Abstract
Background:
In the 2016 update of the World Health Organization Classification of Tumors of the central nervous system, phenotypic and genotypic parameters are integrated in diffuse low-grade glioma (LGG) tumor classification. Implementation of this combined phenotypic–genotypic characterization identifies prognostic relevant subgroups.
Case Description:
We report a case of a 67-year-old patient with an LGG that showed molecular characteristics similar to glioblastoma multiforme (GBM). After gross total tumor resection, the patient received combination therapy (radiotherapy and chemotherapy) according to high-grade glioma treatment protocol.
Conclusion:
The introduction of molecular parameters to the classification of LGG will add a level of objectivity, which will yield biological homogeneous subclasses. Consequently, this will influence patient counseling and clinical decision making regarding treatment protocols.
Keywords: Low-grade glioma, molecular characteristics, treatment, WHO classification
INTRODUCTION
In the 2016 update of the World Health Organization Classification of Tumors of the Central Nervous System phenotypic and genotypic parameters are integrated in diffuse low-grade glioma (LGG) tumor classification. Implementation of this combined phenotypic-genotypic characterization identifies prognostic relevant subgroups
CASE REPORT
A 67-year-old male presented with transient reduced orientation and short-term memory loss. The symptoms lasted for 24 hours during which he also suffered loss of face and object (building) recognition. The patient reported complete amnesia for this episode. Additional amnestic evaluation revealed a period of transient global amnesia of several hours occurring one and a half years ago. His medical history stated diabetes mellitus type II and hypercholesterolemia. Neurological examination at the time of evaluation showed no abnormalities.
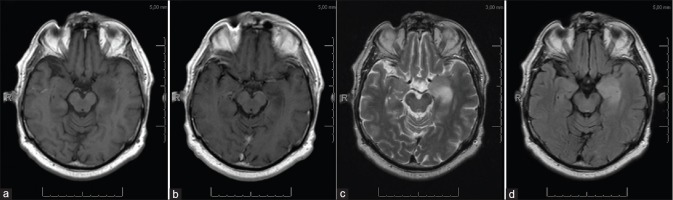
Electroencephalography was performed which showed a normal background activity and no signs of epileptic or epileptiform discharges. Magnetic resonance imaging (MRI) showed a nonenhancing lesion with high signal intensity on T2-weighted images and low signal intensity on T1-weighted images of the left mesial temporal lobe [Figure 1a-d]. Diffusion-weighted imaging revealed no abnormal restricted diffusion. A diffuse low-grade glioma (LGG), dysembryoplastic neurepithelial tumor (DNET), or postictal changes were included in the radiological differential diagnosis. Subsequent MRI scan 1 month later demonstrated an essential unchanged situation, which suggested the lesion to be of glial origin rather than postictal (sub-) cortical changes.
Figure 1.
Preoperative MRI. (a and b) T1-weighted image without and with gadolinium. (c) T2-weighted image. (d) T2-FLAIR
A left-sided anterior temporal lobectomy in combination with resection of the radiologically thickened hippocampus was performed. Intraoperatively, a grayish tumorous tissue of soft consistency was removed suspicious for LGG. A gross total resection was achieved.
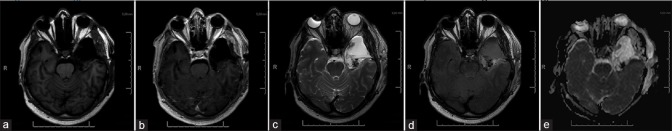
A postoperative MRI scan within 72 hours depicted a small area with an increased T2 signal intensity with no abnormal diffusion restrictions, suspicious for residual tumor [Figure 2a-e]. Postoperatively, the patient suffered subtle dysphasia, which showed complete remission after 3 days. The patient was discharged from the hospital on the fifth postoperative day.
Figure 2.
Post-operative MRI. (a and b) T1-weighted image without and with gadolinium. (c) T2-weighted image, (d) T2-FLAIR, (e) Diffusion-weighted imaging
Histology
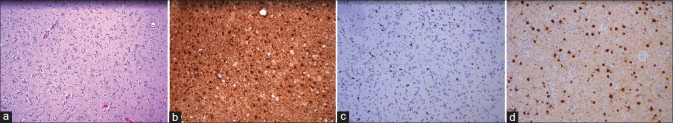
The obtained tissue showed high cellularity with subpopulations of cells with enlarged nuclei and perinuclear clearing in accordance with a “fried-egg” appearance. The NeuN stain identified multiple neurons. Glial cells stained positive with GFAP, and Ki-67-staining showed an increased proliferative activity in the enlarged atypical cells. The histological diagnosis was an LGG, WHO II, molecularly characterized by the absence of: IDH1/2 mutation, 1p/19q codeletion, or MGMT promotor hypermethylation. See Figure 3 for the histology staining.
