Abstract
Background:
Hyperglycemia-mediated oxidative stress implicates in etiology of kidney cell aging and diabetic nephropathy. We evaluated the effects of different doses of resveratrol and quercetin and their combination therapy on aging marker in human kidney cell culture under hyperglycemia condition.
Methods:
Human embryonic kidney cell (HEK-293) was cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 100 mM (18 mg/L) for 24 h. The cells were treated with resveratrol (2.5, 5, 10 μm), quercetin (3, 6, 12 μm), and combination of these (R 2.5 μm, Q 3 μm) and (R 5 μm, Q 6 μm) and (R 10 μm, Q 12 μm) for 48 h, and then, cells were lysed to access RNA and lysate.
Results:
The analysis of data showed that beta-galactosidase enzyme gene expression as an aging marker in all treatment groups has reduced in a dose-dependent manner. Gene expression of Sirtuin1 and thioredoxin (Trx) in all treated groups in comparison to control group increased in a dose-dependent fashion. Trx interacting protein (TXNIP) gene expression decreased in a dose-dependent manner in all treated groups, especially in resveratrol and combination therapy.
Conclusions:
According to the results of this research, quercetin, resveratrol, and especially combination treatments with increased expression levels of antioxidants, can reduce aging markers in HEK cell line in hyperglycemia conditions. These results lead us to use flavonoids such as resveratrol for anti-aging potential.
Keywords: Aging, human embryonic kidney cell-293, quercetin, resveratrol, thioredoxins
Introduction
Hyperglycemia, uncontrolled glucose regulation, is widely recognized as a causal link between diabetes and diabetic complications that induced oxidative stress and it can be implicated in the etiology of diabetic nephropathy.[1,2]
Hyperglycemia which occurs during diabetes type 1 and type 2 causes oxidative stress.[3]
Cellular senescence is programmed biological process activated by normal cells in response to various types of stress that includes telomere uncapping, oxidative stress, oncogene activity, and so on.[4] Once cells enter senescence period, they undergo a series of morphologic and metabolic changes.
Senescence-associated beta galactosidase (SA-beta-GAL) is a widely used marker for cellular senescence that in 1995, Dimri et al. described a candidate biomarker for replicative senescence. The activity of SA-beta-GAL is detectable at pH 6.0 and permits the identification of senescent cells both in culture and in mammalian tissues.[5,6]
Sirtuins (SIRTs) are NAD+ -dependent deacetylases that catalyze the hydrolysis of acetyllysine residues. They play an important role in many physiological and pathophysiological processes, such as aging and metabolic regulations.[7]
Deficiency under various stress conditions, such as metabolic or oxidative stress or hypoxia, is implicated in the pathophysiologies of age-related diseases including diabetes, cardiovascular diseases, and renal diseases.[8]
The sulfur-containing amino acid residues, cysteine, and methionine are sensitive to oxidative stress. Oxidation of the sulfhydryl groups on these amino acids is unique in which oxidative changes can be enzymatically repaired by a number of highly expressed thiol-reducing systems, thioredoxin (Trx), and glutaredoxin. During the reduction of disulfide groups, Trx itself oxidizes, and requires reduction by Trx reductase to restore its activity. In addition to this redox process, the activity of Trx is modulated by an endogenous inhibitor, Trx interacting protein (TXNIP).[9]
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is a bioflavonoid found in green vegetables, onions, apples, legumes, green tea, citrus fruits, red grape wine, and like other flavonoids, acts as a quencher for reactive oxygen species (ROS) generated by any physical or chemical action.[10]
Resveratrol (3, 5, 4’-trihydroxystilbene) is a natural polyphenol extracted from grapes and several plants, characterized as a potently free radical scavenger and antioxidative agent.[11]
In this study, we evaluated the effects of different doses of resveratrol and quercetin and the combination of these two substances under hyperglycemia condition in a human kidney cell cultures; furthermore, we evaluated some oxidative stress and aging markers in these cells.
