Abstract
With rapid advances in understanding molecular pathogenesis of human diseases in the era of genome sciences and systems biology, it is anticipated that increasing numbers of therapeutic genes or targets will become available for targeted therapies. Despite numerous setbacks, efficacious gene and/or cell-based therapies still hold the great promise to revolutionize the clinical management of human diseases. It is wildly recognized that poor gene delivery is the limiting factor for most in vivo gene therapies. There has been a long-lasting interest in using viral vectors, especially adenoviral vectors, to deliver therapeutic genes for the past two decades. Among all currently available viral vectors, adenovirus is the most efficient gene delivery system in a broad range of cell and tissue types. The applications of adenoviral vectors in gene delivery have greatly increased in number and efficiency since their initial development. In fact, among over 2000 gene therapy clinical trials approved worldwide since 1989, a significant portion of the trials have utilized adenoviral vectors. This review aims to provide a comprehensive overview on the characteristics of adenoviral vectors, including adenoviral biology, approaches to engineering adenoviral vectors, and their applications in clinical and preclinical studies with an emphasis in the areas of cancer treatment, vaccination and regenerative medicine. Current challenges and future directions regarding the use of adenoviral vectors are also discussed. It is expected that the continued improvements in adenoviral vectors should provide great opportunities for cell and gene therapies to live up to its enormous potential in personalized medicine.
Keywords: Adenovirus, Adenoviral vector, Cell therapy, Gene transfer, Gene therapy, Oncolytic virus, Regenerative medicine, Vaccine development
Introduction
By using directed gene transfer to treat human disease, gene therapy may hold the potential to revolutionize medicine – in part because this approach is capable of treating the root cause of a disease, not merely its symptoms.1 Despite numerous setbacks in the past decades, gene therapy remains as a field that is constantly growing and developing as scientists look for new strategies to treat some of the most difficult health issues. There has been a long-lasting interest in using viral vectors, especially adenoviral vectors, for gene therapy in the past two decades.1, 2, 3 It is well recognized that poor gene delivery is the limiting factor for most in vivo gene therapies while the only exceptions are where therapeutic viruses can be injected directly into the target site or where they can be introduced into target cells ex vivo.
Adenovirus has received tremendous attention as an effective gene delivery vector and was in fact the first DNA virus to enter rigorous therapeutic development, largely because of its well-defined biology, its genetic stability, its high gene transduction efficiency and its ease of large-scale production.2, 3, 4 Adenovirus (Ad) is a non-enveloped, linear double-stranded DNA virus with 57 identified human Ad serotypes.2, 5 Ad serotypes differ in tropism and are further divided into six subgroups, A–G. Differences in viral capsids delineate tropisms among serotypes. The viral capsid is comprised of capsid proteins, core proteins, and cement proteins. These diverse serotypes can give rise to a vast range of therapeutic candidate viruses. Thus, it is not surprising that adenovirus continues to occupy the center stage in gene therapy arena.2, 3, 4
Compared with other viral gene delivery systems, adenoviral vectors offer significant advantages.2 First, adenovirus is the most effective means of delivering genes in vivo as most human cells express the primary adenovirus receptor and the secondary integrin receptors. Thus are easily infected with adenovirus vectors and consequently yield high levels of the transgene expression.2 Second, the development of gutless adenoviral vectors allows us to circumvent anti-adenoviral vector immunity. Third, despite the concern over safety of their use, there has been extensive experience with adenovirus vectors in many different clinical applications, and the safest dosing and routes of administration are now well established.2 In fact, adenovirus vectors are the most common vector used in clinical trials worldwide and account for >20% of all gene therapy trials (see below). Fourth, adenovirus vectors offer a versatile platform for developing strategies to modify viral capsids in order to enhance therapeutic properties and improve targeting specificity of the virus. Interestingly, some of the inherited shortcomings of adenovirus, such as immunity evoked against the adenovirus capsid and low-level expression of adenovirus genes, may now prove beneficial for the development of anticancer immunotherapies, where inducing immunity against the cancer or directly killing the cancer cell is the goal. Furthermore, the combined immunity against the adenovirus together with the short time of expression is ideal for using the adenovirus as a platform for developing vaccines.2
The past two decades have witnessed many advances in the adenovirus vector system, ranging from its deployment as a vector for transgene delivery and supplementation for vaccination to its use as an oncolytic agent. Together with adeno-associated viruses and lenti/retrovirus vectors, the adenovirus now represents one of the three major viral vector categories in the gene therapy “tool box”. In this review, we will focus on the basic features of adenovirus, namely by the comparison of adenovirus to other viral and non-viral vectors, the common methods to produce adenovirus, the current clinical and preclinical use of adenovirus as gene transfer tools, as well as the existing challenges and opportunities in using adenovirus vectors in gene and cell-based medicine.
Commonly-used methods for gene delivery
A variety of other gene delivery techniques, largely sub-divided between viral and non-viral methodologies, have come into use over the last few decades (Table 1). In addition to adenovirus (AdV), lentivirus (LV), herpes simplex virus (HSV), adeno-associated virus (AAV), and baculovirus have also been studied for use in gene therapies.1, 3 Within the non-viral subclass, techniques utilizing naked DNA injection, electroporation, gene gun, sonoporation, magnetofection, and lipoplexes have also been developed for gene delivery.6, 7, 8, 9
Table 1.
Characteristics of the commonly-used viral vectors.
| Viral system | Adenovirus (Ad5) | AAV | Retrovirus | Lentivirus | HSV-1 | Baculovirus |
|---|---|---|---|---|---|---|
| Genome material | dsDNA | ssDNA | RNA | RNA | dsDNA | dsDNA |
| Genome size | 36 kb | 8.5 kb | 7–11 kb | 8 kb | 150 kb | 80–180 kb |
| Enveloped | No | No | Yes | Yes | Yes | Yes |
| Biosafety level | BSL-2 | BSL-1 | BSL-1/2 | BSL-2/3 | BSL-2 | BSL-1 |
| Insert size | 8–36 kb | 5 kb | 8 kb | 9 kb | 30–40 kb | No limit known |
| Max titer (particles/mL) | 1 × 1013 | 1 × 1011 | 1 × 109 | 1 × 109 | 1 × 109 | 2 × 108 |
| Tropism | Broad, low for blood cells | Broad, low for blood cells | Broad (pan or psuedo-typed) | Broad (pan or psuedo-typed) | Neurons | Some mammalian cells |
| Infectivity | Dividing and non-dividing cells | Dividing and non-dividing cells | Dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells | Dividing and non-dividing cells |
| Transgene expression | Transient | Transient or stable | Stable | Stable | Transient | Transient or stable |
| Vector genome form | Episomal | Episomal (>90%), site-specific integration (<10%) | Integrated | Integrated | Episomal | Episomal or integrated |
| Inflammatory potential | High (low for HC-AdVs) | Low | Low | Low | High | High |
| Advantages | High titers; extremely efficient transduction of most cell types and tissues | Safe transgene delivery; non-inflammatory; non-pathogenic | Persistent gene transfer in dividing cells | Persistent gene transfer in most tissues | Large packaging capacity; strong tropism for neuronal cells | Large cargo sizes; high level of gene expression |
| Drawbacks | Capsid mediates a potent inflammatory response (eliminated in HC-AdVs) | Small packaging capacity; requiring helper AdV for replication and difficult to produce pure viral stocks | Only transduces dividing cells; integration might induce oncogenesis in some applications | Integration might induce oncogenesis in some applications | Inflammatory; no expression during latent infection; transient gene expression in non-neuronal cells | Limited mammalian host range |
While viral vector techniques make use of the natural aptitude of viruses to efficiently deliver genes directly into target cells, these applications do not come without potential for adverse side effects (Table 1). Namely, the use of viruses often present risk for marked immunogenic response, insertional mutagenesis, and cytotoxicity. Furthermore, the transgenic capacities of most viral vectors are largely limited in size.10, 11 On the other hand, though non-viral systems do not exhibit immunotoxic concerns nor significantly constrain transgene size, they are generally far less efficient in delivering transgenes to a target cell.12, 13
Despite great strides in gene delivery innovation over the last 20 years, no single class of gene delivery system has been developed without falling prey to one of these major limitations or potential adverse side effects.14, 15, 16, 17 As such, adjunct techniques, like those of episomal vectors and transposon-based vectors, may be applied in combination with larger gene delivery systems to overcome such obstacles. These complementary techniques may be tailored in combination to enhance stable gene expression and efficient gene transfer; or alternatively, used stand-alone to mitigate side effects and improve the overall safety profile of a system.
Non-viral episomal vectors for safe and persistent gene delivery
The use of episomal vectors provides yet another system for gene delivery with its own set of benefits and limitations. Since these vectors remain extrachromosomal without direct integration into the host genome, they spare host cells the potential side effects of insertional mutagenesis.18, 19, 20, 21 And though the immunogenicity of these systems can be greatly decreased by excluding viral integration, non-viral episomal vectors are generally inefficient transfection agents – much like stand-alone transposon-based systems.21 Transgenes delivered via episomal plasmids classically exhibit transient rates of expression; similar to AdVs and AAV vectors.15 However, recent advances have provided options to circumvent this major drawback by constructing episomal vectors that incorporate genetic material from viruses like the BK virus (BKV), Epstein–Barr Virus (EBV), Bovine Papilloma Virus-1 (BPV-1), and Simian Virus 40 (SV40).21, 22 These viruses, and consequently their derivative episomal constructs, contain trans-activating factors that can be used to uncouple transgene replication from the regulatory mechanisms of the host cell to establish an autonomous ‘episomal replicon’.21, 23, 24
One of the most studied episomal vector systems integrates the SV40 large T-antigen, an oncoprotein and replicative helicase, to confer amplified and sustained transgene expression.25, 26, 27 Similarly, the BKV T antigen has also been used in studies as a trans-activating factor to induce rapid transgene amplification.28 Yet the broad range of interaction exhibited by these trans-acting factors, particularly their inhibition of the p53 tumor-suppressor protein, make them too volatile for application in human gene therapy.21, 28
Non-viral DNA transposons for efficient long-term and footprint-less gene delivery
DNA transposons represent a powerful emerging tool for gene delivery that is unencumbered by many of the toxic and undesirable side effects that burden viral vector modalities.29, 30, 31, 32 Furthermore, most transposon systems are non-immunogenic and are able to deliver transgene cargos between 9 and 10 kb in size for Sleeping Beauty or up to 100 kb for piggyBac transposon.31 Transposon-based systems utilize transposase enzymes to mediate a ‘cut-and-paste’ transfer of transgenes from vector to host genome.33 The transposon vector, carrying a transgene cassette between unique flanking DNA sequences, is commonly delivered to the cell in the form of a circular DNA plasmid.31, 34 Cellular uptake of plasmid molecules by non-viral techniques are far less efficient than that of viral vector techniques; which marks one of the largest limitations faced by stand-alone transposon-based delivery systems.31, 35, 36, 37, 38 However, once inside the cell, transposon systems enable direct integration of transgenes into the host genome with significant reliability.31, 36, 38 We demonstrated that the piggyBac transposon-mediated gene expression is more efficient than that of retroviral vector transduced cells.39
The benefits conferred by direct genomic integration toward the stability and longevity of transgene expression have been described above. Transposons used in mammalian hosts are considered non-autonomous systems. Here, the transposase enzyme must be supplied by a secondary source, since functional transposase enzymes are not inherent to vertebrates.36, 40 Such a non-autonomous system prevents the transposon from independently continuing to excise and re-integrate itself in a haphazard fashion, largely diminishing the risk of oncogenic mutagenesis.36, 37, 40 Given their relatively simple design, composed almost entirely of DNA, the production costs of transposons are considerably lower than those of viral vector systems.29, 31, 38
Lentiviral vectors for stable and long-term gene transfer
The lentivirus is unique among retroviruses given its ability to transduce non-replicating cells in addition to replicating cells.9, 41 As a retrovirus, however, lentiviral vectors contain RNA gene products that must undergo processing by reverse transcriptase enzymes to produce double-stranded DNA proviral transgenes. Most lentiviruses are generally apathogenic in humans and therefore exhibit low levels of immunogenicity.42 The lentiviral vector capacity, at 8 kb, is small like that of AAV vectors. Though dissimilar to AAV vectors and AdVs, lentiviral vectors ultimately integrate transgenes into the host genome through the activity of retroviral integrase enzymes.43, 44 This integration of transgenes into the host genome presents a double-edged sword when considering the efficiency and safety of lentiviral vectors in comparison to AdV and AAV vectors.35
While genomic integration provides the benefits of stable transgene expression in addition to continued expression among daughter cells, the risk of inactivating tumor-suppressor genes, activating oncogenes, or otherwise altering various other vital regulatory genes (a phenomenon termed “insertional mutagenesis”) depends on the site of integration and may potentially lead to cancer.44, 45, 46, 47, 48, 49, 50, 51, 52 The precise integration site of transgenes delivered by lentiviral vectors, though non-random, is more or less unpredictable.53, 54, 55 As such, concerns regarding the development of cancer by insertional mutagenesis certainly exist when considering the use of lentiviral vectors in gene therapy.
