Abstract
High risk neonates experience numerous painful/stressful procedures daily in neonatal intensive care units (NICUs). Accumulated pain and stress have detrimental impact on infants’ neurodevelopment. Few valid tools are available to measure accumulated pain/stressors among NICU infants. The aim of this study was to obtain nurses’ perceptions about severity and acuity levels regarding each painful/stressful procedure that infants may experience in the NICU. The data will support developing a new instrument, the Accumulated Pain/Stressor Scale (APSS) in NICUs. A nationwide online survey was conducted through the U.S. National Association of Neonatal Nurses membership. Respondents were asked to rate the perceived severity of pain/stress associated with 68 procedures using a 5-point Likert scale and to categorize pain/stress as acute or chronic. Modal values were used to determine summary rankings among the procedures. Eighty-four neonatal nurses completed the survey. Among 68 procedures, nearly all were rated as painful/stressful to some degree. Five procedures (7%) had a modal value of 5 (extremely painful/stressful), 9 (14%) had a value of 4, 20 (29%) a value of 3, 30 (44%) a value of 2, and 4 (6%) had a value of 1 (not painful/stressful). Forty-four procedures (65%) were perceived as acute, 6 (9%) as chronic and 18 (26%) as both acute and chronic. Nurse’s perceptions of pain severity and acuity regarding procedures in NICUs are somehow varied. Further studies are needed in developing and validating the scale. The development of the APSS can quantitatively measure the accumulated neonatal pain/stress.
Keywords: accumulative pain, stress, neonatal intensive care unit, instrument, nurses
Background
Evidence has established that newborn infants, including prematurely born infants, have the ability to perceive and experience pain. Moreover, due to immaturity of the descending pathways that inhibit pain impulses, preterm neonates have lower tolerance of painful procedures than full-term infants, potentially leading to more severe consequences (Slater et al., 2010). In the high technology, neonatal intensive care unit (NICU) environment, preterm infants are more likely to receive painful and stressful stimuli and, due to extended hospitalization, tend to receive it over a longer duration. On average, preterm infants experience 12–16 painful procedures per day (Carbajal et al., 2008). Growing concerns have been raised about accumulated and unmanaged pain/stress exposure in early life and its long-term, adverse consequences on the infant brain and neurodevelopment.
Vulnerable infants exposed to intense, repeated, or prolonged painful/stressful procedures in early life are likely to have altered pain pathways and thresholds, altered programming of stress systems, and impaired neuro-behavioral outcomes, including cognitive, memory and behavioral deficits, compared to full-term peers. These deficits may persist into adolescence and adulthood (Beauchamp et al., 2008; Grunau, Holsti, & Peters, 2006; Grunau et al., 2009; Hermann, Hohmeister, Demirakca, Zohsel, & Flor, 2006). When neonatal rats experience persistent peripheral inflammation, similar to multiple heelstick procedures given to human neonates, their spinal neuronal circuits exhibit changes in nociceptive primary afferent axons and show altered responses to sensory stimulation during adulthood (Bhutta et al., 2001; Ruda, Ling, Hohmann, Peng, & Tachibana, 2000). Similarly, repetitive or cumulative exposure to pain and stress is believed to permanently alter a human newborn’s neuronal and synaptic organization (Anand & Scalzo, 2000; Fitzgerald & Beggs, 2001). More seriously, unrelieved excessive pain/stress can alter the structure and function of the developing brain in preterm infants through, for example, reduction of white and subcortical grey matter structures during maturation (Brummelte et al., 2012). These pain/stress exposures may be related to altered IQ in school age children that is mediated by brain microstructural changes (Vinall et al., 2014). Strategies for how to assess and manage cumulative pain/stress in the early life stage remain largely under-investigated and urgently need to be addressed.
More than 40 neonatal pain measurement tools are currently available for research and clinical use, but no single tool has been established as the “gold standard”. Additionally, the majority of instruments were developed to measure acute pain across a short time period using physiological and behavioral cues, such as crying, facial expressions, heart rate variation, increased respiration, and decreased oxygenation (Cong, McGrath, Cusson, & Zhang, 2013) rather than directly quantifying cumulative pain and stress (Ranger, Johnston, & Anand, 2007).
Capturing bio-behavioural response to a painful procedure is challenging because the hypothalamic-pituitary-adrenal (HPA) axis in preterm neonates is not fully developed and physiological cues may not be displayed due to extreme immaturity (Newnham, Inder, & Milgrom, 2009). One study found that only 20% of extremely premature infants cry during a heel stick procedure (Gibbins, Stevens, Beyene, et al., 2008). Meanwhile, some physiological and behavioral indicators e.g., heart rate and crying, may be not specific to pain and could be associated with other conditions such as hunger or fatigue. Factors such as severity of illness, gestational age, frequency of and time since previous painful procedures, and medication use may also affect the ability of preterm infants to respond and could make behavioral variables ineffective in detecting neonatal pain (Gibbins, Stevens, McGrath, et al., 2008; Hatfield & Ely, 2015; Stevens et al., 2007).
