Abstract
Background:
Providing smoking cessation services are special importance to tobacco control programs. To date, Champix is a new expensive medication for smoking cessation available nationally. Champix has both agonist and antagonist activities and can reduce nicotine dependence and withdrawal symptoms. The purpose of this study was to evaluate the duration of using Champix based on its cost.
Methods:
This quasi-experimental study was conducted with smokers presenting to the Tanaffos Smoking Cessation Clinic in Tehran, Iran 2016. Smokers were visited by a physician 3 times at 1-week intervals for counseling. Smokers started to use Champix and stopped smoking in the 2nd week of counseling and were followed up by phone and through regular visits to the clinic at 1, 3, and 6 months postintervention. Some of them did not continue medication for 12 weeks because of its cost.
Results:
A total of 227 smokers including 133 males (58%) with a mean age of 43 years were enrolled of whom 116 (51.1%), 89 (43.6%), and 34 (20.6%) had quit smoking after 1, 3, and 6 months, respectively. Quit rates were significantly higher among those who used Champix for more than 6 weeks, and this rate was not correlated with age, sex, educational level, or nicotine dependence.
Conclusions:
Use of Champix for more than 6 weeks increases the quitting success rate compared with using for a shorter time. The cost of Champix was important for smokers and adding Champix to the list of insurance medication or getting it free of charge is needed.
Keywords: Champix, quit, smoking
Introduction
Each year, tobacco consumption has resulted in death of more than 5 million people, and the rate of morbidity and mortality is expected to exceed 8 million annually by the year 2030.[1] More than a billion people worldwide are addicted to tobacco products. Many of these people would like to quit, but unfortunately, only a small number of them can benefit from assistance in this respect. Thus, authorities in different countries are responsible for providing low-cost and cost-effective interventions and quit programs to help smokers stop smoking.[2] As for any kind of addictive substance, quitting smoking without any outside assistance can be difficult for the majority of smokers. It would be preferred if they overcome their nicotine dependence with the help of their quitting counselor.[3]
Treatment of nicotine dependence is among the main responsibilities of health-care systems worldwide. Several techniques such as a simple medical consultation, pharmaceutical therapy such as nicotine replacement therapy or Champix and over the phone counseling through quit-line have been recommended for this purpose. Repeated consultations during medical visits emphasize the necessity of quitting smoking.[4,5] In addition, counseling provided by health-care workers can significantly increase the quit rate.[6] Such interventions are extremely efficacious because they are provided by health-care professionals for whom people have respect.[7,8] People from and part of a country should have the opportunity to use quit-lines. Expert counselors should be available to assist smokers in quitting through the quit-line. These services are cheap, easily accessible, and confidential and can be accessed at any time since many smokers are not free or interested to call during business hours.[9] In Iran, these services are easy available.[10,11]
Champix (Varenicline) is a new medication for smoking cessation using for 12 weeks. Recent studies have shown that these receptors play a major role in extreme nicotine dependence and craving; Champix initially activates the α4 and β2 subunits, which in turn, moderate nicotinic stimulation at the acumbens nucleus which releases dopamine. If nicotine is consumed during Champix treatment, dopamine release does not increase (antagonist effect). Champix, therefore, has both agonist and antagonist activities and can reduce nicotine dependence and withdrawal symptoms. Champix is rapidly absorbed, and 92% is excreted in the urine. Its half-life is 17 h and it takes 45 min to reach peak concentration.[12] The cost of a course of using Champix is about 200 US$ compare with other medication such as nicotine gum (20 US$) and bupropion (50 US$) in Iran. In general, pharmaceutical therapy is more expensive than listening to physicians’ recommendations or using quit-lines. However, based on evidence, it increases the quit rate 2–3 fold. On the other hand, the cost of medication may be more than the cost of smoking during the same time period, and this is an economical barrier for smokers to use medication.[13]
Many studies on different aspects of quit smoking have been conducted in Iran;[14,15,16,17,18,19,20] however, none have focused on the best duration of using Champix. This study was conducted to evaluate the efficacy of Champix used for different time periods based on its cost and its effectiveness based on the duration of using.