Lentiviruses have been found to demonstrate strong tropism for neural stem cells and have been studied for their utility in delivering genes within CNS tissues ex vivo.43, 44 Accordingly, numerous groups have applied lentiviral vectors within animal models to study potential therapies for Parkinson Disease, Alzheimer Disease, and Huntington's Disease.56, 57, 58, 59, 60, 61 Various methods for modifying and pseudotyping lentiviral vectors to enhance their versatility and specificity among certain cell types, including hematopoietic precursors, neurons, lymphoid cells, and macrophages, have also been reported.1, 62, 63, 64, 65 Alternative studies seeking to enhance transgene expression in a cell-specific manner have incorporated tissue- and cell-specific promoters within transgene constructs and coupled their delivery to a diversity of viral vectors (including AdV, lentivirus, AAV, and HSV).
Adenovirus-associated virus (AAV) for safe and small cargo gene delivery
Adeno-associated virus (AAV) is a small, helper-dependent, single-stranded DNA virus capable of transducing both dividing and non-dividing cells by delivering a predominantly episomal transgene product.66, 67, 68, 69, 70, 71 In comparison to AdVs, AAV vectors provide a safer option for transduction given their naturally diminished pathogenicity and immunogenicity in humans.9, 72 AAV vectors have been found to display the potential for host genome integration. However, in these rare instances, they will reliably integrate in a site-specific manner to the q arm of human chromosome 19 – where expression remains silent unless the AAV lytic cycle is induced by a helper virus.1, 62, 73, 74 Such site-specificity further bolsters the safety profile of AAV vectors.
One considerable disadvantage of AAV vectors is their small transgene capacity of approximately 4.8 kb, which severely restricts the breadth of therapeutic genes that may be delivered via AAV vector.9, 35, 62, 75 AAVs have been studied in the treatment of Hemophilia B, Leber's Congenital Amaurosis, Alpha-1 Antitrypsin Deficiency, and Cystic Fibrosis.72, 76, 77, 78 Surprisingly, some AAV vectors, particularly vectors derived from the AAV9 serotype, have been found to cross the blood–brain barrier and proved capable of transducing cells of the CNS.79, 80, 81, 82, 83 Such breakthroughs hold great clinical promise in developing novel therapeutic options for diseases of the brain and spinal cord.
Herpes simplex virus (HSV) vectors for neural tissue-specific and large cargo gene delivery
The Herpes Simplex Virus (HSV) is a double-stranded DNA virus capable of delivering up to 50 kbp of transgenic DNA when used as a vector; making HSV one of the largest viral vectors under routine study.62, 84, 85, 86 Similar to the adenovirus, pre-existing immunity to HSV infection is prevalent within the general population; however, HSV vectors are largely able to evade inactivation by host immune response.68, 69, 84, 85, 87 HSV vector genomes also remain episomal like those of AdVs. As a result, they are expectedly burdened by the same limitations of transient expression faced by AdVs as discussed above.62, 88 If an HSV vector co-infects a cell with an underlying latent HSV infection, the opportunity for genetic recombination between the vector and wild-type genomes also presents a considerable safety concern.89, 90
One of the most striking characteristics of HSV vectors is their natural tropism for neuronal cells, which contributes to their great therapeutic potential in delivering genes in the selective treatment of nervous system disorders.91, 92 A recent study by Goss et al has evaluated HSV vectors in targeting sensory neurons to express pain reliever genes in patients with chronic pain disorders.93 Though early HSV vectors were limited to transduction of non-dividing cells, modifications have been recently developed to produce replication-conditional HSV vectors that demonstrate significant toxicity within proliferating tumors.94 The neurotropism of HSV vectors in combination with these oncolytic properties has proved valuable in targeting tumors and cancerous cells of neuronal origin.92, 95, 96, 97, 98, 99 Studies have demonstrated the successful used HSV vectors against gliomas, glioblastomas, melanoma and other solid tumors.88, 91, 100, 101
Adenoviral vectors as one of the most efficient gene delivery systems
One of the most salient features of the adenovirus is its prevalence among healthy individuals. As such, a broad pre-existing immunity against Adenovirus is common in the general population.102 Such immunity largely hinders the utility of AdVs derived from the most prevalent serotypes of adenovirus.62 Therefore, investigators have focused on using the rare serotypes 2 and 5 to construct AdVs for gene therapy.35, 42, 103 Of additional concern, the proteins of all AdVs exhibit high immunogenicity. As a result, repeat administrations of AdVs within a single patient are severely limited to prevent risk anaphylactic shock or death.62, 104, 105, 106, 107, 108 Interestingly, the strong immunogenicity of AdVs makes them ideal candidates for applications in oncolysis and vaccination.35, 109, 110 AdVs can be used to trigger antitumoral immunity within the tumor microenvironment,110, 111 and have been studied in the development of vaccines against HIV and influenza, among other pandemic diseases.62, 103, 112
Adenoviral vectors are able to transduce both replicating and quiescent cell populations, making them a valuable tool in delivering transgenes in vivo and within mature tissues.62 In contrast to lentiviral vectors, AdVs are able to deliver larger transgenes up to 8 kbp in size; however, their DNA does not integrate into the host genome, but rather, resides episomally in the host nucleus.113, 114 Such episomal transduction precludes the risks of insertional mutagenesis, without direct integration into the host genome although transgene expression is transient, is vulnerable to cell silencing mechanisms, and is destined for dilution among daughter cells should cell division ensue.62
Nonetheless, it is clear that no single vector system outlined above presents a ubiquitously ideal method for achieving gene delivery across all applications. However, a thorough review of the advantages and limitations characteristic of each modality, with an eye for integrating techniques, enables investigators to devise a highly modulatory strategy to approach the task at hand. By studying these vectors within novel and innovative applications, we may realize the full therapeutic promise these techniques hold for patients. Here, we only focus on the characteristics of adenoviral vector-mediated gene transfer.
The characteristics of adenovirus
The adenoviral genomes are linear, non-segmented double-stranded DNA with sizes ranging from 26 kb to 45 kb in length, depending on the serotype. The genome of the commonly-used human adenovirus type 5 (Ad5) is approximately 36 kb (Fig. 1).3 The DNA is flanked on both ends by hair-pin-like, inverted terminal repeats (ITR), which serve a variety of purposes. One of the roles of ITRs is to act as a self-primer to promote primase-independent DNA synthesis, making them important elements in DNA multiplication. Another function of the ITRs is to facilitate integration into the host genome (Fig. 1). In addition to ITRs, another genetic element of the adenovirus is the packaging signal, which is located on the left arm of the genome and is required for proper viral transcript packaging. Viral transcripts are classified as either early or late. The four early transcriptional units, E1, E2, E3, and E4, are responsible for expressing non-structural proteins (Fig. 1). These proteins have regulatory functions and are involved in viral DNA replication. The late proteins encode for structural components of the Ad virion (Fig. 2A and B).
Figure 1.
The genome structure and major transcript units of human Ad5. The Ad5 genome is composed of 36 kb linear double-stranded DNA, also shown in map units (mu). The Ad5 genes are temporally transcribed as early units (E1, E2, E3 and E4 units) or late unit (L1 to L5) in both directions. The top part of the listed genes are transcribed from left to right, while the lower part of the genes are transcribed from right to left, as indicated by the dotted arrows. E1 gene products are involved in the replication of the virus. The E2 region is sub-divided into E2A and E2B. These proteins provide the machinery for viral DNA replication and the ensuing transcription of late genes, which mostly encode structural proteins for virus packaging. Most of the E3 proteins are involved in modulating the immune response of infected cells and are not essential for viral production in vitro.
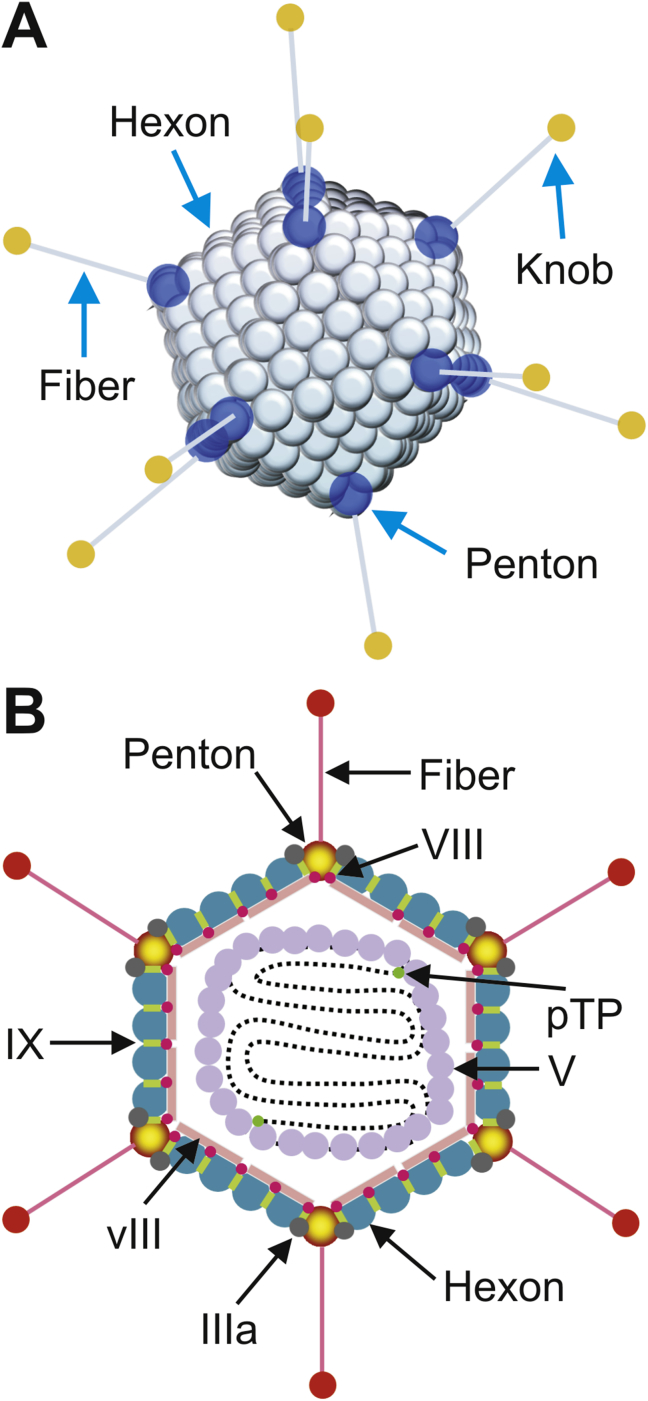
Figure 2.
The structure of adenovirus. (A) Adenovirus is a large, non-enveloped virus presenting icosahedral symmetry. The hexon, penton base, and knobbed fiber, are the most important capsid proteins for gene delivery. (B) Hexon is the major protein forming the 20 triangular faces of the viral capsid. The 240 hexon capsomers in the capsid are trimers, each interacting with six other trimers. The 12 vertices are formed by the penton capsomere, a complex of five copies of the penton base, and three copies of fiber. Each penton capsomere interacts with five hexon capsomeres, one from each of the five faces that converge at the vertex. The knobbed fiber protrudes from the fiber base. In addition, the 5′ termini of adenovirus genome bind covalently to the precursor terminal protein (pTP). The viral genome DNA is wrapped in a histone-like protein and contains the inverted terminal repeats (ITRs), which act as origins of replication.
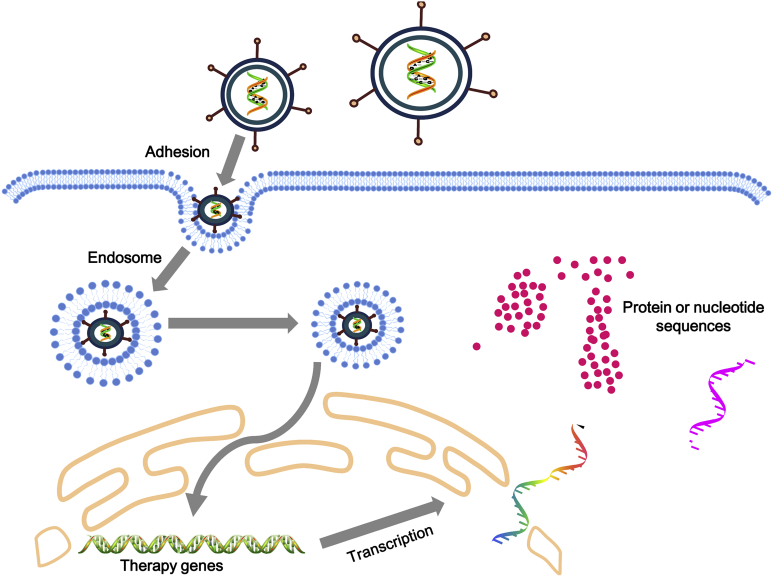
The adenovirus virion has unique viral structure: 90–100 nm in size, non-enveloped, icosahedral particle containing a nucleocapsid (Fig. 2A and B). The viral capsid consists of 252 proteins, involving three distinct types: fiber, penton and hexon based proteins. There are 240 hexon proteins and 12 penton proteins (Fig. 2A and B). Both fiber and penton base proteins are key in receptor binding and internalization, while hexon composes most of the viral capsid. The “spikes” or fibers are associated with each penton base protein of the capsid and facilitates attachment to host cell receptors, including the coxsackie-Ad receptor (CAR) (Fig. 3). After endocytosis, the Ad capsid escapes from the endosome and reaches the nuclear pores via microtubule-mediated translocation (Fig. 3). In nature, wild-type AdVs do not integrate their genomes into host cell chromosomes. With a few exceptions, they replicate as linear, extra-chromosomal DNA elements in the nucleus. After DNA replication, the Ad genomes are packaged into the newly assembled capsids in the nucleus of the infected cell. The release of Ad progeny requires the lysis of the infected cell.