These ambiguities leave neonatal nurses’ challenged by the issue of pain assessment in NICUs. Nurses working in NICUs have widely reported e.g., 34.7% in the U.S. and 57.5% in China, that the pain assessment tools adopted in their units are inaccurate for measuring neonatal pain (Cong et al., 2014). In comparison to acute pain, signs of repeated or cumulative pain tend to be more subtle in preterm infants, leading to under-recognition and under-treatment (Ranger et al., 2007). Young preterm infants may not display “appropriate” signs of pain response when they experience persistent painful or stressful procedures because they can lack the energy reserves to express bio-behavioral responses (Ranger et al., 2007) or because the NICU experience has already led to abnormal brain development and to hypersensitivity to pain stimuli (Ranger & Grunau, 2014).
To determine the impact of repeated and cumulative pain and to provide an evidential basis for its treatment, NICU infants need to be monitored closely. The only tool that we found for assessing cumulative pain/stress in infants that is specifically targeted to the NICU setting is the Neonatal Infant Stressor Scale (NISS) developed in Australia by Newnham, et al. (2009). The NISS provides a systematic way to identify and quantify infant stress. It lists 68 procedures and attaches a severity level to each one. However, in using the NISS, we found that it does not cover many pain/stressors that occur as part of standard practice in NICUs in the United States.
In order to quantitatively and more accurately assess the extent and severity of pain/stress that infants experience in NICUs in the United States, the authors of this paper developed the Accumulated Pain/Stressor Scale (APSS) in NICU. Since it is difficult to differentiate pain and stress in very preterm infants (Brummelte et al., 2015; Grunau et al., 2013), we use the term “painful/stressful” to characterize the full spectrum of events that can cause pain and produce stress in the neonatal population.
The initial version of the APSS consisted of 62 items (unpublished data) within nine categories of painful/stressful event potentially experienced by the NICU infants. The nine categories included blood draw, feeding, imaging, infection, peripheral venous access, central venous access, surgery, respiratory, and “miscellaneous”. A focus group study was conducted to examine clinicians’ perceptions regarding the concept of accumulated pain/stress and to obtain expert judgment of the content validity of the initial APSS scale. Nine neonatologists and five neonatal nurses were recruited from a level IV NICU (the highest level of NICU with the capacities to provide surgical repair of complex congenital or acquired conditions) in the Northeast U.S. and participated in the focus group discussion and completed a survey. Based on results from the focus group (unpublished), the APSS was then revised to a 68-item scale.
The current study is the second stage of the scale development, which reports findings from an evaluation of this revised, 68-item instrument. The objectives were to access a national sample of neonatal clinicians, to explore their perceptions about the acuity and severity of virtually all individual painful/stressful events that occur in NICUs in the U.S., and to use that information to further document the validity of the APSS.
Methods
Design
A descriptive, cross-sectional survey design was used in the study. American neonatal nurses were recruited through the National Association of Neonatal Nurses (NANN) webpage. Inclusion criteria for the participants were: registered nurses over 18 years of age, English speaking, and working in a NICU setting in the United States at the time of survey.
Instrument
The survey questionnaire consisted of 68 painful/stressful procedures and events that are commonly experienced by neonates in the NICU. The procedures were grouped into 9 categories, including daily care, feeding, imaging, blood draw, peripheral venous access, central venous access, respiratory care, surgeries and major procedures, and infection. The participants were asked to classify each of the pain/stressors as acute, chronic, or both, meanwhile, to use a numerical scale to indicate the severity level of each pain/stressor based on the participant’s experience, knowledge, and judgment (1= not painful/stressful; 2 = a little; 3 = moderate; 4 = very; and 5 = extremely painful/stressful). Survey respondents were instructed to draw conclusions from their experiences or memories of the NICU patient population rather than from the experiences of any particular baby.
Procedures
The study protocol was approved by the Institutional Review Board of the corresponding author’s home institution. The survey was uploaded and administered electronically using Qualtrics Survey Software, Version 2015 (Provo, Utah, USA). NANN members were emailed an invitation letter with a link to the Qualtrics survey through the NANN website. The information sheet with informed consent was posted on the survey website as the first question so that the potential participants were able to select “yes” or “no” to acknowledge that they had read the information sheet and gave informed consent before they could continue with the survey questionnaire. Demographic information was collected at the end of the survey questionnaire. After the initial invitation email, two reminder emails were sent 10 days apart. The survey remained available for 30 days following the date of the initial email to the participants. When participants completed the survey, their data were secured within a password-protected account. Once the survey was closed, the data were downloaded for statistical analysis.
Statistical Analysis
Survey data were analysed using the R statistical software package (R 3.22). Descriptive statistical methods were used to summarize responses. This included calculating the mode and the average deviation from the mode (ADFM) for the acuity classification and for the severity ranking for each APSS item. The ADFM is the average of the absolute deviations from the mode. It quantifies dispersion or variability and can be used to describe agreement among the participants scoring each item. Correlation coefficients were also computed to evaluate associations between the severity of pain/stress that nurses rated to each procedure and their ages, educational levels, years of NICU experience, and highest NICU level in which they had worked.
