the default phone option is not desired. (d) screenshot of the invitation procedure for consultants, which often requires knowledge of the consultant's email. On the consultant side, email notifications allow the consultant to click and download the appropriate plugins, for viewing the Web-Ex session. (e) screenshot of the top panel on the screen for requesting or passing desktop sharing control; steps required all before starting the Sakura VisionTek desktop application
A caveat of WebEx as a desktop sharing solution is that it is not optimized for a large-scale telepathology workflow because WebEx is not necessarily a simple push button solution to accommodate the technologically challenged. We acknowledge that our cytotechnologist team has more than enough technical familiarity for mildly complicated computer applications like WebEx. The configuration of WebEx includes an understanding of procedures for inviting consultants through E-mail forwarding mechanisms. Many steps such as panning, menu navigation, and clicking are needed to create a WebEx session and to access the desktop sharing option. The number of sub-processes required for the initiator of a WebEx session can vary as different configuration options are available. The shortest permutation, which includes panning across screens and navigating menus for login and setup, is around six steps. This translates to 45 s for an expert level user. Moreover, inviting consultants to view the desktop image from WebEx requires either an E-mail notification or creating a WebEx meeting ID. The meeting ID from WebEx is arguably a simpler workflow solution but can be problematic. A time expiration is associated with each meeting ID, and no mechanism exists to determine in advance if a session has timed out. For first time consultants, WebEx browser plug-ins should be installed before joining a telepathology session.
Figure 7b-e are screenshots illustrating a representative workflow for a WebEx session directed for dynamic robotic telepathology. Figure 7b shows the creation of a WebEx session from the remote host satellite location with a standard login and password procedure. This initial step is accomplished however by a person at the remote satellite site initiating the WebEx session or another person off-site using remote desktop to login the workstation and then perform the WebEx login and password procedure. Figure 7c and d show the steps needed for screen sharing then invitation of participants which are usually consultant invitees. Figure 7e shows the extra steps needed in passing desktop control to invitees before the final step of the consultant invitee opening the SkVT application. These series of illustrated screenshots emphasize the increased number of required clicks and steps that are not ideal for a scalable remote dynamic robotic telepathology workflow unless the participants have a reasonable degree of technical familiarity as with our cytotechnologist team.
A client-server network solution with a web-based user interface [Figure 8] would provide a major advantage to any telecytology vendor. Client-server architecture obviates the need for desktop sharing by bypassing the multiple tasks required for WebEx configuration. The web-based user interface simplifies the workflow and setup by requiring only a login for viewing and image sharing. Of the current commercially available robotic telepathology solutions, none offers such a network solution. Telepathology workflows that utilize client-server architecture were introduced over a decade before the time of this publication.[4,5,6,7,8,9,10,11,12] Vendors such as Trestle, Nikon, and Zeiss offered client-server architecture operated products that are no longer available commercially.
Figure 8.
Idealized client-server remote viewing and control architecture the client server architecture. This allows for multiple views and control while obviating the need for desktop sharing by bypassing the multiple operational tasks required for WebEx configuration. The web-based user interface simplifies the workflow and setup by requiring only a login for viewing and image sharing
Another glitch discovered during our live implementation is that only one active instance of the SkVT application is allowed to run on the desktop. Thus if an operator forgets to close the application after a viewing session, the next operator cannot open the SkVT desktop application until the previous user logs out. Other functionalities provided by other systems like Mikroscan but not SkVT are thumbnail tracking and recording options, which are useful for retrospective evaluation of discordant adequacy calls. This feature allows us to assess whether regions of interest were missed in cases in which there were errors of omission.
CONCLUSION
In this technical article, we present a detailed description of our implementation of robotic telecytology and its architecture. The benefits and challenges associated with available desktop sharing solutions, as well as information security concerns, were also addressed. Our goal is to provide an extensive review of the implementation process, particularly the technical, cultural, and operational constraints. We believe that our architecture template can serve as an extensible blueprint for other centers interested in implementing robotic microscope solutions without on-site cytotechnologists.
Financial support and sponsorship
MSK Cancer Center Support Grant/Core Grant (P30 CA008748) Presentation at a Meeting: 2016 USCAP and 2016 Pathology Visions.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2017/8/1/32/214166
REFERENCES
- 1.Sirintrapun SJ, Cimic A. Dynamic nonrobotic telemicroscopy via skype: A cost effective solution to teleconsultation. J Pathol Inform. 2012;3:28. doi: 10.4103/2153-3539.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudas R, Vanden Bussche C, Baras A, Ali SZ, Olson MT. Inexpensive telecytology solutions that use the Raspberry Pi and the iPhone. J Am Soc Cytopathol. 2014;3:49–55. doi: 10.1016/j.jasc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Zhao L, Zhang R, Hassell L. FaceTime validation study: Low-cost streaming video for cytology adequacy assessment. Cancer Cytopathol. 2016;124:213–20. doi: 10.1002/cncy.21636. [DOI] [PubMed] [Google Scholar]
- 4.Brauchli K, Christen H, Haroske G, Meyer W, Kunze KD, Oberholzer M, et al. Telemicroscopy by the internet revisited. J Pathol. 2002;196:238–43. doi: 10.1002/path.1036. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan KJ, Burgess JR, Sandberg GD, Myers CP, Bigott TR, Greenspan RB, et al. Use of robotic telepathology for frozen-section diagnosis: A retrospective trial of a telepathology system for intraoperative consultation. Mod Pathol. 2002;15:1197–204. doi: 10.1097/01.MP.0000033928.11585.42. [DOI] [PubMed] [Google Scholar]
- 6.Horbinski C, Fine JL, Medina-Flores R, Yagi Y, Wiley CA. Telepathology for intraoperative neuropathologic consultations at an academic medical center: A 5-year report. J Neuropathol Exp Neurol. 2007;66:750–9. doi: 10.1097/nen.0b013e318126c179. [DOI] [PubMed] [Google Scholar]
- 7.Banach L, Stepien A, Schneider J, Wichrzycka-Lancaster E. Dynamic active telepathology over National Health Laboratory Service network, South Africa: Feasibility study using Nikon Coolscope. Diagn Pathol. 2008;3(Suppl 1):S3. doi: 10.1186/1746-1596-3-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University Health Network experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Dunn BE, Choi H, Recla DL, Kerr SE, Wagenman BL. Robotic surgical telepathology between the iron Mountain and Milwaukee Department of Veterans Affairs Medical Centers: A 12-year experience. Hum Pathol. 2009;40:1092–9. doi: 10.1016/j.humpath.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Słodkowska J, Pankowski J, Siemiatkowska K, Chyczewski L. Use of the virtual slide and the dynamic real-time telepathology systems for a consultation and the frozen section intra-operative diagnosis in thoracic/pulmonary pathology. Folia Histochem Cytobiol. 2009;47:679–84. doi: 10.2478/v10042-010-0009-z. [DOI] [PubMed] [Google Scholar]
- 11.Pantanowitz L, Wiley CA, Demetris A, Lesniak A, Ahmed I, Cable W, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi: 10.4103/2153-3539.104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitkovski T, Bhuiya T, Esposito M. Utility of telepathology as a consultation tool between an off-site surgical pathology suite and affiliated hospitals in the frozen section diagnosis of lung neoplasms. J Pathol Inform. 2015;6:55. doi: 10.4103/2153-3539.168515. [DOI] [PMC free article] [PubMed] [Google Scholar]