Figure 3.
Histology staining. (a) Hematoxylin and eosin, ×100. (b) Glial fibrillary acidic protein (GFAP), ×100. (c) Ki-67 (mib1), ×100. (d) Neuronal nuclei (NeuN), ×100
Further genomic analyses using next generation sequencing revealed no mutation in the ATRX, CDKN2a, CIC, FUBP1, NOTCH1, PTEN, and TP53 coding genes and no mutations in the mutational hotspots of BRAF, H3F3A, EGFR, IDH1/IDH2, and PIK3CA. Copy number variation analyses of chromosomes 1, 7, 9, 10, 12, and 19 showed an imbalance of chromosome 7 including EGFR, CD6, and Met with a loss of chromosome 10.
Treatment
The patient was diagnosed according to the 2016 update of the World Health Organization classification of tumors of the central nervous system; IDH wild-type (IDHwt), and diffuse astrocytoma.[7] Considering the distinct high-grade molecular traits, the patient was treated postoperatively according to the Stupp protocol; concomitant temozolomide 75 mg/m2/day for 49 days and radiotherapy with a total dose up to 59.4 Gy in 33 fractions followed by 6 cycles of temozolomide.[10]
DISCUSSION
Depending on the WHO stage (I–IV), the survival of glioma patients varies from several months to more than 20 years.[10] The prevalence of LGG is lower when compared to the WHO IV glioblastoma multiforme; of all gliomas LGG comprise 15%.[8] Moreover, within the spectrum of LGG, significant variation in mean overall survival is observed, ranging from 5.9 years (astrocytoma) to 11.9 for oligodendroglioma.[8]
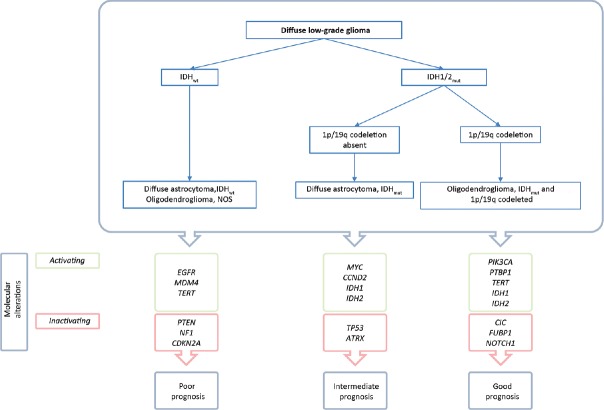
In May 2016, the WHO presented an update of the classification of tumors of the central nervous system.[7] Here, for the first time, phenotypic and genotypic parameters were integrated in diffuse glial tumor classification. Every LGG is characterized according to the presence or the absence of mutations in the isocitrate dehydrogenase 1 or 2 genes (IDH) and complete or incomplete deletion of both the short arm of chromosome 1 and of the long arm of chromosome 19 (1p/19q co-deletion). Implementation of this combined phenotypic–genotypic characterization identifies subgroups that correlate with overall survival and treatment responses. Figure 1 shows a flowchart of the classification system adapted to the molecular classification of LGG, as suggested by The Cancer Genome Atlas Research Network.[1] Here, we will present a concise overview of several of the identified molecular markers in LGG with prognostic and biological relevance.
LGG and IDH
The first segregation of diffuse LGG is based on the presence of IDH1/2 gene mutation [Figure 4]. These mutations occur at a single amino acid residue of IDH1, arginine 132, which is most commonly mutated to histidine (R132H).[4] IDH is a cytosolic enzyme involved in the decarboxylation of isocitrate, producing α-ketoglutarate and CO2. A mutation in the IDH1 gene (IDHmut) allows the homodimeric enzyme to reduce α-ketoglutarate in 2-hydroxygluterate (2-HG). Accumulation of the 2-HG metabolite is associated with the dedifferentiation of gliomas.[4] In addition, an IDH gene mutation causes hypermethylation of specific DNA loci (CpG islands), resulting in a significantly different gene expression profile compared to wild type IDH LGG.[11] Specifically, hypermethylation of the DNA-repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) promotor gene downregulates the expression of this enzyme, and therefore increases tumor susceptibility to alkylating agents such as temozolomide.
Figure 4.