Results
National Survey
Eighty-four neonatal nurses from the U.S. participated in the national survey. The majority were female (98.8%), non-Hispanic White (95.2%), had a baccalaureate or higher degree (89.2%), and had worked in a Level III (having the capacity to provide comprehensive care for infants born <32 weeks gestation and <1500 g and critically ill infants) or Level IV NICU (96.4%). The mean age of the neonatal nurses was 50.2 ± 10.8 years, with an average of 22.9 ± 10.9 years working experience in the NICU. Table 1 summarizes demographic characteristics of the participants.
Table 1.
Demographic Characteristics of Neonatal Nurses (N = 84)
| Demographic Characteristics | Frequency | Percentage (%) |
|---|---|---|
| Age (years) (n = 81) | ||
| ≤40 | 14 | 17.3 |
| 41–50 | 20 | 24.7 |
| 51–60 | 37 | 45.7 |
| ≥61 | 10 | 12.3 |
| NICU practice (years) (n = 83) | ||
| ≤10 | 16 | 19.3 |
| 11–20 | 17 | 20.5 |
| 21–30 | 26 | 31.3 |
| ≥31 | 24 | 28.9 |
| Gender (n = 84) | ||
| Male | 1 | 1.2 |
| Female | 83 | 98.8 |
| Race (n = 82) | ||
| White | 80 | 97.6 |
| Asian | 2 | 2.4 |
| Ethnicity (n = 82) | ||
| Hispanic | 2 | 2.4 |
| Non-Hispanic | 80 | 97.6 |
| Education (n = 84) | ||
| Diploma/Associate | 9 | 10.8 |
| Baccalaureate | 28 | 33. |
| Master | 35 | 41.7 |
| Doctoral | 12 | 14.3 |
| Level of NICU (n = 84) | ||
| Level II | 3 | 3.6 |
| Level III | 43 | 51.2 |
| Level IV | 38 | 45.2 |
Acuity of Perceived Pain/Stressors
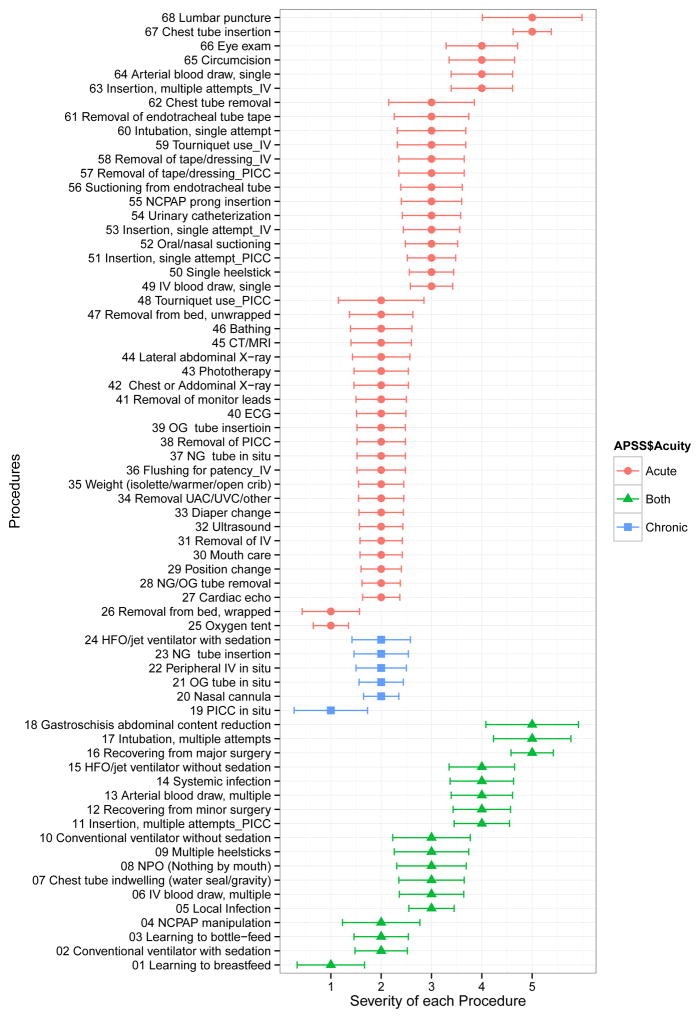
Figure 1 depicts the acuity classification based on the mode of the acuity classifications for each item. Forty-four (64.7%) painful/stressful events were considered to be acute, including events from 8 of the 9 categories: blood draw, feeding, imaging, peripheral venous access, central venous access, procedures, respiratory, and miscellaneous. Six (8.8%) events were considered to be chronic: orogastric tube in situ (feeding), nasogastric tube in situ (feeding), peripheral venous access in situ (peripheral venous access), peripherally inserted central catheter (PICC) in situ (central venous access), nasal cannula (respiratory), and high frequency oscillatory (HFO)/jet ventilator with sedation (respiratory). Eighteen (26.5%) events from 6 categories (blood draw, feeding, infection, procedure, respiratory event and peripheral venous access) were considered to be both acute and chronic.