Figure 3.
Adenovirus-mediated gene delivery to mammalian cells. The initial step of adenovirus infection is gaining access to the host cell, and the 12 spikes of the capsid are adhesion receptors, which recognize and bind to specific glycoprotein receptors, such as CAR on the target cell membrane, with the irreversible binding of cell-surface integrins to the penton at the base of the spike. This leads to endocytosis, and the cell-surface membrane invaginates, forming a pit. This pit invaginates and pinches off as a vesicle in the cytoplasm, coated by clathrin and containing the virus. The vesicles are sent to endosome. When the endosome becomes more acidic, the virus is uncoated as the outer capsid disassembles, revealing the viral DNA-protein core. The shed spikes have a toxic function and breach the endosome membrane, allowing the viral core to escape from the endosome into the cytosol of the host cell. Subsequently, the transgenes or nucleic acids are expressed in the target cells.
The evolution of three generations of adenoviral vectors
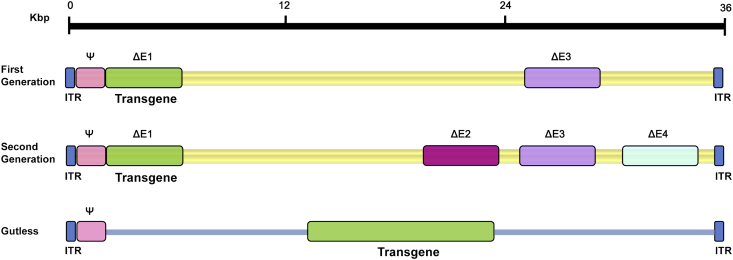
The use of adenoviral vectors (AdVs, mostly derived from human AdV5) has become more efficient with time and experience. To establish safety in use, the first improvements were made by eliminating essential viral-replication genes (Fig. 4). First generation AdVs are those stripped of regulatory genes E1a and E1b – the first transcriptional regulatory factors to be produced during the viral life cycle. The depletion of this gene resulted in replication-deficient AdV with an initial transgene cloning capacity of 5.2 kb.5 Looking to improve, researchers wanted to increase cloning capacity and continue to decrease capacity for viral replication. Thus, second generation AdVs describe vectors with deletion of other non-structural genes (E2/E3/E4) in addition to the original E1 gene absent in first generation vectors (Fig. 4). While the second generation of vectors demonstrated increased cloning capacity and reduced cytotoxicity, they still triggered immune responses in vivo resulting in the reduced yields of transduced cells.5 Born from this struggle to develop less immunogenic vectors, a third generation of AdVs emerged, termed high-capacity adenoviral vectors (HC-AdVs), also known as gutless AdVs or helper-dependent AdVs (HD-AdVs) (Fig. 4). The HC-AdV is stripped of all viral coding sequences, resulting in a vector with only 5′ and 3′ ITRs in addition to a packaging signal, thus providing a larger capacity for transgenic cloning sequences (36 kb). Because of this structure, they are often referred to as “gutless adenoviral vectors.” Furthermore, the structure of the HC-AdV minimizes cytotoxicity, thus enabling prolonged expression of therapeutic genes, rendering the HC-AdV the most promising AdV to use for gene therapy to date.
Figure 4.
Three generations of adenoviral vectors. The first generation vectors are deficient in the E1 and E3 regions and have a maximum capacity of 8.2 kb for introducing of a therapeutic gene. The second generation vectors are deficient in E2 and E4, in addition to the deletion of the E1 and E3 regions. Gutless Ad vectors, also known as helper-dependent adenoviruses (HD-Ad), or high-capacity adenoviruses (HC-Ad), can be created to prevent the problem of Ad-created cellular immune response. HD-AdVs are helper-dependent because they depend on a helper adenovirus to be able to produce. HD-AdVs are of high capacity because they allow insertions of up to 36 Kb. Gutless Ad maintain only the 5′ and 3′ ITRs and the packaging signal (Ψ), an essential for final assembly of the virion.
High-capacity adenoviral vectors (HC-AdVs) are beneficial because they lack the viral elements that can cause an immune response in the host. HC-AdV's are deemed “helper-dependent” because while these vectors lack viral genetic components, they also lack the necessary packaging components. A complementary virus, or helper virus (HV), is used to provide the necessary proteins in trans for the packaging of an Ad-based vector.115 The HV is not packaged along with the desired HC-AdV because it has its packaging sequences flanked by loxP recognition sites, which is sufficiently excised by Cre recombinase so that the helper virus DNA remains unpackaged. While the packaging ability of the HV is stunted, the HV is replicated at normal levels and can thus express all of the functions necessary in trans for replication and packaging of a vector genome containing the appropriate cis-acting elements.
Optimization of the delivery system is essential for its efficiency as a gene therapy tool because HC-AdV is often needed in large quantities. Large-scale production of HC-Ad via HVs can be optimized through modification of HV with “stuffer” sequences to enhance growth characteristics. Such stuffer sequences help to optimize HC-AdV replication, increase the total production of HC-AdVs, and reduce the incidence of HV contamination.116, 117
The HC-Ad system relies on minimal HV contamination. Another way to minimize contamination is by alternative methods of selection against the HV. The traditional method is through a Cre/loxP system, but Ng described a FLP/FRT system that was shown to be equivalent with respect to HC-AdV amplification efficiency and HV contamination levels.118 This provides scientists with a second recombinase system for HC-AdV production, which will enhance the utility and flexibility of HC-AdVs. The Cre/loxP system requires final, density-based separation of HC-AdV and residual HV by ultracentrifugation to reduce contamination levels.119 This step hinders the optimization of large-scale production. FLP recombinase has shown to mediate maximum levels of excision (about 100% versus 80% for Cre) and, without any purification, showed the same levels of residual contamination as Cre after ultracentrifugation, rendering the FLP/FRT system as a promising strategy in optimizing HC-Ad production.
Approaches to the efficient productions of recombinant adenoviral vectors
Although the use of recombinant adenoviruses has been widespread, the initial generation of a given adenoviral vector (mostly the first generation AdVs) remains a technically challenging and time-consuming process.1, 3, 120, 121 In the past decades, numerous efforts were devoted to the development of techniques for rapid and efficient generation of recombinant AdVs.1, 3, 121, 122, 123, 124, 125
The classical method for generating E1-deleted AdVs utilizes homologous recombination between two DNA vectors, one carrying sequences mapping to the very left end of the adenovirus genome and the gene of interest, and the other carrying sequences that slightly overlap the 3′-most viral sequences on the first molecule and continue to the right end of the adenovirus genome.123, 124 This recombination takes place inside E1-expressing cells such as HEK-293 cells, generating the desired recombinant viral DNA (Fig. 5A). While functional, this approach is rarely used now because its AdV generation process is extremely inefficient. An early alternative to this method involved an in vitro direct ligation of the transgene-containing fragment to the linear E1/E3-deleted adenoviral genome DNA through a restriction enzyme site, such as ClaI site.126 However, due to the large size of Ad genome, unique and useful restriction sites are limited, making the construction of Ad vectors relatively labor intensive. Nonetheless, the direct ligation approach was significantly improved when additional unique restriction sites, such as I-CeuI, SwaI and PI-SceI were engineered and added to the E1 deletion region.127 However, this ligation approach is not commonly used because the efficiency is low and the recombinant virus often requires purification of contaminating wild-type and transgene-null viruses.
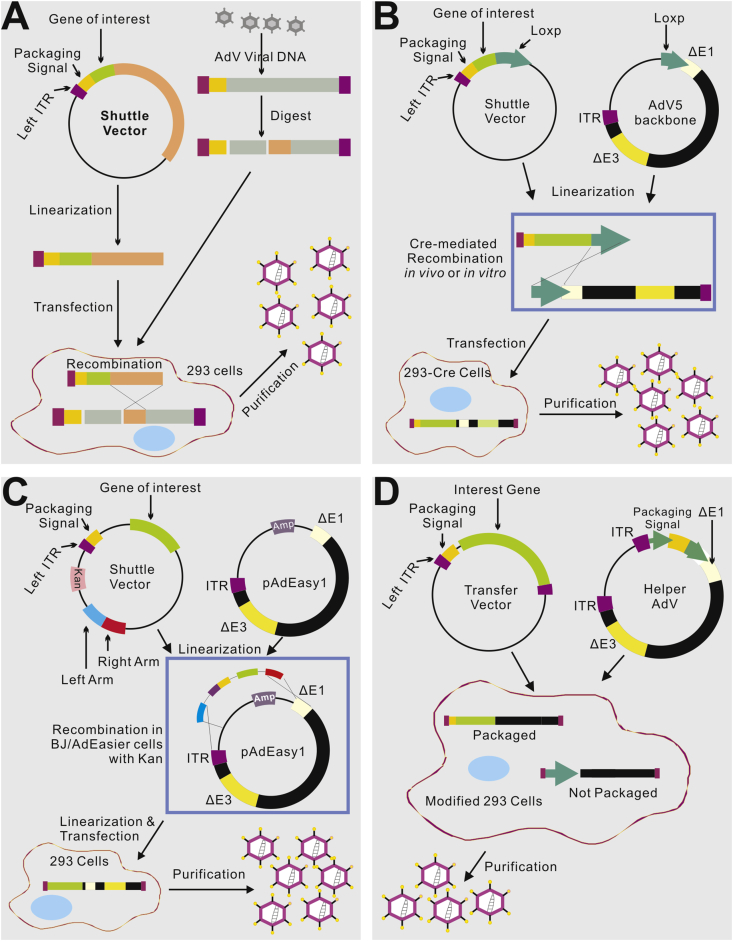
Figure 5.
Four commonly-used methods to generate and produce adenovirus vectors for gene delivery. (A) The traditional method – recombination in HEK-293 cells. The gene of interest (GOI) is first cloned into a shuttle vector, which contains 5′-ITR, packaging signal and homologous regions to adenoviral genome. Adenoviruses are generated in HEK-293 cells through recombination between shuttle vector and adenoviral backbone vector, which is unable to produce virus by its self. (B) Cre/LoxP-mediated recombination. The GOI is cloned into a shuttle vector that contains LoxP site(s). Cre recombinase-mediated recombination occurs with a LoxP-containing adenoviral backbone vector in vitro or 293-Cre cells, leading to the generation of adenoviruses. (C) The AdEasy system. The GOI is subcloned into a shuttle vector that contains 5′-ITR and packaging signal, as well as a kanamycin-containing bacterial replication unit flanked with homologous arms. Recombinant adenoviral plasmids are generated through homologous recombination between the linearized shuttle vector and ampicillin-resistant adenoviral backbone vector, such as pAdEasy1, in the bacterial strain BJ5183 cells under kanamycin selection. The resultant adenoviral plasmids are linearized and used for adenovirus production in HEK-293 cells. (D) The use of helper adenovirus for the production of HC-AdVs (or HD-AdVs, or Gutless AdVs). The GOI is cloned into a transfer vector that contains both ITRs and packaging signal only. Adenoviruses are generated with a helper adenovirus, which will not be packaged due to the deletion of packaging signal in the modified HEK-293 cells, usually through Cre/LoxP or FLP/FRT excision system.
To overcome the low efficiency of homologous recombination in HEK-293 cells, the Cre-lox site-specific recombination system was later developed to generate AdVs (Fig. 5B).128, 129 This system requires three components (1) a recombinant adenovirus that contains two loxP sites flanking the packaging signal; (2) a shuttle vector which contains the left ITR, the packaging signal, the expression cassette and a loxP site; and (3) a 293-Cre cell line which expresses the Cre recombinase (Fig. 5B). When the shuttle vector carrying the gene of interest and the viral DNA are co-transfected into the 293-Cre cells, an intramolecular recombination occurs between the two loxP sites present in the viral DNA to generate an adenovirus genome which is able to replicate and express viral genes, but is unable to be packaged. A second recombination occurs between the loxP site of this product and that on the shuttle vector, generating the desired recombinant virus (Fig. 5B). A similar method using FLIP/FRT system was later developed.118 The major disadvantage of this method is the presence of the parental AdV in the preparation, which is difficult to completely remove from the viral preparations even after multiple passages in 293-Cre cells. Thus, this method requires a careful verification of the identity of the recombinant virus.