Methods
This quasi-experimental study was conducted on smokers presenting to the Tanaffos smoking cessation clinic in Tehran 2016. The sampling method was first-come first-serve and all data were collected from their files retrospectively. Having a history of daily smoking and willing to quit were inclusion and not willing for continuing treatment for any reason was exclusion criteria of this study. All smokers who provided written informed consent were visited by a physician three times with 1-week intervals. They all received similar information and instructions for quitting. Smokers were provided with Champix and advised to use it for at least 10 weeks including 2 weeks for starter pack 0.5 and 1 mg, 4 weeks for the first maintenance pack 1 mg, and 4 weeks for the second maintenance pack 1 mg. Using or stop using of Champix was depending on patients because of its cost.
All smokers presented for assessment of quit outcome during their 1st month of abstinence. In addition, they were followed up by phone 3 and 6 months after abstinence. Patients decided to stop using of Champix whenever they want because of its cost so they were divided into three groups of 2 weeks with starter pack, 2–6 weeks with starter pack plus first maintenance pack, and more than 6 weeks with starter pack plus first and second maintenance packs based on the duration of using Champix to evaluate the effect of duration of treatment. This categorization was done after 6 months of using Champix according their files. Thus, matching of independent variables in the 3 groups was done during the analysis phase.
Demographic characteristics, smoking status, results of the Fagerström Test for Nicotine Dependence (FTND), and level of exhaled carbon monoxide (CO)[21,22,23,24] were recorded for all smokers in their medical files. Frequency distribution, Chi-square test for difference in frequency of quitting between males and females and smokers of different educational levels, t-test, and ANOVA were used for data analysis. P < 0.05 was considered statistically significant.
Results
A total of 227 smokers participated in this study, of which 133 (58.4%) were male. The mean age of smokers was 43.1 years (range: 18–86 years), and the median age was 42 with interquartile range 4. In terms of level of education, 96 smokers (42%) had a bachelor's or higher degree. The mean smoking experience was 21.6 ± 11 years (range: 2–60 years). They gained a mean score of 5.5 ± 2.8 (range: 0–10) in FTND, and a mean score of 27.5 ± 12.8 (range: 6–94) in exhaled CO test. There was no significant difference in three groups by monthly income.
Successful quit rate was 51.1% (116 individuals) at 1st month, 43.6% (89 individuals) at 3 months, and 20.6% (34 individuals) at 6 months.
Smokers were divided into three groups (76 in group 1, 77 in group 2, and 74 in group 3) based on the duration of using Champix. The only reason of difference duration using or not continuing using Champix by patients was the cost.
Table 1 shows the data for independent variables and the significant differences between the three groups in these variables. Table 2 shows the quit outcome at 1, 3, and 6 months follow-ups in the three groups.
Table 1.
Comparison of the independent variables between the three groups based on the duration of using Champix, Tehran 2016
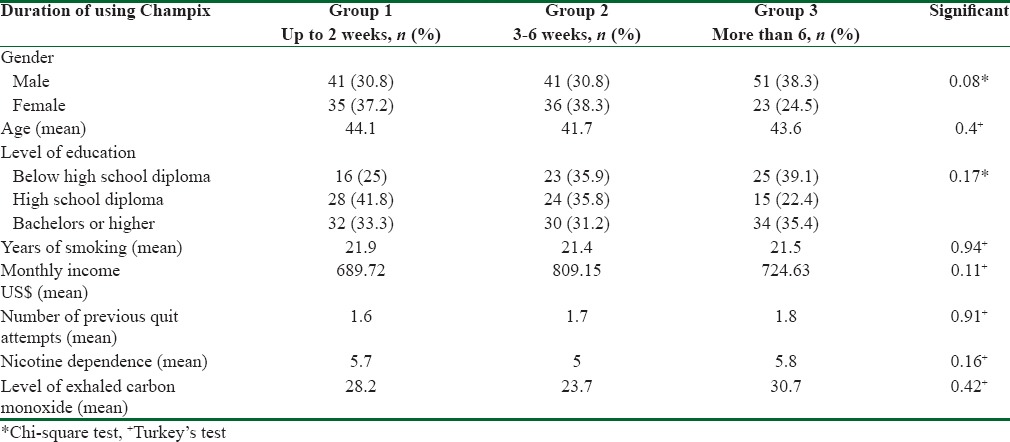
Table 2.