Figure 1. Severity and Acuity of each procedure.
Graph shows mode (dot) and average deviation of mode (error bar) of the severity of each painful/stressful procedure on a 5-point scale. The procedures were grouped into acute (red color), both acute and chronic (green color) and chronic (blue color) based on the mode of acuity.
The ADFM for the acuity of each event ranged from 0.02 to 0.93. The events with lowest ADFM (representing relatively high agreement among survey respondents) included cardiac echo (0.02), CAT scan and magnetic resonance imaging (MRI) (0.02), electrocardiogram (ECG) (0.08), ultrasound (0.10) and peripheral IV insertion with single attempt (0.11), whereas the ones with highest ADFM (representing lowest agreement) included peripheral IV insertion with multiple attempts (0.93), nasal continuous positive airway pressure (NCPAP) prong insertion (0.92), local infection (0.90), PICC line insertion with multiple attempts (0.87) and learning to bottle feed (0.83).
Severity of Perceived Pain/Stressors
Figure 1 also represents the mode and ADFN of the severity level of pain/stress for each event. Four (4.8%) events were most frequently ranked as 1 on the 5-point Likert scale (1 = not painful/stressful; 5 = extremely painful/stressful), 30 (35.7%) events as level 2, 20 (23.8%) events as level 3, 9 (10.7%) events as level 4, and 5 (6.0%) events as level 5. The four events that were scored as not painful/stressful were learning to breastfeed, oxygen tent, PICC line in situ, and removal from bed (wrapped). The events that were considered as most painful/stressful included gastroschisis abdominal content reduction, lumbar puncture, recovering from major surgery, chest tube insertion, and intubation with multiple attempts.
The ADFM for severity score ranged from 0.35 to 0.99 (Figure 1). Examples of the events with lowest ADFM (and so with the strongest agreement among respondents) were oxygen tent (0.35), nasal cannula (0.35), cardiac echo (0.37), chest tube insertion (0.38), and NG/OG tube removal (0.38). Examples of the events with highest ADFM (and so the weakest agreement) were lumbar puncture (0.99), gastroschisis abdominal reduction (0.92), chest tube removal (0.85), tourniquet use for PICC line insertion (0.85), and conventional ventilation without sedation (0.77).
For each participant, the mean severity score of all the events within each of the 9 categories was calculated. Spearman correlations between the mean score of each category and the demographic characteristics were then computed. The results showed no significant correlation between the mean perceived severity of any category and participants’ age, years of experiences as a neonatal nurse, or level of education. However, there was a correlation between the highest NICU level where a participant had worked and the mean perceived pain/severity scores assigned to the blood draw category (r = 0.249, p < 0.05). Correlations between the mean score assigned to the blood draw category and the other 8 pain/stress categories were not statistically significant.
Summarizing Pain/Stressors using the APSS
As explained above, the 68 painful/stressful events and procedures included in the APSS are classified into nine caregiving categories. Based on the survey results, within the nine categories, each item was assigned to a pain/stress severity level and to one of three acuity levels, acute (A), both acute and chronic (B), and chronic (C) (see Table 2). To document infant pain/stressors using the APSS, clinicians can tally the acute (A) procedures, and tally and record the hours/minutes of both acute and chronic (B), or chronic (C) events, depending on the course of the events for each shift. The recorded frequency or duration of each event can be weighted through multiplication by the assigned pain severity level for that event. A total score for each acuity level (A, B, and C) can be calculated for each day by summing the weighted frequency or duration for each acuity level.
Table 2.