Methods
Reagents
Resveratrol, quercetin, and penicillin and streptomycin antibiotic were obtained from Sigma (Sigma, Aldrich, USA) company. Human embryonic kidney cell (HEK-293) was purchased from Stem cell technology research center (Iran, Tehran). DMEM containing 25 mM (4.5 mg/L) glucose, fetal bovine serum (FBS) and trypsin-ethylenedinitrilotetraacetic acid were obtained from Gibco (USA). Dimethyl sulfoxide was purchased from the Biomedical company (USA). Primer sequences to measure gene expression by real time-polymerase chain reaction (PCR) technique were ordered from Bioneer (Korea) company.
Human embryonic kidney cell culture
HEK-293 cells was cultured in DMEM containing 25 mM (4.5 mg/L) glucose, 10% FBS (v/v), 1% penicillin, and streptomycinantibiotic. High glucose treatment was performed by culturing cells in DMEM containing 100 mM (18 mg/L) glucose and for the indicated times.
Treatment procedure
Cells were seeded in 6-well plates with DMEM containing 25 mM glucose and 10% FBS. After cell confluence reached at 60%–80%, the medium was replaced with DMEM containing 100 mM glucose and 2% FBS. Twenty-four hours later, the cells were cultured in DMEM containing 100 mM glucose and treated with resveratrol (2.5, 5, 10 μm), quercetin (3, 6, 12 μm), and combination of these two substances (R 2.5 μm, Q 3 μm) and (R 5 μm, Q 6 μm) and (R 10 μm, Q 12 μm) after 24 h medium and treatments was replaced. 24 h later, the cells were lysed by lysis buffer.
RNA extraction and real-time polymerase chain reaction
Total RNA was extracted from cells using the TRIZOL reagent. cDNA was synthesized by thermo science kit. Real-time PCR was performed using cDNA single strand. Briefly, reverse-transcribed cDNA in triplicate samples were checked for beta-galactosidase, SIRT1, TXN, and TXNIP mRNA levels with SYBR Green PCR master kit (Jena Bioscience Germany). PCR primers for target cDNAs were: Beta-Galactosidase: 5’-AACAGGCAGCAACATCACAG (Forward) and 5’-GCAAGTATATCATAGAGGGAGGAA (Reverse);
TXN: 5’-AAGAAGGGACAAAAGGTGGGT (Forward), and 5’-GCAGATGGCAACTGGGTTTATG (Reverse); TXNIP: 5’-AGCCAGCCAACTCAAGAGAC (forward), and 5’-AGCAGACACAGGTGCCATTA. Beta-actin primers were: 5’-TGGCACCCAGCACAATGAA (forward) and 5’-CTAAGTCATAGTCCGCCTAG (reverse) and SIRT1: 5’-CACCAGAAAGAACTTCACCACCAG (forward) and 5’-ACCATCAAGCCGCCTACTAATCTG (reverse). Relative levels of target gene mRNA expression were calculated using the 2−ΔΔCT method. Amplification of the target gene cDNA was normalized to beta-actin expression.
Statistical analyses
The experiments were repeated three times in each group and data are expressed as mean ± standard deviation statistical analysis is carried out with SPSS software version 16 (SPSS, Chicago) in the significant level α = 0.05 and using one-way ANOVA and post-Tukey tests.
Results
We evaluated the effects of different doses of resveratrol and quercetin and the combination of these two substances under the conditions hyperglycemia in a human kidney cell cultures; moreover, we evaluated some oxidative stress and aging markers in this cells.
The effect of resveratrol and quercetin on aging markers
Kidney cells in high glucose condition showed increased level of the aging marker; beta-galactosidase enzyme [Figure 1]. The analysis of data showed that beta-galactosidase enzyme gene expression in all three treatment groups including resveratrol, quercetin, and the combination group was reduced in a dose-dependent manner. In combination therapy groups, high dose of resveratrol and quercetin showed notable effect on aging marker [Figure 1].
Figure 1.