Frequency of quitting at 1, 3, and 6 months among smokers presenting to the smoking cessation clinic based on the duration of using Champix in 2016
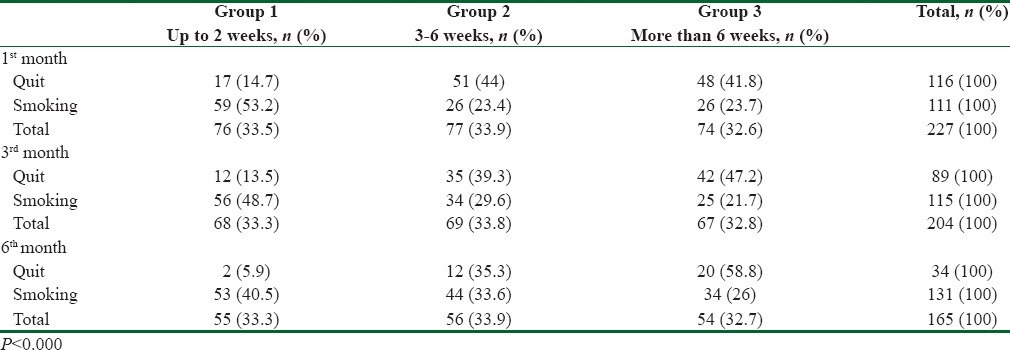
In group 3, quit rate at 1, 3, and 6 months was 64.8% (48 from 74 smokers), 62.6% (42 from 67 smokers), and 37% (20 from 54 smokers), respectively higher than two other group significantly (P < 0.000).
Chi-square test failed to find a statistically significant difference in frequency of quitting between males and females and smokers of different educational levels. Independent t-test did not show a significant correlation between frequency of quitting and age. No significant difference was detected based on FTND and CO expiratory test score using the ANOVA and Tukey's test between the three groups or the Chi-square test between smokers in terms of quitting.
Discussion
The present study was conducted to compare the efficacy of various durations of Champix and suggested that long-term use of it improves quit rate. In this study, we were focus on the cost of Champix for smokers, so it is very important for our health system to concern about cost-effectiveness of this intervention which was shown before[13] to cover the cost of medication in smoking cessation cervices nationally as a middle-income country. This issue may apply for other medication also to increase tendency toward quit smoking generally. It might be generated a hypothesis for testing with a randomized clinical trial in near future.
Our finding showed that all smokers tried to use Champix first with starter pack which cost about 40 US$, but 151 person (66.5%) used first maintenance pack which cost about 75 US$ plus previous cost, and only 74 person (32.5%) had second maintenance pack with about 190 US$ totally. As the only reason for not continuing using Champix was cost, it might related with smokers’ willingness for not paying much medication.
The study illustrated that almost half the smokers successfully quit smoking in the 1st month of treatment, but this trend fell by 20% at 6 months later. Several studies reveal that relapse after cessation occurs commonly within this time of abstinence.[25] Relapse usually happens during the first 6 months of abstinence, especially in the 1st month.[26] However, in the present study, a difference in using Champix was a key factor, and this finding should be further evaluated in future studies. An important finding of the present study was the high-quit success rate and continued abstinence in smokers who used Champix longer. This finding has also been noted in meta-analysis and systematic review[27,28] that using medication for longer period had better outcomes in quit smoking compare with whom that using it for a short time and should be taken into consideration by the authorities in tobacco control programs to advice smokers who use medication for longer periods of time.[29]
According to not significant difference in monthly income between 3 groups and since the only reason to stop the consumption of Champix is the high price for patients, health-care workers have to give them more information about the benefits of this cost comparing to hazards of failure in quit smoking. This issue is cited in the study of Fernández de Bobadilla Osorio et al.[30] The cost of quit smoking methods was assessed in nationwide studies of Heydari et al.[10,11] and according to physicians and patients low-cost of smoking cessation treatment was an effective factor in quit smoking; however, in the only clinical trial of Champix in Iran,[12] this factor was not seen and assessed because it was given to patients free of charge. It was the only difference between these two studies that showed cost of medication was important for using Champix continuously.