Accumulated Pain/Stressor Scale (APSS): Result of Nation Survey
| Pain/stressors Categories | Pain Level (Frequency) | Acuity (Frequency) | ||
|---|---|---|---|---|
| Daily care | Level | A | B | C |
| Removal from bed, wrapped | 1 (45.2%) – 2 (40.5%) | X | ||
| Removal from bed, unwrapped | 2 | X | ||
| Removal of monitor leads | 2 | X | ||
| Removal UAC/UVC/other | 2 | X | ||
| Diaper change | 2 | X | ||
| Position change | 2 | X | ||
| Mouth care | 2 | X | ||
| Weight (isolette/warmer/open crib) | 2 | X | ||
| Bathing | 2 (38.1%) – 3 (36.9%) | X | ||
| Phototherapy | 2 | X | ||
| Feeding | Level | A | B | C |
| NG tube insertion | 2 | X | ||
| OG tube insertion | 2 | X | ||
| NG/OG tube removal | 2 | X | ||
| Learning to breast-feed | 1 (42.9%) – 2 (40.5%) | X (31%) | X (35.7%) | |
| Learning to bottle-feed | 2 | X (32.1%) | X (36.9%) | |
| NPO (Nothing by mouth) | 2 (38.1%) – 3 (39.3%) | X | ||
| NG tube in situ | 2 | X | ||
| OG tube in situ | 2 | X | ||
| Imaging | Level | A | B | C |
| Cardiac echo | 2 | X | ||
| ECG | 2 | X | ||
| CT/MRI | 2 | X | ||
| Chest or Anterior-Posterior abdominal X-ray | 2 | X | ||
| Lateral abdominal X-ray | 2 | X | ||
| Ultrasound | 2 | X | ||
| Blood Draw | Level | A | B | C |
| Heelstick, single stick | 3 | X | ||
| IV blood draw, single | 3 | X | ||
| Arterial blood draw, single | 4 | X | ||
| Heelstick, multiple sticks | 3(38.1%) – 4 (36.9%) | X | ||
| IV blood draw, multiple | 3 | X | ||
| Arterial blood draw, multiple | 4 | X | ||
| Peripheral IV | Level | A | B | C |
| Tourniquet use | 2 (34.5%) – 3 (35.7%) | X | ||
| IV Insertion, single attempt | 3 | X | ||
| IV Insertion, multiple attempts | 4 | X (40.5%) | X (45.2%) | |
| IV Flushing for patency | 2 | X | ||
| Removal of IV | 2 | X | ||
| Removal of IV tape/dressing | 3 | X | ||
| Peripheral IV in situ | 2 | X | ||
| Central venous access | Level | A | B | C |
| Tourniquet use | 2 (36.9%) – 3 (32.1%) | X | ||
| PICC Insertion, single attempt | 3 | X | ||
| PICC Insertion, multiple attempt | 4 | X | ||
| Removal of PICC | 2 | X | ||
| Removal of PICC tape/dressing | 3 | X | ||
| PICC in situ | 1 (38.1%) – 2 (36.9%) | X | ||
| Respiratory | Level | A | B | C |
| Oxygen tent | 1 | X | ||
| Intubation, single attempt | 3 | X | ||
| Oral/nasal suctioning | 3 | X | ||
| Endotracheal tube suctioning | 3 | X | ||
| Endotracheal tube tape removal | 2 (31%) – 3 (33.3%) | X | ||
| NCPAP prong insertion | 2 (36.9%) – 3 (41.7%) | X (39.3%) | X (35.7%) | |
| Chest tube removal | 3 (31%) – 4 (29.8%) | X | ||
| Chest tube insertion | 5 | X | ||
| Conventional ventilator with sedation | 2 | X (40.5%) | X (39.3%) | |
| NCPAP manipulation | 2 (38.1%) – 3 (36.9%) | X | ||
| Chest tube indwelling (water seal/gravity) | 3 | X | ||
| Conventional ventilator without sedation | 3 | X | ||
| HFO (high frequency oscillating ventilator)/jet ventilator without sedation | 4 | X | ||
| Intubation, multiple attempts | 5 | X | ||
| Nasal cannula in situ | 2 | X (33.3%) | X (35.7%) | |
| HFO/jet ventilator with sedation | 2 | X (40.5%) | X (41.7%) | |
| Surgeries/Procedures | Level | A | B | C |
| Urinary catheterization | 3 | X | ||
| Eye exam | 4 | X | ||
| Circumcision | 4 | X | ||
| Lumbar puncture | 4 (32.1%) – 5 (35.7%) | X | ||
| Recovering from minor surgery | 3 (36.9%) – 4 (41.7%) | X | ||
| Recovering from major surgery (e.g., cardiac, abdominal, neural) | 5 | X | ||
| Gastroschisis abdominal content reduction | 5 | X | ||
| Infection | Level | A | B | C |
| Local | 3 | X | ||
| Systemic | 3 (36.9%) – 4 (38.1%) | X | ||
Acuity: A = Acute; B = Both Acute and Chronic; C = Chronic
Document each procedure that the infant has experienced in each shift.
Both Acuity and Pain level were assigned based on the mode of the frequencies of responses of each procedure; if the difference between two frequencies of the procedure is less than 5%, both of the two most frequent scored pain levels or acuities are displayed. The frequency of the most common responses was indicated in the parentheses.
Discussion
Neonatal nurses participating in this national survey perceived that almost all (64 out of 68) of the procedures in the APSS are painful and stressful to neonatal infants to some extent. On average, the events and procedures included in the daily care, feeding, and imaging categories were perceived as only “a little painful/stressful”. In contrast, procedures related to blood draw and medical procedures were considered “very painful/stressful”. These results are consistent with findings from Newnham’s study that procedures in nutrition, radiology, and nursing are perceived as less stressful while medical procedures and surgery are perceived as most stressful (Newnham et al., 2009).
No correlations were found between nurses’ demographic characteristics and the perceived pain/stress level for each category, except the relationship between the highest NICU level in which a nurse has worked and the nurse’s perception of pain/stress level for blood draw. Nurses who have taken care of high-risk infants in a higher level of NICU viewed blood draw procedures as having a higher level of pain, a phenomenon which may be related to their experience or pain management training in such units.