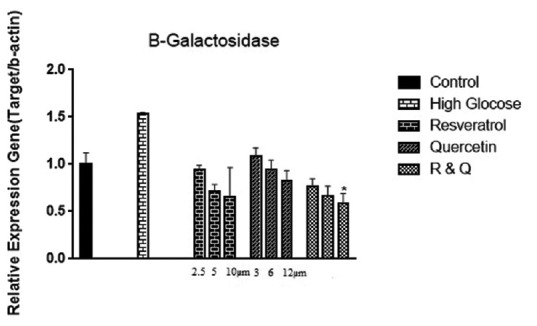
The effect of resveratrol, quercetin, and combination treatment on gene expression of beta-galactosidase in hyperglycemia condition compared with control (nontreated cells). Values are shown as mean ± standard deviation of triplicate wells. *Significant (P ≤ 0.05) difference between treated human embryonic kidney-293 and untreated control cells
The effect of resveratrol and quercetin on markers of oxidative stress
The analysis of data showed that gene expression of SIRT1 has increased in all treated groups compared to the high glucose control group. The increased expression in the combination group of resveratrol and quercetin was remarkable [Figure 2].
Figure 2.
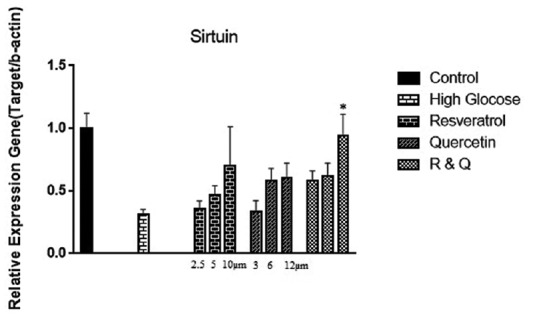
The effect of resveratrol, quercetin, and combination treatment on gene expression of Sirtuin1 in hyperglycemia condition compared with control (nontreated cells). Values are shown as mean ± standard deviation of triplicate wells. *Significant (P ≤ 0.05) difference between treated human embryonic kidney-293 and untreated control cells
Furthermore, gene expression of Trx in all treated groups compared to the high glucose control group in a dose-dependent manner augmented. Trx gene expression significantly increased in high doses of resveratrol and combination group [Figure 3].
Figure 3.
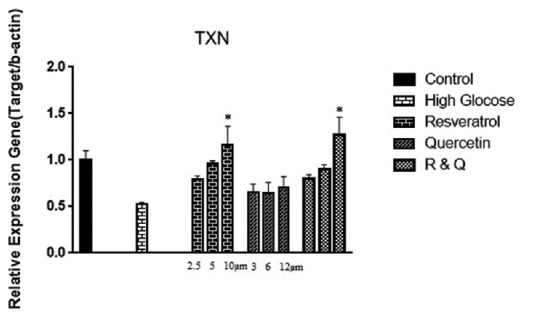
The effect of resveratrol, quercetin, and combination treatment on gene expression of thioredoxin in the hyperglycemia condition compared with control (nontreated cells). Values are shown as mean ± standard deviation of triplicate wells. *Significant (P ≤ 0.05) difference between treated human embryonic kidney-293 and untreated control cells
TXNIP gene expression decreased in a dose-dependent manner in all treated groups.
TXNIP gene expression significantly increased in high doses of resveratrol and combination group [Figure 4].
Figure 4.
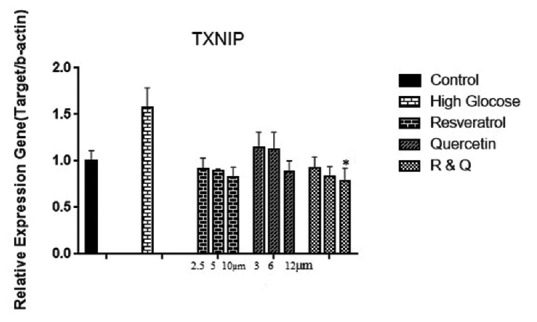
The effect of resveratrol, quercetin, and combination treatment on gene expression of thioredoxin interacting protein in hyperglycemia condition compared with control (nontreated cells). Values are shown as mean ± standard deviation of triplicate wells. *Significant (P ≤ 0.05) difference between treated human embryonic kidney-293 and untreated control cells
Discussion
Diabetic nephropathy is one of the microvascular diseases related to diabetes that under conditions of chronic hyperglycemia in diabetes can cause damage to kidney cells. In this study, we evaluated the effects of different doses of resveratrol and quercetin and the combination of them under the conditions hyperglycemia in a human kidney cell cultures. Besides, we evaluated some oxidative stress and aging markers in these cells. Resveratrol alone and also with quercetin could improve oxidative stress status and aging marker level.