Last, however, we know that pharmaceutical therapy is an expensive intervention for quit smoking but based on evidence, it increases the quit rate 2–3 fold,[13] and this is a cost-effectiveness intervention in the health system. The health-care system must concern about this and try to promote its cost benefit in general population and smokers to use medication or add it to the list of insurance medication.
Conclusions
The cost of Champix was important for smokers and using Champix for more than 6 weeks increases the quitting success rate compared with using for a shorter time.
Recommendation
Adding Champix to the list of insurance medication or getting it free of charge in smoking cessation services is needed.
What does this paper add?
Use of Champix with physician counseling on quitting is usually accepted by the smokers.
Longer use of Champix might be a factor for quit smoking.
The free of charge Champix is important for patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 3.Jones JM. Smoking Habits Stable; Most Would Like to Quit. 2006. Jul 18, [Last accessed on 2007 Dec 06]. Available from: http://www.gallup.com/poll/23791/Smoking-Habits-Stable-Most-Would-Like-Quit.aspx .
- 4.Solberg LI, Maciosek MV, Edwards NM, Khanchandani HS, Goodman MJ. Repeated tobacco-use screening and intervention in clinical practice: Health impact and cost effectiveness. Am J Prev Med. 2006;31:62–71. doi: 10.1016/j.amepre.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 5.West R, Sohal T. “Catastrophic” pathways to smoking cessation: Findings from national survey. BMJ. 2006;332:458–60. doi: 10.1136/bmj.38723.573866.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiore MC. Treating Tobacco Use and Dependence: A Public Health Service Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Press Briefing; 2008. [Last accessed on 2016 May 13]. Available from: https://www.ahrq.gov/professionals/clinicians/guidelines/tobacco/index.html . [Google Scholar]
- 7.Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, Rutten-van Mölken MP. Cost-effectiveness of face-to-face smoking cessation interventions: A dynamic modeling study. Value Health. 2005;8:178–90. doi: 10.1111/j.1524-4733.2005.04008.x. [DOI] [PubMed] [Google Scholar]
- 8.Bao Y, Duan N, Fox SA. Is some provider advice on smoking cessation better than no advice? An instrumental variable analysis of the 2001 National Health Interview Survey. Health Serv Res. 2006;41:2114–35. doi: 10.1111/j.1475-6773.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen L. Impact of a telephone helpline for smokers who called during a mass media campaign. Tob Control. 2000;9:148–54. doi: 10.1136/tc.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heydari G, Ebnahmadi A, Masjedi MR, Lando H. Quit smoking experts’ opinions toward quality and results of quit smoking methods provided in tobacco cessation services centers in Iran. Int J Prev Med. 2015;6:74. doi: 10.4103/2008-7802.162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heydari G, Ebnahmadi A, Masjedi MR, Lando H. Assessment of different quit smoking methods selected by patients in tobacco cessation centers in Iran. Int J Prev Med. 2015;6:81. doi: 10.4103/2008-7802.164118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: Parallel, randomised efficacy trial in Iran. Int J Tuberc Lung Dis. 2012;16:268–72. doi: 10.5588/ijtld.11.0183. [DOI] [PubMed] [Google Scholar]
- 13.Hammond D, Fong GT, McNeill A, Borland R, Cummings KM. Effectiveness of cigarette warning labels in informing smokers about the risks of smoking: Findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii19–25. doi: 10.1136/tc.2005.012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heydari G, Marashian M, Ebn Ahmady A, Masjedi M, Lando HA. Which form of nicotine replacement therapy is more effective for quitting smoking? A study in Tehran, Islamic Republic of Iran. East Mediterr Health J. 2012;18:1005–10. doi: 10.26719/2012.18.10.1005. [DOI] [PubMed] [Google Scholar]