One of the most popular methods to overcome the inefficiency of homologous recombination in HEK-293 cells is to take advantage of the highly efficient homologous recombination in microorganisms, such as yeast and bacterial cells.121, 122, 123, 124, 125, 130, 131, 132 As a result, AdV generation has been drastically facilitated by the techniques exploiting the efficient homologous recombination in bacterial cells. Chartier et al developed a system in which the large adenovirus plasmid is linearized in the E1 or near the E3 region. This DNA molecule is introduced into a RecA+ E. coli strain together with a DNA fragment containing the gene of interest flanked by E1 or E3 sequences. Recombination between both DNA molecules generates a plasmid containing the expression cassette in the desired region.131 Alternatively, a co-integrate plasmid is first obtained by recombination between a large adenovirus plasmid and a small shuttle vector that contains the gene of interest and is selected for in the presence of two antibiotics. The final recombinant plasmid is generated via a second homologous recombination event that excises it from the co-integrated B. subtilis sacB sequences, which encodes a protein that kills the bacterium in the presence of sucrose.132
We further improved the bacterial recombination system and developed the highly simplified and more user-friendly AdEasy system (Fig. 5C).121, 122, 125 Our system was designed to introduce expression cassettes specifically into the E1 region and thus facilitate the recovery of the recombinant E. coli clones. Specifically, the large adenovirus plasmid is introduced into E. coli BJ5183 cells as a supercoiled DNA in order to increase transformation efficiency, and a kanamycin-resistance gene, provided by the shuttle vector containing the expression cassette, is used to select the recombinant clones. The recombinant plasmid DNA is purified, and the viral chromosome is released by restriction digestion and transfected into the appropriate cell line.122, 125 In the AdEasy system, the backbone vector, containing most of the adenoviral genome, is used in supercoiled form, obviating the need to enzymatically manipulate it. The recombination is performed in E. coli rather than in mammalian cells, as the process takes advantage of the highly efficient homologous recombination machinery present in bacteria. We also engineered multiple vectors to allow inclusion of up to 10 kb of transgene sequences and/or multiple transgenes to be produced from the same virus. These modifications resulted in highly efficient viral production systems that can often obviate the need for plaque purification and can significantly decrease the time required to generate usable viruses. In fact, the AdEasy has been one of the most commonly-used AdV making systems worldwide.122, 125
While the generation of HC-AdVs or helper-dependent AdVs is more straightforward, the technical challenge is to remove contaminating helper AdVs from the final adenoviral preparations and to obtain relatively pure HC-AdVs. One of the most efficient methods for producing HC-AdV is the use of Cre/loxP system (Fig. 5D).115 The HC-AdV genome contains the adenoviral ITRs and ψ packaging signal and a noncoding eukaryotic “stuffer” DNA fragment that is needed to bring the vector genome size within the size range (27.7 kb–37 kb) for efficient packaging into virions.115 On the other hand, the helper virus (HV) is E1-deleted and bears a packaging signal flanked by loxP sites. Following infection of 293-Cre cells, this allows the packaging signal to be excised from the HV genome, rendering the HV genome unpackageable but still able to undergo DNA replication and thus complement the replication and encapsidation of the HC-AdV genome in trans.115 Alternative HC-AdV production systems based on FLP/FRT site-specific recombination system have also been developed and similar outcomes were obtained.118, 133 While packaging signal excision was relatively efficient in either the Cre/LoxP or FLP/FRT system, it was not complete, leading to HV contamination, albeit at low levels.129 Further studies showed significant reduction in HV contamination was achieved using producer cells that expressed much higher levels of Cre.129 Because the genome size of the HC-AdV (∼30 kb) and the HV (∼36 kb) are engineered to be different, they can be physically separated by CsCl ultracentrifugation which provided an additional method to purify the HC-AdV from residual HV. Nonetheless, removing residual HV remains the top priority regarding the production and use of HC-AdVs.
It is noteworthy that, while critical to develop techniques for efficient AdV production, the improvement of AdV infection efficiency and packaging efficiency should also significantly benefit the AdV production process. We found that adenovirus-mediated gene transfer in mesenchymal stem cells can be significantly enhanced by the cationic polymer polybrene.134 Furthermore, we have demonstrated that overexpression of Ad5 precursor terminal protein (pTP) accelerates recombinant adenovirus packaging and amplification in HEK-293 packaging cells.135 More recently, we have engineered the RAPA cell line for rapid adenovirus production and amplification.136 By assessing human Ad5 genes (E1A, E1B19K/55K, pTP, DBP, and DNA Pol) and host factor OCT1 for their contributions to adenovirus production, we demonstrated that overexpression of both E1A and pTP led to a significant increase in adenovirus amplification, whereas other transgene combinations did not significantly affect adenovirus amplification. When E1A and pTP were stably expressed in 293T cells, the resultant RAPA line showed high efficiency in adenovirus amplification and production.136 Furthermore, adenovirus production efficiency in RAPA cells was dependent on the amount of transfected DNA. Under optimal transfection conditions high-titer adenoviruses were obtained within 5 days of transfection.136 Therefore, a combination of using the AdEasy system and the RAPA cell line should significantly expedite AdV production.
Adenovirus-mediated gene therapy for monogenic diseases
The first in vivo application of AdVs for genetic therapy in humans was reported in 1992 with the successful transfer and expression of alpha-1 antitrypsin (A1AT) cDNA within hepatocytes of a patient with alpha-1 antitrypsin deficiency.137 Simultaneously, A1AT gene transfer methods were under development in native lung tissues using an E1−E3− serotype 5 adenoviral vector.2 Soon thereafter, with the identification and characterization of the CFTR gene underlying cystic fibrosis, and the known tropism of the adenovirus for respiratory epithelium, studies investigating the transduction of CFTR cDNA via AdV in humans were soon underway.138 With each subsequent administration, investigators came to recognize that a response by the patients' humoral and cellular immunity to adenoviral vectors was responsible for this transient, 1–2 week expression of AdV transgenes, while also leading to adverse immunogenic events in other patients.139, 140 The death of one patient under experimental treatment for ornithine-transcarbamylase deficiency using an AdV vector in 1999,107 followed by the development of leukemia in two patients with X-linked severe combined immunodeficiency in 2002, safety concerns surrounding the application of viral vectors in human subjects lead to a sharp decline in studies over the next decade.52, 73 Much of the research focus in that time then shifted toward developing less immunogenic viral vectors. Hence, the utility of AAV and lentiviral vectors emerged.141
Despite these initial setbacks, AdVs have returned to the spotlight of human gene therapy in recent years. Adenoviral vectors now represent the most commonly studied viral vectors among clinical trials currently underway worldwide (Table 2).142 The potent immunogenic properties that once undermined the use of AdVs in sustained treatment of genetic deficiencies have since been leveraged to purposefully evoke host immunity, thereby highlighting the adenovirus as an ideal vaccine carrier and antitumoral agent.
Table 2.
The registered gene therapy clinical trials.
| Vector system | Number of registered trials | % |
|---|---|---|
| Adenovirus | 505 | 21 |
| Adeno-associated virus | 173 | 7.2 |
| Retrovirus | 449 | 18.6 |
| Naked/Plasmid DNA | 414 | 17.2 |
| Lentivirus | 144 | 6 |
| Vaccinia Virus | 125 | 5.2 |
| Herpes Simplex Virus | 89 | 3.7 |
| Poxvirus | 70 | 2.9 |
| Other | 440 | 18 |
| Total (as of March 2017) | 2409 | |
Note: Vectors used in clinical gene therapy trials registered with the international gene therapy clinical trial registry up to March 2017.
Source: Gene Therapy Clinical Trials Worldwide (provided by the Journal of Gene Medicine); http://www.wiley.co.uk/genmed/clinical/.
Adenoviral vectors as anticancer agents
There are three main therapeutic categories to which AdVs are applied as anticancer therapies. In the first, replication-defective (RD) AdVs are used for their immunogenic properties to deliver immune-related genes/epitopes directly to tumor cells in order to attract and induce a local antitumoral immune response.143, 144, 145, 146, 147, 148, 149, 150 In the second category of strategies, replication-competent (RC) AdVs (also called conditionally replicative Ad, CRAds; or oncolytic AdVs) may be used to preferentially replicate within cancer cells and achieve oncolysis by executing the natural lytic life cycle of the virus in cancer cells (Fig. 6).2, 151 Lastly, either RD or RC AdVs may be used to deliver and/or overexpress tumor-suppressor genes or cytotoxic/suicide genes within cancerous cells to directly induce an intrinsic cytotoxic cascade, cause cell cycle arrest, or trigger apoptosis.151, 152
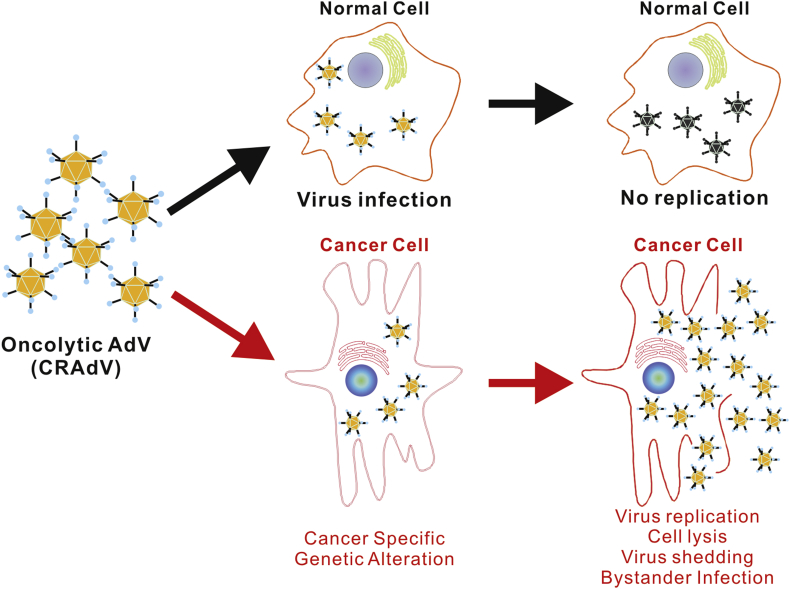
Figure 6.
The anticancer effect of oncolytic adenoviruses (CRAdVs). The oncolytic adenoviruses infect both normal and cancer cells with similar efficiency. In cancer cells cancer-specific genetic changes activate the expression of Ad5 E1 genes, which initiates viral replication cascade, leading to virus production and cancer cell lysis. The packaged viruses are released and infect neighboring cancer cells to achieve bystander killing effect. However, normal cells lack the cancer-specific activation of viral replication, and thus no Ad5 viruses are reproduced. As the result, normal cells are spared by oncolytic adenoviruses.
Two RD adenoviral vector systems, Advexin and Gendicine, have produced considerable data among a number of clinical trials. Advexin is an E1−E3− Ad5 vector expressing p53 from a Cytomegalovirus (CMV) promoter, while Gendicine is very similar except that it expresses p53 from a Rous Sarcoma Virus promoter.151, 152 In numerous studies, including phase I and II trials, Advexin was judged to be safe within the settings of colorectal cancer, hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC), prostate cancer, breast cancer, ovarian cancer, bladder cancer, glioma, and squamous cell carcinoma of the head and neck (HNSCC).153, 154, 155, 156 In a phase III trial comparing Advexin vs. Advexin + Methotrexate in the treatment of recurrent HNSCC, this vector system proved to be well tolerated with evidence of antitumoral activity.157 In October of 2003, the State Food and Drug Administration of China approved Gendicine for its use in combination with chemotherapy to treat HNSCC, making Gendicine the first commercially available gene therapy vector in the world.152, 158, 159, 160, 161 More than 7000 patients have been treated with Gendicine while post-market studies have reported extremely low rates of vector shedding and a mild side effect profile, even after intravascular administration.158, 159, 162 Gendicine has since been further studied in 16 controlled clinical trials in China for the treatment of advanced and unresectable cancers, including HCC, NSCLC, malignant glioma, and epithelial ovarian carcinoma.64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79 Even among patients who failed conventional chemotherapy/radiation, Gendicine still displays synergistic effects in combination with these therapies while overall demonstrating better response and survival rates than those of control groups.65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83
The advantages of RC AdVs in targeted cancer therapy become evident given that cancer cells are generally more conducive to Ad replication than quiescent or non-cancerous cells.163 Furthermore, additional cancer-targeting modifications have been developed to support efficient oncolytic replication of AdVs in cancer cells while limiting their replication in healthy cells.151, 164 In this way, many conditionally-replicative AdVs have been developed to target high-replicating cell populations or cell populations with specific oncogenic mutations.165 In 1996, ONYX-015 became the first replication-selective AdV to enter clinical trials.166 With a partial E1B gene deficiency that inhibits the replication of ONYX-015 in healthy cells expressing the p53 tumor suppressor, this oncolytic AdV exclusively replicates and amplifies in p53-deficient cancer cells.73, 167, 168 In phase I and II trials, ONYX-015 has demonstrated safety and tolerance when administered via intratumoral, intravascular, or intraperitoneal injection in patients with various advanced cancers.166, 169 Furthermore, available results have since shown ONYX-015 is most effective in conjunction with conventional chemotherapy.168, 170 In November of 2005, the replication-selective AdV, Oncorine (or “H101”), was approved in China for the treatment of cancer.151, 161 Similarly, this oncolytic AdV also possesses a 55 kb E1B deletion, and has demonstrated significantly higher response rates in combination with traditional chemotherapy when compared to chemotherapy alone.171
In light of such promising findings, numerous other clinical trials are currently underway to evaluate newer generations of oncolytic AdVs.73, 151, 172, 173, 174 These newer generations incorporate suicide genes (such as HSV thymidine kinase)175 or cytotoxic pro-drug converting properties under the control of tissue or tumor-specific promoters (like the promoter of the prostate antigen PSA)176, 177, 178 to further enhance the efficacy, potency, and specificity of these vector systems.179, 180 Additionally, advances in our understanding of adenoviral capsid antigens and immunogenic properties will undoubtedly reveal a broadened versatility of these vectors in vaccination efforts. Though current AdV systems have achieved some notable clinical successes, many challenges still largely impede the widespread use of AdVs in human gene therapy. Improvements in clinical efficacy and safety are needed to bring these innovations from the bench to the bedside, and thus require continued studies and trials despite their notorious debut to the clinical arena.