Events that were classified as chronic by neonatal nurses tended to be less painful/stressful, with the modal severity level generally falling at 1 (not painful/stressful) or 2 (a little painful/stressful). Seventeen out of 68 events were perceived as both acute and chronic by clinicians. One of the reasons for this result may be that there is still no established definition of chronic pain in the neonatal population (van Dijk & Tibboel, 2012). The concept of “pediatric chronic pain” has been defined as persistent or recurrent discomfort that lasts longer than an expected course of acute illness or injury, usually longer than 3 to 6 months (American Pain Society, 2012). However, this criterion usually cannot be applied to a neonatal population because the neonatal period is usually shorter than 3 months for most infants.
Although several studies have been conducted to conceptualize neonatal chronic pain, a consensus remains elusive (Pillai Riddell et al., 2009; van Ganzewinkel, Anand, Kramer, & Andriessen, 2014). Riddel’s study listed potential examples of chronic pain in infancy, including repetitive acutely painful procedures such as cardiac patients with multiple surgeries, infants exposed to daily heel lances, and needle sticks (Pillai Riddell et al., 2009). Ganzewinkel also concluded that experiencing “daily episodes of continuous or recurrent pain sensations” constitutes a potential etiology for chronic pain (van Ganzewinkel et al., 2014). Our findings are consistent with those of previous studies in that some events, including multiple heelsticks and other blood draws, were perceived as both acute and chronic due to having initially high severity and long duration.
Agreement varied on the severity level perceived by neonatal nurses for individual painful/stressful events. Procedures that were viewed as less painful/stressful, such as oxygen tent, nasal cannula, cardiac echo, and NG/OG tube removal, tended to have higher agreement (small ADFM) among nurses, whereas procedures that were viewed as more painful/stressful, such as lumbar puncture, gastroschisis abdominal reduction and conventional ventilation without sedation, tended to have higher disagreement. This could be explained by the fact that the no specific clinical circumstances for events in the APSS were provided to the survey respondents. In an open-ended question included at the end of the survey, 24 of the 84 participants commented on the lack of specific circumstances of the procedures when rating perceived pain/stress experienced by neonates. Multi-dimensional factors that may influence potential pain/stress responses and consequences include neonates’ state and health conditions, the professional experience of the clinician who performs the procedure, whether comfort measures or analgesia are given, and the diverse pain management techniques and protocols used in different units and hospitals. In light of the results, the more severe painful/stressful a procedure is, the more varied the clinical and caregiving factors that affect pain/stress assessment and outcomes. To prevent harmful long-term effects on infant health, clinicians need to address management of different levels of painful/stressful procedures in the NICU.
One of the limitations of this study is a failure to fully account for variability in pain/stress experience across different neonates’ conditions and across different institutional contexts. However, the majority of existing pain measurement tools applied to the neonatal population, including assessments based on physiological and behavioral cues, have the same limitation (Krechel & Bildner, 1995; Newnham et al., 2009; Schiavenato et al., 2013; Stevens, Johnston, Petryshen, & Taddio, 1996). The current study attempted to moderate this limitation by using a national sample of NICU nurses in the expectation that including a diverse group of respondents would enhance validity and generalizability of the APSS. Differences in perceived pain/stress levels for each item that may be due to contextual factors were quantified and the mode was used to represent the severity level of each event under the majority of circumstances.
Another way in which the present study failed to account for differences in how neonates experience pain/stress in the NICU setting is that the survey questionnaire did not attempt to assess variation in nurses’ perceptions of pain/stress among different post-conceptional age groups. Mixed findings have been reported regarding the relationship of preterm infants’ gestational and postnatal ages and their bio-behavioral pain responses (Sellam, Cignacco, Craig, & Engberg, 2011). In one recent study, an infant’s postmenstrual age was not found to be associated with any kind of behavioral or physiological pain responses (Sellam, Engberg, Denhaerynck, Craig, & Cignacco, 2013). Analogously, Newnham’s study showed that the stress levels that were perceived by clinicians among different postconceptional age groups (< 28, 28–32, and 32–37 weeks respectively) were similar (Newnham et al., 2009).
Pharmacological and non-pharmacological interventions are also factors that impact the severity of each painful procedure and their roles as potential moderating factors were not reflected in the APSS. Pain interventions in the NICU are still not consistently used in clinical practice due to multiple factors, such as side effects of opioid medication, lack of standard pain protocols, and variability in clinicians’ individual practices (Gibbins et al., 2015; Green, Darbyshire, Adams, & Jackson, 2014; Stevens et al., 2011). Therefore, further study is needed to calibrate the severity of painful events experienced by infants with concurrent administration of analgesic interventions.