Resveratrol and quercetin are a family of polyphenols which their antioxidant and their anti-diabetic properties have been proven. Quercetin reduces blood glucose levels in diabetic rats.[12]
Quercetin treatment caused a reduction in polyuria (~45%) and glycemia (~35%), that abolished hypertriglyceridemia and had significant effects on renal function such as decreased proteinuria and high plasma levels of uric acid, urea, and creatinine, which were accompanied by beneficial effects on the structural changes of the kidney, especially glomerulosclerosis. This study showed a decrease in oxidative stress and apoptosis in diabetic nephropathy mice.[13]
Resveratrol protects against oxidative stress and exhibits anti-inflammation properties, which may contribute to its beneficial effects on the early stage of diabetic nephropathy.[14]
Resveratrol ameliorated renal injury and enhanced mitochondrial biogenesis with Mn-super oxide dismutase dysfunction in the kidney of mice. Resveratrol has antioxidative activities through Adenosine monophosphate-activated protein kinase/SIRT1-independent pathway.[15]
Beta-galactosidase enzyme is considered as a cellular aging marker, and data analysis showed that the expression of this enzyme in HEK cells was increased in hyperglycemia. The present study showed that in all groups treated with resveratrol and quercetin and combination treatment with dose-dependent manner decreased the expression of this gene and the most significant effect was observed in the combination treatment group. In 2008, Verzola was studied on the aging process in the kidneys of patients with type 2 diabetic nephropathy. Data analysis showed that expression of SA-beta-GAL increased in the kidney of patients with type 2 diabetes. The staining of SA-beta-GAL in the early stages of diabetic renal tubular disease is approximately three times higher than the control group.[16]
Other marker of aging is senescence marker protein30 (SMP30), SMP30, anti-aging marker protein, a 34 kDa cytosolic protein that plays an important role in calcium homeostasis, oxidative stress, and ascorbic acid biosynthesis. Based on our previous study, the expression of SMP30 anti-aging factor decreased in high glucose medium. Treatment with different concentrations of resveratrol, quercetin, and notably resveratrol/quercetin induced the mRNA expression of SMP30 in HEK-293 cells.[17]
The first SIRT was discovered in the body of the SIRT protein and was named SIRT1. Since then, six SIRT proteins have been found (SIRT2 to SIRT7).[18]
SIRT1 deficiency under various stress conditions, such as metabolic or oxidative stress or hypoxia, is implicated in the pathophysiologies of age-related diseases including diabetes, cardiovascular diseases, and renal diseases.[8] Various studies have shown that resveratrol can prevent reduce SIRT1 levels in hyperglycemia conditions.[19]
Other study showed chronic hyperglycemia accelerated aging process through a novel SIRT1 and p300 regulated pathway.[20]
The analysis of data in the present study showed that gene expression of SIRT1 increased in all treated groups compared to the control group with a high concentration of glucose in a dose-dependent manner. The increased expression in the combination group of resveratrol and quercetin was remarkable.
Increased levels of ROS by hyperglycemia can induce the apoptosis of renal cells and diabetic nephropathy.[21] To protect cells against oxidative stress, cells possess defensive mechanisms such as intracellular antioxidants. Although enzymes in the antioxidants defense mechanisms of the cell are induced under hyperglycemic conditions the oxidative stress remains high. Furthermore, there are endogenous inhibitor of antioxidants such as TXNIP.[22]
High glucose significantly increases intracellular ROS levels in human aortic endothelial cells. In addition, high glucose can reduce the antioxidant activity of Trx. This study showed that glucose enhances the expression of TXNIP, a Trx inhibitory protein, through p38 mitogen-activated protein kinase.[23]
In this study, the expression levels of Trx as an antioxidant had a significant increase in all treated groups compared with the control group, also, the TXNIP expression levels decreased in all groups compared to the control group. In this regard, a study on diabetic rats showed that TXNIP expression levels significantly increased in diabetic mice.[22]
Conclusions
According to the results of this research, it can be concluded that resveratrol, quercetin and especially their combination improves expression levels of antioxidants and aging markers in HEK cell line in hyperglycemia conditions.