WHO 2016 simplified algorithm for classification of LGG, adapted to Brat et al. (2015)
IDH mutations are present in almost 90% of the diffuse LGG and are correlated with a favorable, therapy independent, survival compared to IDHwt LGG: 13.1 years compared to 5.1 years.[1,6] Furthermore, an IDH mutation is predictive for the response of the LGG to multimodal treatment strategies, as was recently shown that the addition of chemotherapy to radiotherapy increases progression-free and overall survival in diffuse LGG compared to radiotherapy alone.[2]
IDHmut with 1p/19q codeletion
Subsequent to IDHmut/IDHwt segregation, LGGs are classified according to the presence or absence of 1p/19q codeletion. A strong correlation exists between the presence an IDH mutation and 1p/19q codeletion with the histological oligodendroglioma with a correspondence rate of 95% for WHO II tumors. Strikingly, mutations in the coding gene for TElomerase Reverse Transcriptase (TERT) is found in 96% of this subclass. TERT gene mutations cause an activation of this enzyme.[5] However, it is not associated with an increased telomere length in diffuse glioma, and is therefore unlikely to contribute to the maintenance of unlimited replicative potential.[3] However TERT mutation status is associated with prognosis in WHO 2016 defined groups.[9] Patients with a IDHmut/1p/19q co-deleted LGG have significant progression-free and overall survival compared with their 1p/19q IDH native counterparts.[2]
IDHmut without 1p/19q codeletion
Almost all diffuse LGGs without a 1p/19q codeletion harbor mutations in the Tumor protein (TP) 53 coding gene and the majority harbor inactivating mutations in the ATRX gene.[1] Dysfunction of the TP-53 gene, as known for the Li-Fraumeni syndrome, causes a loss of tumor suppressive capabilities of TP-53. For LGG, it is hypothesized that, after the acquisition of an IDH mutation, a tumor cell either acquires a 1p/19q codeletion or a mutation in TP-53. This theory is further supported by the observation that TERT and ATRX mutation are mutually exclusive and result in different subclasses: IDHmut with (TERT) or without (ATRX) 1p/19q codeletion. Interestingly, not TERT but ATRX mutations were associated with increased telomere length in a pan-glioma analysis, suggesting an alternative lengthening of telomeres.[3]
LGG-IDHwt
LGG with an IDH wild type comprise 10% of all diffuse LGG WHOII tumors. Identification of LGG-IDHwt is of the upmost importance as the majority of this subgroup shows molecular similarities with GBM tumors, with consequent worse prognosis and treatment response.[1] Mutations in the genes encoding for EGFR, PTEN, and NF1 are observed in both LGG-IDHwt and GBM.[12] Moreover, numerical and structural chromosomal abnormalities such as a trisomy of chromosome 7 and a loss of chromosome 10 are more frequently observed in high-grade glial tumors.
CONCLUSION
A subclass of diffuse LGG shows a molecular profile similar to high-grade glioma and is associated with a poor overall survival. Therefore, additional molecular characterization is necessary to identify this subgroup. The 2016 update of the WHO Classification of diffuse LGG facilitates subclass segregation and therefore formally introduces molecular diagnostic results as part of routine neuropathological practice with direct clinical consequences. The presented case illustrates that molecular characterization, beyond the scope of the WHO classification, highly influences adjuvant treatment strategies, and is therefore an example of personalized medicine. Further research will have to show whether the identified subclass-specific genetic aberrations, such as EGFR amplification, will aid to the development of targeted therapy for low-grade gliomas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Tim A. M. Bouwens van der Vlis, Email: tim.bouwens@mumc.nl.
Ann Hoeben, Email: ann.hoeben@mumc.nl.
Jan C. Beckervordersandforth, Email: jan.beckervordersandforth@mumc.nl.
Linda Ackermans, Email: linda.ackermans@mumc.nl.
Daniëlle B. P. Eekers, Email: danielle.eekers@maastro.nl.
Rianne M. J. Wennekes, Email: ri.wennekes@zuyderland.nl.
Olaf E. M. G. Schijns, Email: o.schijns@mumc.nl.
REFERENCES
- 1.Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372:2481–98. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374:1344–55. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164:550–63. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang FW, Bielski CM, Rinne ML, Hahn WC, Sellers WR, Stegmeier F, et al. TERT promoter mutations and monoallelic activation of TERT in cancer. Oncogenesis. 2015;4:e176. doi: 10.1038/oncsis.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–61. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 8.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 9.Pekmezci M, Rice T, Molinaro AM, Walsh KM, Decker PA, Hansen H, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1690-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 11.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–83. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]