- 15.Heydari G, Jianfar G, Alvanpour A, Hesami Z, Talischi F, Masjedi MR. Efficacy of telephone quit-line for smokers in Iran: 12 months follow up results. Tanaffos. 2011;10:42–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Tobacco Advisory Group of the Royal College of Physicians. Nicotine Addiction in Britain; a Report of the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Physicians of London; 2000. [Last accessed on 2007 Dec 06]. Available from: http://www.rcplondon.ac.uk/pubs/books/nicotine . [Google Scholar]
- 17.Heydari G, Masjedi M, Ahmady AE, Leischow SJ, Lando HA, Shadmehr MB, et al. A comparative study on tobacco cessation methods: A quantitative systematic review. Int J Prev Med. 2014;5:673–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Heydari G, Ebnahmadi A, Masjedi MR, Lando H. Utilization of a telephone interactive voice-response tobacco cessation support service in the Islamic Republic of Iran. East Mediterr Health J. 2014;20:254–9. [PubMed] [Google Scholar]
- 19.Heydari G, Ebnahmadi A, Masjedi MR, Lando H. Status and costs of smoking cessation in countries of the Eastern Mediterranean Region. East Mediterr Health J. 2012;18:1102–8. doi: 10.26719/2012.18.11.1102. [DOI] [PubMed] [Google Scholar]
- 20.Heydari G, Marashian M, Emami H. Efficacy of nicotine patch in combination with trazodone in smoking cessation. Tannafos. 2010;9:50–57. [Google Scholar]
- 21.Wagena EJ, Zeegers MP, van Schayck CP, Wouters EF. Benefits and risks of pharmacological smoking cessation therapies in chronic obstructive pulmonary disease. Drug Saf. 2003;26:381–403. doi: 10.2165/00002018-200326060-00002. [DOI] [PubMed] [Google Scholar]
- 22.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–505. [PubMed] [Google Scholar]
- 23.Sachs DP, Benowitz NL, Silver KJ. Aoki M, Hisamichi S, Tominaga S. Smoking and Health. Amsterdam: Elsevier; 1987. Effective use of nicotine polacrilex (Nicorette) in patients with chronic obstructive pulmonary disease; pp. 793–95. [Google Scholar]
- 24.Glover ED, Glover PN, Franzon M, Sullivan CR, Cerullo CC, Howell RM, et al. A comparison of a nicotine sublingual tablet and placebo for smoking cessation. Nicotine Tob Res. 2002;4:441–50. doi: 10.1080/1462220021000018443. [DOI] [PubMed] [Google Scholar]
- 25.Wadgave U, Nagesh L. Nicotine replacement therapy: An overview. Int J Health Sci (Qassim) 2016;10:425–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Hand S, Edwards S, Campbell IA, Cannings R. Controlled trial of three weeks nicotine replacement treatment in hospital patients also given advice and support. Thorax. 2002;57:715–8. doi: 10.1136/thorax.57.8.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King JL, Pomeranz JL, Merten JW. A systematic review and meta-evaluation of adolescent smoking cessation interventions that utilized nicotine replacement therapy. Addict Behav. 2016;52:39–45. doi: 10.1016/j.addbeh.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Prochaska JJ. Nicotine replacement therapy as a maintenance treatment. JAMA. 2015;314:718–9. doi: 10.1001/jama.2015.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med. 2002;162:1267–76. doi: 10.1001/archinte.162.11.1267. [DOI] [PubMed] [Google Scholar]
- 30.Fernández de Bobadilla Osorio J, Sánchez-Maestre C, Brosa Riestra M, Arroyo O, Sanz de Burgoa V, Wilson K. Cost effectiveness analysis of varenicline (Champix) for the treatment of smoking in Spain. An Med Interna. 2008;25:342–8. doi: 10.4321/s0212-71992008000700006. [DOI] [PubMed] [Google Scholar]