Therefore, resveratrol and quercetin can be used in pharmacology industry as supplementary treatment in recovery and treatment of stress caused by high glucose and prevention of complications of diabetes including diabetic nephropathy.
Financial support and sponsorship
This investigation was supported by Cardio Vascular Research Center, Birjand University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This investigation was supported by Grant No. 773 from the office of Vice Chancellor for Research, Birjand University of Medical Sciences.
References
- 1.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Pang S, Deng B, Qian L, Chen J, Zou J, et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int J Biochem Cell Biol. 2012;44:629–38. doi: 10.1016/j.biocel.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 3.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–8. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–76. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Cristofalo VJ. SA beta Gal staining: Biomarker or delusion. Exp Gerontol. 2005;40:836–8. doi: 10.1016/j.exger.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Geng YQ, Guan JT, Xu XH, Fu YC. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem Biophys Res Commun. 2010;396:866–9. doi: 10.1016/j.bbrc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Schirmer H, Pereira TC, Rico EP, Rosemberg DB, Bonan CD, Bogo MR, et al. Modulatory effect of resveratrol on SIRT1, SIRT3, SIRT4, PGC1α and NAMPT gene expression profiles in wild-type adult zebrafish liver. Mol Biol Rep. 2012;39:3281–9. doi: 10.1007/s11033-011-1096-4. [DOI] [PubMed] [Google Scholar]
- 8.Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin Sci (Lond) 2013;124:153–64. doi: 10.1042/CS20120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Advani A, Gilbert RE, Thai K, Gow RM, Langham RG, Cox AJ, et al. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol. 2009;20:730–41. doi: 10.1681/ASN.2008020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109:8–14. doi: 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Wen D, Huang X, Zhang M, Zhang L, Chen J, Gu Y, et al. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS One. 2013;8:e82336. doi: 10.1371/journal.pone.0082336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 13.Gomes IB, Porto ML, Santos MC, Campagnaro BP, Pereira TM, Meyrelles SS, et al. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014;13:184. doi: 10.1186/1476-511X-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CC, Chang CY, Wu YT, Huang JP, Yen TH, Hung LM. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci. 2011;18:47. doi: 10.1186/1423-0127-18-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634–43. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1563–73. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- 17.Mahboob Z, Hemmati M, Khorashadizadeh M, Gholami M. Additive effects of resveratrol and resveratrol/quercetin in prevention of hyperglycemia-mediated cell death through downregulation of NADPH oxidase and RAGE expression. J Pancreas. 2017;18:26–32. [Google Scholar]
- 18.Dong YJ, Liu N, Xiao Z, Sun T, Wu SH, Sun WX, et al. Renal protective effect of sirtuin 1. J Diabetes Res 2014. 2014:843786. doi: 10.1155/2014/843786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitada M, Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev 2013. 2013:568093. doi: 10.1155/2013/568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagener FA, Dekker D, Berden JH, Scharstuhl A, van der Vlag J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis. 2009;14:1451–8. doi: 10.1007/s10495-009-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada Y, Fukagawa M. A possible role of thioredoxin interacting protein in the pathogenesis of streptozotocin-induced diabetic nephropathy. Kobe J Med Sci. 2007;53:53–61. [PubMed] [Google Scholar]
- 23.Li X, Rong Y, Zhang M, Wang XL, LeMaire SA, Coselli JS, et al. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun. 2009;381:660–5. doi: 10.1016/j.bbrc.2009.02.132. [DOI] [PMC free article] [PubMed] [Google Scholar]