Adenoviral vectors for vaccine development
Adenoviral vectors have been incorporated into the development of vaccines against tuberculosis,181 HIV,182, 183, 184 Ebola,185, 186, 187, 188, 189, 190 malaria,191 and influenza.192, 193 Different from most traditional vaccines, immunization techniques using recombinant viral vectors trigger both a robust cytotoxic T-cell response in addition to a humoral response.62, 194, 195 This cytotoxic T-cell response is far more efficient in destroying virus-infected cells, intracellular pathogens, and cancerous cells – while also expanding protective immunity against strains of similar pathogens that express homologous epitopes.196
In the early 2000s, Merck began manufacturing a mixed-valent vaccine product composed of three Ad5-based vectors expressing several HIV-1 proteins.184 This product was evaluated in the notable STEP study, which began enrolling volunteer subjects from around the world in 2004.184, 197 Early findings suggested the vaccine was well tolerated, even upon repeat intramuscular injections, and was able to induce T-cell responses to HIV antigens.182, 183, 193 However, the study was prematurely stopped in 2007 when subjects in the treatment group revealed higher rates of HIV infection than those in the placebo group.151 Though multiple theories have surfaced to explain the increased trend for HIV acquisition in this treatment arm (particularly among men-who-have-sex-with-men, men who were uncircumcised, and/or those with pre-existing Ad5 neutralizing antibodies prior to vaccination),103, 184 none such theories have undergone direct investigation, and so this phenomenon remains to be completely understood.103, 153
Adenoviral vaccine regimens based upon sequential exposure to HIV antigens and/or different serotypes (techniques suggested to induce more profound T-cell responses) then became the focus of investigation in subsequent years.151, 198 An NIH-sponsored phase II clinical trial (called the “HVTN trial”) began in 2009 and exposed subjects first to a DNA-based primer vaccine expressing HIV proteins to then be followed by a booster Ad5 vector vaccine expressing the same proteins. Yet again, this trial was brought to an early end in 2013 when the vaccine failed to reduce the rate of HIV infection or attenuate disease contracted by subjects during the trial.199 Most recently, such “prime-boost” strategies continue to be employed among techniques that utilize heterologous vectors and/or heterologous inserts with the aim of creating improved coverage against HIV.200, 201 In comparison to homologous pairing strategies, studies by Walsh et al have shown that vaccination strategies using heterologous vector pairs (particularly Ad5/Ad35 vectors) and inserts (particularly EnvA/EnvB inserts) improve T-cell response rates and induce significantly higher numbers of total T-cell epitopes, all with well accepted tolerance.200 Similarly, Baden et al found that both humoral and cellular immune responses to HIV-1 can be augmented with either homologous or heterologous (vector and insert) adenoviral booster vaccines using novel vector serotypes, Ad26 and Ad35, without adverse events and despite pre-existing vector immunity.201
The charge to design an Ebola vaccine became of paramount international priority when the WHO declared an epidemic in 2014. Accordingly, a surge of research and collaboration efforts have accelerated vaccine development in recent years, leading to nearly a dozen candidate vaccines now in various stages of clinical trials. Among three of the key candidate vaccines designated by the WHO, two are derived from adenoviral vectors, namely, ChAd3-ZEBOV1 from GlaxoSmithKline and Ad26-ZEBOV/MVA-BN-Filo2 from Johnson & Johnson.185 In a phase I trial, the ChAd3-ZEBOV vaccine was safe and well-tolerated.202 At low doses, found tolerable in a small cohort of macaques,203 a single test dose of this same vaccine was shown to induce antibody and T-cell responses in humans detectable as far out as 6 months post-inoculation.187 Since February of 2015, the ChAd3-ZEBOV vaccine has been tested in thousands of healthy volunteers in Liberia while proceeding into phase II and phase III efficacy trials.185, 187 With similar findings, the Ad26-ZEBOV/MVA-BN-Filo vaccine advanced from phase I trials in the UK in 2013 prior to the West African outbreak, with subsequent phase II and III studies relocated to West Africa upon the outbreak in 2014.189, 204, 205 And though this vaccine has demonstrated rapid and durable protection against Ebola in macaques, conclusive evidence supporting the efficacy of this vaccine in human subjects has not yet been reported.206, 207, 208, 209 Lastly, another Ebola vaccine candidate using an Ad-C5 vector has been tested in phase 1 trials among healthy Chinese adults, showing safe and immunogenic findings, though with insufficient clinical efficacy in individuals with pre-existing immunity to the adenovirus.190, 210, 211, 212
Adenovirus-mediated gene delivery in regenerative medicine
The use of adenoviral vectors for genetic engineering has opened new areas of investigation in most areas of medicine. From basic science laboratories to multi-center clinical trials, this promising field has captured the attention of the scientific community. The number of clinical trials alone has skyrocketed in the last two decades. The breadth of these trials will be discussed in the next section to show the diverse nature of potential clinical applications. These clinical trials have been possible due to the efforts in basic and translational studies across many disciplines. The volume of preclinical studies that have paved the way for these clinical trials is tremendous. It is beyond the scope of this review to highlight, even superficially, the many areas explored by these studies as they touch nearly every medical discipline. Instead, we will focus on several preclinical advances in the field of bone tissue engineering that have led to clinical trials in this area. We highlight this limited area to demonstrate how cumulative efforts, at the basic and translational levels, have made clinical use possible. A look at advances in genetic engineering across other disciplines shows a similar path from benchtop to bedside. In this way, a look at the evolution of bone tissue engineering advancements is loosely representative of how genetic engineering has evolved in other areas of medicine.
Successful bone tissue engineering requires four key components: osteoinduction, osteoblast differentiation, osteoconduction, and mechanical stimulation.213, 214, 215, 216, 217 In general, preclinical developments in bone tissue engineering have centered on augmenting growth factors necessary for the first three of these elements. The majority of these studies have utilized adenoviral vectors largely because transient expression is more advantageous than constitutive expression in bone tissue engineering.213, 218 This approach utilizes adenoviral vectors containing genes that code for essential osteoinductive growth factors.219, 220 Efforts aim to induce osteogenic differentiation, facilitate ossification, and encourage integration with surrounding bone tissue. Osteoblastic differentiation can be induced by a variety of osteoinductive growth factors both in vivo and in vitro. PDGFs (Platelet Derived Growth Factors), FGFs (Fibroblast Growth Factors), IGFs (Insulin-Like Growth Factors), EGF, Wnts, retinoic acids, NOTCH, and BMPs (Bone Morphogenetic Proteins) are some of the many factors that have displayed osteogenic activity in studies.220, 221, 222, 223, 224, 225, 226 At least 15 BMPs have been identified in humans and mice.219, 220, 227 They display a spectrum of osteogenic activity, with BMP-2, BMP-6, BMP-7, and BMP-9 among the most osteogenic.213, 219, 220, 228, 229, 230 With their potent osteogenic action on the most important steps in the bone formation cycle from osteoblast to osteocyte, many of these BMPs were an early focus for researchers.231, 232, 233
Early bone regeneration studies focused on rhBMPs (recombinant human BMPs.) However, difficulties with stability, cost, and efficacy limited the more widespread adoption of this strategy for meeting bone engineering goals.213 Investigators were able to largely overcome these difficulties by implementing a gene therapy approach by transducing BMP with an array of vectors, most commonly adenoviral vectors. In 1999, Lieberman et al compared rhBMP-2 and BMP-2 transduced via adenoviral vector gene transfer of bone marrow cells in their ability to heal skeletal defects in a rat model. The results strongly favored the gene transfer group, which demonstrated increased quality and quantity of bone formation.234 Other studies followed that supported a gene therapy approach to improving bone engineering techniques via BMP.235
After establishing the merits of utilizing gene therapy to study bone engineering, the techniques were refined over the following years to accumulate a large body of work in which many factors in the bone formation cascade were studied for the potency of their osteogenic effect.219, 228, 229, 230 Other key aspects were also studied including mesenchymal stem cell source, injection sites, skeletal defect sites, and direct in vivo vs. ex vivo approaches. Many of these efforts have been instrumental in advancing bone engineering to humans via clinical trials and in some cases FDA approval for clinical use. BMP-2 has been used in clinical trials examining its use in spinal fusion and in the repair of tibial fractures.236, 237 BMP-7 has been used in clinical trials for the management of tibial nonunion fractures and spinal fusion.238, 239 This work, among others, led to FDA approval for recombinant BMP-7 for use in nonunion of long bones and in anterior spinal fusions. Other clinical uses for BMPs are likely on the horizon. While not currently in clinical use, BMP-9 has demonstrated more robust osteoinduction than any other BMPs in several preclinical animal models.228, 240, 241
AdV-mediated gene delivery has also been used in numerous preclinical studies involved other aspects of regenerative medicine and tissue engineering. We demonstrated that AdV-mediated expression of Sox9 significantly improved the healing of intervertebral degenerative discs in a rabbit model.242 Adenoviral vectors were shown to deliver transgenes effectively into the flexor tendon of rabbit injury model.243 Furthermore, we showed that adenovirus-mediated gene transfer of BMP14 expedited tendon-healing and increased tendon tensile strength in a rat model of Achilles tendon injury.244 In this animal model, no adverse immunological response to the adenoviral vector was detected in the host tissue, and the local production of BMP-14 did not induce unwelcome bone or cartilage formation within the healing tendon.244
Challenges and future directions
The use of AdVs in gene therapy comes with its challenges. Some of the major obstacles surrounding the use of viral vectors include reactions with the immune system, viral longevity, vector packaging capacity, and contamination with HV. As previously discussed, the removal of viral components and the emergence of HC-AdV have significantly reduced immune reaction among other benefits. In addition to preventing tissue damage and inflammation, the immune reduction also helps to prolong the lifespan of the virus. One major challenge relating to the prolonged expression of HC-AdV is due to previous immunization against Ad. To troubleshoot this issue, HC-AdV longevity can be optimized by encoding novel tetracycline-dependent (TetOn)-regulatory elements.245 With expression tightly regulated by doxycycline, the HC-Ad-TetOn vectors demonstrated better regulation and effectiveness than constitutively active HC-AdV in the presence of an immune response.246 The lack of viral protein genes also provides increased packaging capacity, as previously discussed. However, even the presence of viral packaging machinery can impose challenges, namely those related to the issue of having too little unique enzyme sites for transgene insertion. To overcome this issue, Shi and colleagues demonstrated efficacy of HD-AdVs that have a unique transgene insertion site, while maintaining tissue-specificity.247
Another issue surrounding the use of AdV for gene therapy is contamination with helper virus (HV). Previously discussed strategies for reducing contamination have proven to be successful, but contamination levels can still be too high to use the AdV for human purposes. To combat this issue, one group has constructed a HV (AdTetCre) in which the packaging signal is flanked by loxP sites that can be excised by a chimeric MerCreMer recombinase encoded in the same viral genome. The MerCreMer expression was under control using a TetOn system248 and was able to produce titers with significant numbers of infectious units and with <0.1% HV contamination. Self-inactivating HVs based on virally encoded recombinases are promising tools for reducing contamination in the production of HC-AdV.
Additional challenges include cellular barriers and viral targeting and transport. Promising research has demonstrated that expression levels of AdV receptors do not correlate with their transduction efficiency or biological function.249 Further research related to the challenges faced by HC-AdV should be conducted to improve transduction yields and overall efficacy.
In summary, the use of AdVs for gene therapy and gene delivery tools has produced promising results among numerous breakthrough studies for human applications. More importantly, AdVs may be used in many emerging fields of biomedical research. For example, the HC-AdVs can be used to effectively deliver engineered CRISPR/Cas9 systems to targeted cells and/or tissues for efficient genome editing,250, 251 which cannot be effectively accomplished by other viral systems. Furthermore, the HC-AdVs can be used to deliver anti-PD1/PD-L1 immune checkpoint therapeutics for cancer treatment.252, 253 While the use of AdV in gene therapy has shown substantial promise and increasing potential, future work will need to be done regarding the current pitfalls of using the vector. Although great strides have been made, improvements regarding reactions with the immune system, viral longevity, vector packaging capacity, and contamination with HV will need to be made to continue making breakthroughs. Nevertheless, the evolution of the adenoviral vector as a tool for transfer of genetic material has revolutionized the way physicians and scientists can approach treating even the most debilitating diseases.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
Research in the authors' laboratories was supported in part by research grants from the National Institutes of Health (AT004418, DE020140 to TCH and RRR), the US Department of Defense (OR130096 to JMW), the Scoliosis Research Society (TCH and MJL), and the 973 Program of the Ministry of Science and Technology (MOST) of China (# 2011CB707906 to TCH). CSL and EMF were recipients of the Pritzker Summer Research Fellowship funded through the National Institute of Health (NIH) T-35 training grant (NIDDK). RZ, ZZ and YS were recipients of the Predoctoral Fellowship from the China Scholarship Council. WZ, YW, XW and KW were recipients of the Postdoctoral Fellowship from the China Scholarship Council. The reported work was also supported in part by The University of Chicago Cancer Center Support Grant (P30CA014599) and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000430. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Russell R. Reid, Email: rreid@surgery.bsd.uchicago.edu.