Assessing cumulative pain and stress is critical and necessary in neonatal care given the strong association between repeated painful/stressful procedures in early life and subsequent adverse neuro-developmental outcomes in preterm infants (Grunau, 2002; Provenzi et al., 2015). However, existing pain assessment tools mostly focus on acute, one-time pain events. To our knowledge, only two instruments currently in use attempt to evaluate cumulative pain and stressors, the Neonatal Infant Stressor Scale (Newnham et al., 2009) and the Procedural Load Index (Schiavenato et al., 2013). Both tools were developed using experts recruited from local regions, which may limit their generalizability to NICUs in United States.
Implications for Nursing Practice and Research
This study describes the perception of pain intensity for the most commonly encountered procedures/events in NICUs and uses this information as part of the validation process for the Accumulated Pain/Stressor Scale (APSS), an instrument designed to quantify cumulative pain/stress experienced by NICU infants. Based on the results, NICU nurses should be aware of the level of pain associated with each procedure/event as well as the cumulative pain/stress that infants experience daily. When sufficiently validated, the APSS scale will provide nurses guidance in delivering pain management for each infant and in advocating for infants to avoid unnecessary painful clinical procedures. Awareness of cumulative pain/stress scores will also provide NICU nurses’ the ability to identify the infants with increased risk of adverse neurobehavioral development, and to offer comforting measures, such as skin-to-skin contact, in order to promote more favorable developmental outcomes.
Existing evidence shows that cumulative pain/stressor is strongly predictive of infants’ neurobehavioral development. However, the inavailability of an established and accurate assessment tool increases the difficulty of the future research exploring the undesirable consequences resulting from cumulative pain/stress or demonstrating the benefits of protective strategies that control or compensate for it. This study provides researchers a new approach for measuring cumulative pain/stress. With further validation, the APSS scale should be able to guide clinical practice for individual NICU infants and to support research that more conclusively documents effects of cumulative pain/stress in preterm infants and the effectiveness of protocols for its management.
Conclusion
The new APSS scale will provide a systematic tool for evaluating cumulative pain/stress experienced by preterm infants in NICUs. By documenting pain/stressors using the APSS, clinicians can calculate the total frequency of acute (A) events and weighted scores for the severity of those events and the duration of both acute and chronic (B) or chronic (C) events, as well as durations that are weighted by severity. This information can guide the delivery or pain management and patient care, promoting neurodevelopmental outcomes later in life. Future studies are needed to further validate the APSS, especially expert judgment that address the impact of procedures/events with highly variable classifications by NICU nurses concerning chronicity or severity. In addition, larger studies need to be conducted applying the APSS scale in infant populations to test the reliability and validity of the instrument and, when necessary, to modify it in order to enhance its accuracy. Cut-off points for the scale that predict developmental consequences will also need to be explored and tested. With further support from the evidence, the APSS will be used to guide clinical pain practice and to support research into the effects of pain and its management.
Supplementary Material
Acknowledgments
This publication was supported by the National Institute of Nursing Research of the National Institutes of Health (NIH-NINR) under Award Number 1K23NR014674-01 and Affinity Research Collaboratives award through University of Connecticut Institute for Systems Genomics.
Footnotes
Conflict of interest statement:
There are no declared conflicts of interest for any of this study’s authors.
Contributor Information
Wanli Xu, University of Connecticut School of Nursing, 231 Glenbrook Rd, Storrs, 06269-4026.
Stephen Walsh, Associate Professor, University of Connecticut School of Nursing, 231 Glenbrook Rd, Storrs, 06269-4026, Phone: 860-486-0544.
Xiaomei Cong, Associate Professor, University of Connecticut School of Nursing, 231 Glenbrook Road, Unit 2026, Storrs, CT 06269-2026.
References
- Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77(2):69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131(Pt 11):2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73(1–2):51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Chau CM, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, Grunau RE. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Psychoneuroendocrinology. 2015;51:151–163. doi: 10.1016/j.psyneuen.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, … Miller SP. Procedural pain and brain development in premature newborns. [Comparative Study Research Support, Non-U.S. Gov’t] Ann Neurol. 2012;71(3):385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, … Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. Jama. 2008;300(1):60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- Cong X, McGrath JM, Delaney C, Chen H, Liang S, Vazquez V, … Dejong A. Neonatal nurses’ perceptions of pain management: survey of the United States and China. Pain Manag Nurs. 2014;15(4):834–844. doi: 10.1016/j.pmn.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7(3):246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Gibbins S, Stevens B, Beyene J, Chan PC, Bagg M, Asztalos E. Pain behaviours in Extremely Low Gestational Age infants. [Evaluation Studies Research Support, Non-U.S. Gov’t] Early Hum Dev. 2008;84(7):451–458. doi: 10.1016/j.earlhumdev.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Gibbins S, Stevens B, Dionne K, Yamada J, Pillai Riddell R, McGrath P, … Johnston C. Perceptions of health professionals on pain in extremely low gestational age infants. Qual Health Res. 2015;25(6):763–774. doi: 10.1177/1049732315580105. [DOI] [PubMed] [Google Scholar]
- Gibbins S, Stevens B, McGrath PJ, Yamada J, Beyene J, Breau L, … Ohlsson A. Comparison of pain responses in infants of different gestational ages. [Comparative Study Multicenter Study] Neonatology. 2008;93(1):10–18. doi: 10.1159/000105520. [DOI] [PubMed] [Google Scholar]
- Green J, Darbyshire P, Adams A, Jackson D. It’s agony for us as well: Neonatal nurses reflect on iatrogenic pain. Nurs Ethics. 2014 doi: 10.1177/0969733014558968. [DOI] [PubMed] [Google Scholar]
- Grunau RE. Early pain in preterm infants. A model of long-term effects. Clin Perinatol. 2002;29(3):373–394. vii–viii. doi: 10.1016/s0095-5108(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Cepeda IL, Chau CM, Brummelte S, Weinberg J, Lavoie PM, … Turvey SE. Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] PLoS One. 2013;8(9):e73926. doi: 10.1371/journal.pone.0073926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11(4):268–275. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, … Johannesen D. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield LA, Ely EA. Measurement of acute pain in infants: a review of behavioral and physiological variables. Biol Res Nurs. 2015;17(1):100–111. doi: 10.1177/1099800414531448. [DOI] [PubMed] [Google Scholar]
- Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125(3):278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth. 1995;5(1):53–61. doi: 10.1111/j.1460-9592.1995.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: the Neonatal Infant Stressor Scale. Early Hum Dev. 2009;85(9):549–555. doi: 10.1016/j.earlhumdev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Pillai Riddell RR, Stevens BJ, McKeever P, Gibbins S, Asztalos L, Katz J, … Din L. Chronic pain in hospitalized infants: health professionals’ perspectives. [Multicenter Study Research Support, Non-U.S. Gov’t] J Pain. 2009;10(12):1217–1225. doi: 10.1016/j.jpain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Provenzi L, Fumagalli M, Sirgiovanni I, Giorda R, Pozzoli U, Morandi F, … Montirosso R. Pain-related stress during the Neonatal Intensive Care Unit stay and SLC6A4 methylation in very preterm infants. Front Behav Neurosci. 2015;9:99. doi: 10.3389/fnbeh.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Pain Manag. 2014;4(1):57–67. doi: 10.2217/pmt.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. [Review] Semin Perinatol. 2007;31(5):283–288. doi: 10.1053/j.semperi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289(5479):628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Schiavenato M, Antos SA, Bell FA, Freedman BR, Kozak AJ, Kroot TB, … Carney LH. Development of a scale for estimating procedural distress in the newborn intensive care unit: the Procedural Load Index. Early Hum Dev. 2013;89(9):615–619. doi: 10.1016/j.earlhumdev.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Sellam G, Cignacco EL, Craig KD, Engberg S. Contextual factors influencing pain response to heelstick procedures in preterm infants: what do we know? A systematic review. [Review] Eur J Pain. 2011;15(7):661 e661–615. doi: 10.1016/j.ejpain.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Sellam G, Engberg S, Denhaerynck K, Craig KD, Cignacco EL. Contextual factors associated with pain response of preterm infants to heel-stick procedures. [Randomized Controlled Trial] Eur J Pain. 2013;17(2):255–263. doi: 10.1002/j.1532-2149.2012.00182.x. [DOI] [PubMed] [Google Scholar]
- Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage. 2010;52(2):583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12(1):13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Stevens B, McGrath P, Gibbins S, Beyene J, Breau L, Camfield C, … Yamada J. Determining behavioural and physiological responses to pain in infants at risk for neurological impairment. [Multicenter Study Research Support, Non-U.S. Gov’t] Pain. 2007;127(1–2):94–102. doi: 10.1016/j.pain.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Stevens B, Riahi S, Cardoso R, Ballantyne M, Yamada J, Beyene J, … Ohlsson A. The influence of context on pain practices in the NICU: perceptions of health care professionals. [Research Support, Non-U.S. Gov’t] Qual Health Res. 2011;21(6):757–770. doi: 10.1177/1049732311400628. [DOI] [PubMed] [Google Scholar]
- van Dijk M, Tibboel D. Update on pain assessment in sick neonates and infants. [Review] Pediatr Clin North Am. 2012;59(5):1167–1181. doi: 10.1016/j.pcl.2012.07.012. [DOI] [PubMed] [Google Scholar]
- van Ganzewinkel CJ, Anand KJ, Kramer BW, Andriessen P. Chronic pain in the newborn: toward a definition. Clin J Pain. 2014;30(11):970–977. doi: 10.1097/AJP.0000000000000056. [DOI] [PubMed] [Google Scholar]
- Vinall J, Miller SP, Bjornson BH, Fitzpatrick KP, Poskitt KJ, Brant R, … Grunau RE. Invasive procedures in preterm children: brain and cognitive development at school age. [Research Support, Non-U.S. Gov’t] Pediatrics. 2014;133(3):412–421. doi: 10.1542/peds.2013-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.