Tong-Chuan He, Email: tche@uchicago.edu.
References
- 1.Kay M.A., Glorioso J.C., Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 2.Crystal R.G. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther. 2014;25(1):3–11. doi: 10.1089/hum.2013.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breyer B., Jiang W., Cheng H. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1(2):149–162. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- 4.Seymour L.W., Fisher K.D. Adenovirus: teaching an old dog new tricks. Hum Gene Ther. 2011;22(9):1041–1042. doi: 10.1089/hum.2011.2517. [DOI] [PubMed] [Google Scholar]
- 5.Rauschhuber C., Noske N., Ehrhardt A. New insights into stability of recombinant adenovirus vector genomes in mammalian cells. Eur J Cell Biol. 2012;91(1):2–9. doi: 10.1016/j.ejcb.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Jin L., Zeng X., Liu M., Deng Y., He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 2014;4(3):240–255. doi: 10.7150/thno.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Li W., Ma N., Steinhoff G. Non-viral gene delivery methods. Curr Pharm Biotechnol. 2013;14(1):46–60. [PubMed] [Google Scholar]
- 8.Manjila S.B., Baby J.N., Bijin E.N., Constantine I., Pramod K., Valsalakumari J. Novel gene delivery systems. Int J Pharm Investig. 2013;3(1):1–7. doi: 10.4103/2230-973X.108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katare D.P., Aeri V. Progress in gene therapy: a review. Int J Toxicol Pharm Res. 2010;1(2):33–41. [Google Scholar]
- 11.Gardlík R., Pálffy R., Hodosy J., Lukács J., Turna J., Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11(4):RA110–121. [PubMed] [Google Scholar]
- 12.Robertson E.S., Ooka T., Kieff E.D. Epstein-Barr virus vectors for gene delivery to B lymphocytes. Proc Natl Acad Sci U S A. 1996;93(21):11334–11340. doi: 10.1073/pnas.93.21.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirai H., Satoh E., Osawa M. Use of EBV-based Vector/HVJ-liposome complex vector for targeted gene therapy of EBV-associated neoplasms. Biochem Biophys Res Commun. 1997;241(1):112–118. doi: 10.1006/bbrc.1997.7776. [DOI] [PubMed] [Google Scholar]
- 14.Ohe Y., Zhao D., Saijo N., Podack E.R. Construction of a novel bovine papillomavirus vector without detectable transforming activity suitable for gene transfer. Hum Gene Ther. 1995;6(3):325–333. doi: 10.1089/hum.1995.6.3-325. [DOI] [PubMed] [Google Scholar]
- 15.Verma I.M., Somia N. Gene therapy – promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 16.Mountain A. Gene therapy: the first decade. Trends Biotechnol. 2000;18(3):119–128. doi: 10.1016/s0167-7799(99)01416-x. [DOI] [PubMed] [Google Scholar]
- 17.Luo D., Saltzman W.M. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18(1):33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 18.Doerfler W., Schubbert R., Heller H. Integration of foreign DNA and its consequences in mammalian systems. Trends Biotechnol. 1997;15(8):297–301. doi: 10.1016/S0167-7799(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 19.Garrick D., Fiering S., Martin D.I., Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18(1):56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- 20.Remus R., Heller H., Schmitz B., Schell G., Doerfler W., Kämmer C. Insertion of foreign DNA into an established mammalian genome can alter the methylation of cellular DNA sequences. J Virol. 1999;73(2):1010–1022. doi: 10.1128/jvi.73.2.1010-1022.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrhardt A., Haase R., Schepers A., Deutsch M.J., Lipps H.J., Baiker A. Episomal vectors for gene therapy. Curr Gene Ther. 2008;8(3):147–161. doi: 10.2174/156652308784746440. [DOI] [PubMed] [Google Scholar]
- 22.Van Craenenbroeck K., Vanhoenacker P., Haegeman G. Episomal vectors for gene expression in mammalian cells. Eur J Biochem. 2000;267(18):5665–5678. doi: 10.1046/j.1432-1327.2000.01645.x. [DOI] [PubMed] [Google Scholar]
- 23.Belt P.B., Jongmans W., de Wit J., Hoeijmakers J.H., van de Putte P., Backendorf C. Efficient cDNA cloning by direct phenotypic correction of a mutant human cell line (HPRT-) using an Epstein-Barr virus-derived cDNA expression vector. Nucleic Acids Res. 1991;19(18):4861–4866. doi: 10.1093/nar/19.18.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazda O., Satoh E., Yasutomi K., Imanishi J. Extremely efficient gene transfection into lympho-hematopoietic cell lines by Epstein-Barr virus-based vectors. Methods. 1997;204(2):143–151. doi: 10.1016/s0022-1759(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich T.D., Bedner E., Darzynkiewicz Z., Lehman J.M. Distinct patterns of MCM protein binding in nuclei of S phase and rereplicating SV40-infected monkey kidney cells. Cytom Part Soc Anal Cytol. 2018;68(1):10–18. doi: 10.1002/cyto.a.20185. [DOI] [PubMed] [Google Scholar]
- 26.Valls E., de la Cruz X., Martínez-Balbás M.A. The SV40 T antigen modulates CBP histone acetyltransferase activity. Nucleic Acids Res. 2003;31(12):3114–3122. doi: 10.1093/nar/gkg418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darimont C., Zbinden I., Avanti O. Reconstitution of telomerase activity combined with HPV-E7 expression allow human preadipocytes to preserve their differentiation capacity after immortalization. Cell Death Differ. 2003;10(9):1025–1031. doi: 10.1038/sj.cdd.4401273. [DOI] [PubMed] [Google Scholar]
- 28.Harris K.F., Christensen J.B., Imperiale M.J. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J Viol. 1996;70(4):2378–2386. doi: 10.1128/jvi.70.4.2378-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Visner G.A. Applications of Sleeping Beauty transposons for nonviral gene therapy. IUBMB Life. 2007;59(6):374–379. doi: 10.1080/15216540701435722. [DOI] [PubMed] [Google Scholar]
- 30.Ehrhardt A., Xu H., Huang Z., Engler J.A., Kay M.A. A direct comparison of two nonviral gene therapy vectors for somatic integration: in vivo evaluation of the bacteriophage integrase phiC31 and the Sleeping Beauty transposase. Mol Ther. 2005;11(5):695–706. doi: 10.1016/j.ymthe.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz-López M., García-Pérez J.L. DNA transposons: nature and applications in genomics. Curr Genomics. 2010;11(2):115–128. doi: 10.2174/138920210790886871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackett P.B., Aronovich E.L., Hunter D. Efficacy and safety of Sleeping Beauty transposon-mediated gene transfer in preclinical animal studies. Curr Gene Ther. 2011;11(5):341–349. doi: 10.2174/156652311797415827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skipper K.A., Andersen P.R., Sharma N., Mikkelsen J.G. DNA transposon-based gene vehicles-scenes from an evolutionary drive. J Biomed Sci. 2013;20 doi: 10.1186/1423-0127-20-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivics Z., Izsvák Z. Sleeping beauty transposition. Microbiol Spectr. 2015;3(2) doi: 10.1128/microbiolspec.MDNA3-0042-2014. MDNA3-0042-2014. [DOI] [PubMed] [Google Scholar]
- 35.Wang D., Gao G. State-of-the-art human gene therapy: part I. Gene Deliv Technol Discov Med. 2014;18(97):67–77. [PMC free article] [PubMed] [Google Scholar]
- 36.Grabundzija I., Irgang M., Mátés L. Comparative analysis of transposable element vector systems in human cells. Mol Ther. 2010;18(6):1200–1209. doi: 10.1038/mt.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plasterk R.H. Molecular mechanisms of transposition and its control. Cell. 1993;74(5):781–786. doi: 10.1016/0092-8674(93)90458-3. [DOI] [PubMed] [Google Scholar]
- 38.Ivics Z., Izsvák Z. Transposons for gene therapy! Curr Gene Ther. 2006;6(5):593–607. doi: 10.2174/156652306778520647. [DOI] [PubMed] [Google Scholar]
- 39.Chen X., Cui J., Yan Z. Sustained high level transgene expression in mammalian cells mediated by the optimized piggyBac transposon system. Genes Dis. 2015;2(1):96–105. doi: 10.1016/j.gendis.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yant S.R., Park J., Huang Y., Mikkelsen J.G., Kay M.A. Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells. Mol Cell Biol. 2004;24(20):9239–9247. doi: 10.1128/MCB.24.20.9239-9247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakuma T., Barry M.A., Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443(3):603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 42.Ertl H.C.J. Progress in the development of hepatitis C virus vaccines. Mol Ther. 2012;20(4):697–698. doi: 10.1038/mt.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lachmann R.H., Efstathiou S. The use of herpes simplex virus-based vectors for gene delivery to the nervous system. Mol Med Today. 1997;3(9):404–411. doi: 10.1016/S1357-4310(97)01106-4. [DOI] [PubMed] [Google Scholar]
- 44.Federici T., Kutner R., Zhang X.Y. Comparative analysis of HIV-1-based lentiviral vectors bearing lyssavirus glycoproteins for neuronal gene transfer. Genet Vaccines Ther. 2009;7:1. doi: 10.1186/1479-0556-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hacein-Bey-Abina S., Hauer J., Lim A. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363(4):355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anson D.S. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet Vaccines Ther. 2004;2(1) doi: 10.1186/1479-0556-2-9. 1479-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laufs S., Gentner B., Nagy K.Z. Retroviral vector integration occurs in preferred genomic targets of human bone marrow-repopulating cells. Blood. 2002;101(6):2191–2198. doi: 10.1182/blood-2002-02-0627. [DOI] [PubMed] [Google Scholar]
- 48.Hacein-Bey-Abina S., Von Kalle C., Schmidt M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 49.Hacein-Bey-Abina S., Le Deist F., Carlier F. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346(16):1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 50.Fischer A., Hacein-Bey-Abina S., Lagresle C., Garrigue A., Cavazana-Calvo M. Gene therapy of severe combined immunodeficiency disease: proof of principle of efficiency and safety issues. Gene therapy primary immunodeficiencies retrovirus lentivirus genome. Bull Acad Natl Med. 2005;189(5):779–785. discussion 786–788. [PubMed] [Google Scholar]
- 51.Fox J.L. US authorities uphold suspension of SCID gene therapy. Nat Biotechnol. 2003;21(3):217. doi: 10.1038/nbt0303-217. [DOI] [PubMed] [Google Scholar]
- 52.Buckley R.H. Gene therapy for SCID-a complication after remarkable progress. Lancet. 2002;360(9341):1185–1186. doi: 10.1016/S0140-6736(02)11290-6. [DOI] [PubMed] [Google Scholar]
- 53.Wu X., Burgess S.M., Sci C. Integration target site selection for retroviruses and transposable elements. Cell Mol Life. 2004:19–20. doi: 10.1007/s00018-004-4206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cereseto A., Giacca M. Integration site selection by retroviruses. AIDS Rev. 2004;6(1):13–21. [PubMed] [Google Scholar]
- 55.Engelman A. The roles of cellular factors in retroviral integration. Curr Top Microbiol Immunol. 2003;281:209–238. doi: 10.1007/978-3-642-19012-4_6. [DOI] [PubMed] [Google Scholar]
- 56.Cockrell A.S., Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36(3):184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 57.Kafri T., Clifton N.J. Gene delivery by lentivirus vectors an overview. Methods Mol Biol. 2004;246:367–390. doi: 10.1385/1-59259-650-9:367. [DOI] [PubMed] [Google Scholar]
- 58.Balaggan K.S., Ali R.R. Ocular gene delivery using lentiviral vectors. Gene Ther. 2011;19(2):145–153. doi: 10.1038/gt.2011.153. [DOI] [PubMed] [Google Scholar]
- 59.Wong L., Goodhead L., Prat C., Mitrophanous K.A., Kingsman S.M., Mazarakis N.D.F. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum Gene Ther. 2006;17(1):1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 60.Azzouz M., Martin-Rendon E., Barber R.D. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease. J Neurosci. 2002;22(23):10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Betchen S.A., Kaplitt M. Future and current surgical therapies in Parkinson's disease. Curr Opin Neurol. 2003;16(4):487–493. doi: 10.1097/01.wco.0000084227.82329.ae. [DOI] [PubMed] [Google Scholar]
- 62.Vannucci L., Lai M., Chiuppesi F., Ceccherini-Nelli L., Pistello M. Viral vectors: a look back and ahead on gene transfer technology. New Microbiol. 2013;36(1):1–22. [PubMed] [Google Scholar]
- 63.Dropulić B. Lentiviral vectors: their molecular design, safety, and use in laboratory and preclinical research. Hum Gene Ther. 2011;22(6):649–657. doi: 10.1089/hum.2011.058. [DOI] [PubMed] [Google Scholar]
- 64.Howarth J.L., Lee Y.B., Uney J.B. Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells) Cell Biol Toxicol. 2010;26(1):1–20. doi: 10.1007/s10565-009-9139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mátrai J., Chuah M.K., VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18(3):477–490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samulski R.J., Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. 2014;1(1):427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 67.Balakrishnan B., Jayandharan G.R. Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr Gene Ther. 2014;14(2):86–100. doi: 10.2174/1566523214666140302193709. [DOI] [PubMed] [Google Scholar]
- 68.Waehler R., Russell S.J., Curiel D.T. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nayak S., Herzog R.W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2009;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarty D.M., Monahan P.E., Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 71.Walther W., Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60(2):249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 72.Teramato S., Ishii T., Matsuse T. Crisis of adenoviruses in human gene therapy. Lancet. 2000;355(9218):1911–1912. doi: 10.1016/S0140-6736(05)73358-4. [DOI] [PubMed] [Google Scholar]
- 73.Giacca M., Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release. 2012;161(2):377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Smith R.H. Adeno-associated virus integration: virus versus vector. Gene Ther. 2008;15(11):817–822. doi: 10.1038/gt.2008.55. [DOI] [PubMed] [Google Scholar]
- 75.Flotte T.R. Birth of a new therapeutic platform: 47 years of adeno-associated virus biology from virus discovery to licensed gene therapy. Mol Ther. 2013;21(11):1976–1981. doi: 10.1038/mt.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai C.M., Lai Y.K., Rakoczy P.E. Adenovirus and adeno-associated virus vectors. DNA Cell Biol. 2002;21(12):895–913. doi: 10.1089/104454902762053855. [DOI] [PubMed] [Google Scholar]
- 77.Flotte T., Carter B., Conrad C. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7(9):1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 78.Kay M.A., Manno C.S., Ragni M.V. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24(3):257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 79.Duque S., Joussemet B., Riviere C. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17(7):1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang B., Li S., Wang H. Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10. Mol Ther. 2014;22(7):1299–1309. doi: 10.1038/mt.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H., Yang B., Mu X. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011;19(8):1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagabhushan Kalburgi S., Khan N.N., Gray S.J. Recent gene therapy advancements for neurological diseases. Discov Med. 2013;15(81):111–119. [PMC free article] [PubMed] [Google Scholar]
- 84.Lachmann R. J. Herpes simplex virus-based vectors. Int Pathol. 2004;85(4):177–190. doi: 10.1111/j.0959-9673.2004.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hukkanen V. Herpesvirus vectors in gene therapy. Open Virol J. 2010;4:94–95. doi: 10.2174/1874357901004010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu W., Liu Z., Liu L. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett. 2007;432(1):13–18. doi: 10.1016/j.neulet.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 87.Burton E.A., Fink D.J., Glorioso J.C. Gene delivery using herpes simplex virus vectors. DNA Cell Biol. 2002;21(12):915–936. doi: 10.1089/104454902762053864. [DOI] [PubMed] [Google Scholar]
- 88.Lentz T.B., Gray S.J., Samulski R.J. Viral vectors for gene delivery to the central nervous system. Neurobiol Dis. 2011;48(2):179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Silva S., Bowers W.J. Herpes virus amplicon vectors. Viruses. 2009;1(3):594–629. doi: 10.3390/v1030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marconi P., Argnani R., Epstein A.L., Manservigi R. HSV as a vector in vaccine development and gene therapy. Adv Exp Med Biol. 2009;655:118–144. doi: 10.1007/978-1-4419-1132-2_10. [DOI] [PubMed] [Google Scholar]
- 91.Marconi P., Manservigi R., Epstein A.L. HSV-1-derived helper-independent defective vectors, replicating vectors and amplicon vectors, for the treatment of brain diseases. Curr Opin Drug Discov Devel. 2010;13(2):169–183. [PubMed] [Google Scholar]
- 92.Manservigi R., Argnani R., Marconi P. HSV recombinant vectors for gene therapy. Open Virol J. 2010;4:123–156. doi: 10.2174/1874357901004010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goss J.R., Krisky D., Wechuck J., Wolfe D. Herpes simplex virus-based nerve targeting gene therapy in pain management. J Pain Res. 2014;7:71–79. doi: 10.2147/JPR.S36619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agarwalla P.K., Aghi M.K., Clifton N.J. Oncolytic herpes simplex virus engineering and preparation. Methods Mol Biol. 2012;797:1–19. doi: 10.1007/978-1-61779-340-0_1. [DOI] [PubMed] [Google Scholar]
- 95.Berto E., Bozac A., Marconi P. Development and application of replication-incompetent HSV-1-based vectors. Gene Ther. 2005;1(12 Suppl):S98–S102. doi: 10.1038/sj.gt.3302623. [DOI] [PubMed] [Google Scholar]
- 96.Burton E.A., Wechuck J.B., Wendell S.K., Goins W.F., Fink D.J., Glorioso J.C. Multiple applications for replication-defective herpes simplex virus vectors. Stem Cells. 2001;19(5):358–377. doi: 10.1634/stemcells.19-5-358. [DOI] [PubMed] [Google Scholar]
- 97.Goins W.F., Goss J.R., Chancellor M.B., de Groat W.C., Glorioso J.C., Yoshimura N. Herpes simplex virus vector-mediated gene delivery for the treatment of lower urinary tract pain. Gene Ther. 2009;16(4):558–569. doi: 10.1038/gt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolfe D., Mata M., Fink D.J. A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther. 2009;16(4):455–460. doi: 10.1038/gt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goss J.R., Harley C.F., Mata M. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol. 2002;52(5):662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 100.Pulkkanen K.J., Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12(4):585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 101.Wong J., Lee C., Zhang K., Rennie P.S., Jia W. Targeted oncolytic herpes simplex viruses for aggressive cancers. Curr Pharm Biotechnol. 2012;13(9):1786–1794. doi: 10.2174/138920112800958751. [DOI] [PubMed] [Google Scholar]
- 102.Davison A.J., Benko M., Harrach B. Genetic content and evolution of adenoviruses. J Gen Virol. 2003;84(Pt 11):2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 103.Lasaro M.O., Ertl H.C.J. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17(8):1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401(6753):517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 105.Bett A.J., Prevec L., Graham F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67(10):5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reid T., Warren R., Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9(12):979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raper S.E., Chirmule N., Lee F.S. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80(1-2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 108.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286(5448):2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 109.Choi I.K., Yun C.O. Recent developments in oncolytic adenovirus-based immunotherapeutic agents for use against metastatic cancers. Cancer Gene Ther. 2013;20(2):70–76. doi: 10.1038/cgt.2012.95. [DOI] [PubMed] [Google Scholar]
- 110.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cerullo V., Pesonen S., Diaconu I. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70(11):4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 112.Kaufmann J.K., Nettelbeck D.M. Virus chimeras for gene therapy, vaccination, and oncolysis: adenoviruses and beyond. Trends Mol Med. 2012;18(7):365–376. doi: 10.1016/j.molmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 113.Ramos-Kuri M., Rapti K., Mehel H. Dominant negative Ras attenuates pathological ventricular remodeling in pressure overload cardiac hypertrophy. Biochim Biophys Acta. 2015;1853(11 Pt A):2870–2884. doi: 10.1016/j.bbamcr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vorburger S.A., Hunt K.K. Adenoviral gene therapy. Oncologist. 2002;7(1):46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- 115.Parks R.J., Chen L., Anton M., Sankar U., Rudnicki M.A., Graham F.L. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93(24):13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sandig V., Youil R., Bett A.J., Franlin L.L., Oshima M., Maione D. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc Natl Acad Sci U S A. 2000;97(3):1002–1007. doi: 10.1073/pnas.97.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy P., Viii A. Sustained human factor in hemophilia following systemic delivery of a gutless adenoviral vector. Mol Ther. 2002;5(1):63–73. doi: 10.1006/mthe.2001.0510. [DOI] [PubMed] [Google Scholar]
- 118.Ng P. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol Ther. 2001;3(5):809–815. doi: 10.1006/mthe.2001.0323. [DOI] [PubMed] [Google Scholar]
- 119.Medico E., Gambarotta G., Gentile A., Comoglio P.M., Soriano P. A gene trap vector system for identifying transcriptionally responsive genes. Nat Biotechnol. 2001;19(6):579–582. doi: 10.1038/89343. [DOI] [PubMed] [Google Scholar]
- 120.Kalburgi S., Khan N.N., Gray S.J. Nagabhushan Recent gene therapy advancements for neurological diseases. Discov Med. 2013;15(81):111–119. [PMC free article] [PubMed] [Google Scholar]
- 121.He T.C. Adenoviral vectors. Curr Protoc Hum Genet. 2004:12.4.1–12.4.25. doi: 10.1002/0471142905.hg1204s40. Chapter 12: Unit 12–14. [DOI] [PubMed] [Google Scholar]
- 122.He T.C., Zhou S., da Costa L.T., Yu J., Kinzler K.W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mizuguchi H., Kay M.A., Hayakawa T. Approaches for generating recombinant adenovirus vectors. Adv Drug Deliv Rev. 2001;52(3):165–176. doi: 10.1016/s0169-409x(01)00215-0. [DOI] [PubMed] [Google Scholar]
- 124.Danthinne X., Imperiale M.J. Production of first generation adenovirus vectors: a review. Gene Ther. 2000;7(20):1707–1714. doi: 10.1038/sj.gt.3301301. [DOI] [PubMed] [Google Scholar]
- 125.Luo J., Deng Z.L., Luo X. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2(5):1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 126.Berkner K.L., Sharp P.A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mizuguchi H., Kay M.A. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther. 1998;9(17):2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- 128.Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M.L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71(3):1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ng P., Evelegh C., Cummings D., Graham F.L. Cre levels limit packaging signal excision efficiency in the Cre/lox Adenoviral vector system. J Virol. 2002;76(9):4181–4189. doi: 10.1128/JVI.76.9.4181-4189.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ketner G., Spencer F., Tugendreich S., Connelly C., Hieter P. Efficient manipulation of the human adenovirus genome as an infectious yeast artificial chromosome clone. Proc Natl Acad Sci U S A. 1994;91(13):6186–6190. doi: 10.1073/pnas.91.13.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chartier C., Degryse E., Gantzer M., Dieterle A., Pavirani A., Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70(7):4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crouzet J., Naudin L., Orsini C. Recombinational construction in Escherichia coli of infectious adenoviral genomes. Proc Natl Acad Sci U S A. 1997;94(4):1414–1419. doi: 10.1073/pnas.94.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Umana P., Gerdes C.A., Stone D. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol. 2001;19(6):582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- 134.Zhao C., Wu N., Deng F. Adenovirus-mediated gene transfer in mesenchymal stem cells can be significantly enhanced by the cationic polymer polybrene. PLoS One. 2014;9(3):e92908. doi: 10.1371/journal.pone.0092908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu N., Zhang H., Deng F. Overexpression of Ad5 precursor terminal protein accelerates recombinant adenovirus packaging and amplification in HEK-293 packaging cells. Gene Ther. 2014;21(7):629–637. doi: 10.1038/gt.2014.40. [DOI] [PubMed] [Google Scholar]
- 136.Wei Q., Fan F., Liao J. Engineering the rapid adenovirus production and amplification (RAPA) cell line to expedite the generation of recombinant adenoviruses. Cell Physiol Biochem. 2017;41:2383–2398. doi: 10.1159/000475909. [DOI] [PubMed] [Google Scholar]
- 137.Jaffe H.A., Danel C., Longenecker G. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 138.Rosenfeld M.A., Yoshimura K., Trapnell B.C. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 139.Yang Y., Nunes F.A., Berencsi K., Furth E.E., Gönczöl E., Wilson J.M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang Y., Jooss K.U., Su Q., Ertl H.C., Wilson J.M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3(2):137–144. [PubMed] [Google Scholar]
- 141.Kotterman M.A., Chalberg T.W., Schaffer D.V. Viral vectors for gene therapy: translational and clinical outlook. Annu Rev Biomed Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 142.Ginn S.L., Alexander I.E., Edelstein M.L., Abedi M.R., Wixon J. Gene therapy clinical trials worldwide to -an update. J Gene Med. 2013;15(2):65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 143.Wu Q., Xia D., Carlsen S., Xiang J. Adenovirus-mediated transgene-engineered dendritic cell vaccine of cancer. Curr Gene Ther. 2005;5(2):237–247. doi: 10.2174/1566523053544272. [DOI] [PubMed] [Google Scholar]
- 144.Tagawa M., Kawamura K., Ueyama T. Cancer therapy with local oncolysis and topical cytokine secretion. Front Biosci Libr. 2008;13:2578–2587. doi: 10.2741/2867. [DOI] [PubMed] [Google Scholar]
- 145.Matthews K.S., Alvarez R.D., Curiel D.T. Advancements in adenoviral based virotherapy for ovarian cancer. Adv Drug Deliv Rev. 2009;61(10):836–841. doi: 10.1016/j.addr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 146.Fukazawa T., Matsuoka J., Yamatsuji T. Adenovirus-mediated cancer gene therapy and virotherapy (Review) Int J Mol Med. 2010;25(1):3–10. [PubMed] [Google Scholar]
- 147.Aguilar L.K., Guzik B.W., Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112(8):1969–1977. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 148.Aurisicchio L., Ciliberto G. Genetic cancer vaccines: current status and perspectives. Expert Opin Biol Ther. 2012;12(8):1043–1058. doi: 10.1517/14712598.2012.689279. [DOI] [PubMed] [Google Scholar]
- 149.Duarte S., Carle G., Faneca H., de Lima M.C.P., Pierrefite-Carle V. Suicide gene therapy in cancer: where do we stand now? Cancer Lett. 2012;324(2):160–170. doi: 10.1016/j.canlet.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 150.Deisseroth A., Tang Y., Zhang L., Akbulut H., Habib N. TAA/ecdCD40L adenoviral prime-protein boost vaccine for cancer and infectious diseases. Cancer Gene Ther. 2012;20(2):65–69. doi: 10.1038/cgt.2012.87. [DOI] [PubMed] [Google Scholar]
- 151.Wold W.S.M., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13(6):421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16(9):1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 153.Wold W.S.M., G. M . Fields Virology. vol. 1. Wolters Kluwer Health Lippincott Williams Wilkins; Philidelphia: 2013. Adenoviruses; pp. 1345–1374. [Google Scholar]
- 154.Nemunaitis J. Head and neck cancer: response to p53-based therapeutics. Head Neck. 2011;33(1):131–134. doi: 10.1002/hed.21364. [DOI] [PubMed] [Google Scholar]
- 155.Roth J.A. Adenovirus p53 gene therapy. Expert Opin Biol Ther. 2006;6(1):55–61. doi: 10.1517/14712598.6.1.55. [DOI] [PubMed] [Google Scholar]
- 156.Senzer N., Nemunaitis J. A review of contusugene ladenovec (Advexin) p53 therapy. Curr Opin Mol Ther. 2009;11(1):54–61. [PubMed] [Google Scholar]
- 157.Nemunaitis J., Clayman G., Agarwala S.S. Biomarkers predict p53 gene therapy efficacy in recurrent squamous cell carcinoma of the head and neck. Clin Cancer Res. 2009;15(24):7719–7725. doi: 10.1158/1078-0432.CCR-09-1044. [DOI] [PubMed] [Google Scholar]
- 158.Tian G., Liu J., Zhou J.S.R., Chen W. Multiple hepatic arterial injections of recombinant adenovirus p53 and 5-fluorouracil after transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a pilot phase II trial. Anticancer Drugs. 2009;20(5):389–395. doi: 10.1097/CAD.0b013e32832a2df9. [DOI] [PubMed] [Google Scholar]
- 159.Ma G., Shimada H., Hiroshima K., Tada Y., Suzuki N., Tagawa M. Gene medicine for cancer treatment: commercially available medicine and accumulated clinical data in China. Drug Des Devel Ther. 2009;2:115–122. doi: 10.2147/dddt.s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi J., Zheng D. An update on gene therapy in China. Curr Opin Mol Ther. 2009;11(5):547–553. [PubMed] [Google Scholar]
- 161.Shirakawa T. The current status of adenovirus-based cancer gene therapy. Mol Cells. 2008;25(4):462–466. [PubMed] [Google Scholar]
- 162.Li Y., Li B., Li C.J., Li L.J. Key points of basic theories and clinical practice in rAd-p53 ( Gendicine ™) gene therapy for solid malignant tumors. Expert Opin Biol Ther. 2015;15(3):437–454. doi: 10.1517/14712598.2015.990882. [DOI] [PubMed] [Google Scholar]
- 163.Vaillancourt M.T., Atencio I., Quijano E., Howe J.A., Ramachandra M. Inefficient killing of quiescent human epithelial cells by replicating adenoviruses: potential implications for their use as oncolytic agents. Cancer Gene Ther. 2005;12(8):691–698. doi: 10.1038/sj.cgt.7700840. [DOI] [PubMed] [Google Scholar]
- 164.Ferrari R., Pellegrini M., Horwitz G.A., Xie W., Berk A.J., Kurdistani S.K. Epigenetic reprogramming by adenovirus e1a. Science. 2008;321(5892):1086–1088. doi: 10.1126/science.1155546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vähä-Koskela M.J., Heikkilä J.E., Hinkkanen A.E. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254(2):178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ganly I., Kirn D., Eckhardt G. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6(3):798–806. [PubMed] [Google Scholar]
- 167.Kim J., Cho J.Y., Kim J.H., Jung K.C., Yun C.O. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther. 2002;9(9):725–736. doi: 10.1038/sj.cgt.7700494. [DOI] [PubMed] [Google Scholar]
- 168.Nemunaitis J., Ganly I., Khuri F. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an EkD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60(22):1B–55B. [PubMed] [Google Scholar]
- 169.Liu T.C., Galanis E., Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 170.Khuri F.R., Nemunaitis J., Ganly I. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 171.Yu W., Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7(2):141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 172.Chira S., Jackson C.S., Oprea I. Progresses towards safe and efficient gene therapy vectors. Oncotarget. 2015;6(31):30675–30703. doi: 10.18632/oncotarget.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.DeWeese T.L., van Li S., der Poel H. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61(20):7464–7472. [PubMed] [Google Scholar]
- 174.Small E.J., Carducci M.A., Burke J.M. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14(1):107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 175.Fukuda K., Abei M., Ugai H. E1A, E1B double-restricted replicative adenovirus at low dose greatly augments tumor-specific suicide gene therapy for gallbladder cancer. Cancer Gene Ther. 2008;16(2):126–136. doi: 10.1038/cgt.2008.67. [DOI] [PubMed] [Google Scholar]
- 176.Li X., Zhang Y.P., Kim H.S. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65(5):1941–1951. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- 177.Kim E., Kim J.H., Shin H.Y. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther. 2003;14(15):1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- 178.Rodriguez R., Schuur E.R., Lim H.Y., Henderson G.A., Simons J.W., Henderson D.R. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57(13):2559–2563. [PubMed] [Google Scholar]
- 179.Nettelbeck D.M., Rivera A.A., Balagué C., Alemany R., Curiel D.T. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62(16):4663–4670. [PubMed] [Google Scholar]
- 180.Conrad C., Miller C.R., Ji Y. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12(3):284–294. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 181.Smaill F., Jeyanathan M., Smieja M. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med. 2013;5(205):205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 182.McElrath M.J., De Rosa S.C., Moodie Z. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harro C., Sun X., Stek J.E., Immunol C.V.I. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin Vaccine. 2009;16(9):1285–1292. doi: 10.1128/CVI.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buchbinder S.P., Mehrotra D.V., Duerr A. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mennechet F.J.D., Tran T.T.P., Eichholz K., van Kremer E.J., de Perre P. Ebola virus vaccine: benefit and risks of adenovirus-based vectors. Expert Rev Vaccines. 2015;14(11):1471–1478. doi: 10.1586/14760584.2015.1083429. [DOI] [PubMed] [Google Scholar]
- 186.De Santis O., Audran R., Pothin E. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016;16(3):311–320. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 187.Green C.A., Scarselli E., Sande C.J. Chimpanzee adenovirus-and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med. 2015;7(300):300ra126. doi: 10.1126/scitranslmed.aac5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Milligan I.D., Gibani M.M., Sewell R. Safety and immunogenicity of novel adenovirus type 26-and modified vaccinia ankara-vectored Ebola vaccines: clinical trial. JAMA. 2016;315(15):1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 189.Zhou Y., Sullivan N.J. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine. Curr Opin Immunol. 2015;35:131–136. doi: 10.1016/j.coi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 190.Zhu F.C., Hou L.H., Li J.X. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385(9984):2272–2279. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 191.Hill A.V., Reyes-Sandoval A., O'Hara G. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6(1):78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 192.Scallan C.D., Tingley D.W., Lindbloom J.D., Toomey J.S., Tucker S.N., Immunol C.V.I. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin Vaccine. 2013;20(1):85–94. doi: 10.1128/CVI.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Peters W., Brandl J.R., Lindbloom J.D. Oral administration of an adenovirus vector encoding both an avian influenza A hemagglutinin and a TLR3 ligand induces antigen specific granzyme B and IFN-γ T cell responses in humans. Vaccine. 2013;31(13):1752–1758. doi: 10.1016/j.vaccine.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 194.Alexander J., Ward S., Mendy J. Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin. Plos One. 2012;7(2):e31177. doi: 10.1371/journal.pone.0031177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiuppesi F., Vannucci L., De Luca A. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J Virol. Jun 2012;86(12):6563–6574. doi: 10.1128/JVI.00302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wei C.J., Boyington J.C., McTamney P.M. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329(5995):1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 197.Gray G., Buchbinder S., Duerr A., Curr A., Opin H.I.V. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5(5):357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu J., O'Brien K.L., Lynch D.M. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hammer S.M., Sobieszczyk M.E., Janes H. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369(22):2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Walsh S.R., Moodie Z., Fiore-Gartland A.J., Heterologous H.I.V., Increases T. Vaccination with 1 envelope sequences and heterologous adenovirus vectors cell responses to conserved regions: HVTN 083. J Infect Dis. 2016;213(4):541–550. doi: 10.1093/infdis/jiv496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Baden L.R., Karita E., Mutua G. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: trial. Ann Intern Med. 2016;164(5):313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ewer K., Rampling T., Venkatraman N. Chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med. 2016;374(17):1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stanley D.A., Honko A.N., Asiedu C. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med. 2014;20(10):1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 204.Deen G.F., Knust B., Broutet N. Ebola in semen of Ebola virus disease survivors-preliminary report. N Engl J Med. 2015:1–7. doi: 10.1056/NEJMoa1511410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Varkey J.B., Shantha J.G., Crozier I. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372(25):2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Martínez M.J., Salim A.M., Hurtado J.C., Kilgore P.E. Ebola virus infection: overview and update on prevention and treatment. Infect Dis Ther. 2015;4(4):365–390. doi: 10.1007/s40121-015-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Biswas S., Choudhary P., Elias S.C. Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. Plos One. 2014;9(9):e107903. doi: 10.1371/journal.pone.0107903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mast T.C., Kierstead L., Gupta S.B. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2009;28(4):950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 209.Abbink P., Lemckert A.A.C., Ewald B.A. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81(9):4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Swenson D.L., Wang D., Luo M., Immunol C.V.I. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine. 2008;15(3):460–467. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pratt W.D., Wang D., Nichols D.K., Immunol C.V.I. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine. 2010;17(4):572–581. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ledgerwood J.E., Costner P., Desai N. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29(2):304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 213.Luo J., Sun M.H., Kang Q. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5(2):167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 214.Luther G., Wagner E.R., Zhu G. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 215.Lamplot J.D., Qin J., Nan G. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 216.Shenaq D.S., Rastegar F., Petkovic D. Mesenchymal progenitor cells and their orthopedic applications: forging a path towards clinical trials. Stem Cells Int. 2010;2010:519028. doi: 10.4061/2010/519028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rastegar F., Shenaq D., Huang J. Mesenchymal stem cells: molecular characteristics and clinical applications. World J Stem Cells. 2010;2(4):67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 219.Luu H.H., Song W.X., Luo X. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 220.Deng Z.L., Sharff K.A., Tang N. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 221.Chen L., Jiang W., Huang J. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Min Res. 2010;25(11):2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu X., Qin J., Luo Q. Cross-talk between EGF and BMP9 signalling pathways regulates the osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2013;17(9):1160–1172. doi: 10.1111/jcmm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang H., Wang J., Deng F. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang W., Deng Z.L., Chen L. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5(7):e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liao J., Wei Q., Zou Y. Notch signaling augments BMP9-induced Bone formation by promoting the osteogenesis-angiogenesis coupling process in mesenchymal stem cells (MSCs) Cell Physiol Biochem. 2017;41(5):1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]
- 226.Wang J., Liao J., Zhang F. Nel-Like Molecule-1 (Nell1) is regulated by bone morphogenetic protein 9 (BMP9) and potentiates BMP9-induced osteogenic differentiation at the expense of adipogenesis in mesenchymal stem cells. Cell Physiol Biochem. 2017;41(2):484–500. doi: 10.1159/000456885. [DOI] [PubMed] [Google Scholar]
- 227.Reddi A.H. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Jt Surg Am. 2001;83-A(Suppl 1(Pt 1)):S1–S6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- 228.Cheng H., Jiang W., Phillips F.M. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Jt Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 229.Kang Q., Song W.X., Luo Q. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kang Q., Sun M.H., Cheng H. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 231.Sakou T. Bone morphogenetic proteins: from basic studies to clinical approaches. Bone. 1998;22(6):591–603. doi: 10.1016/s8756-3282(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 232.Einhorn T.A., Lee C.A. Bone regeneration: new findings and potential clinical applications. J Am Acad Orthop Surg. 2001;9(3):157–165. doi: 10.5435/00124635-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 233.Barnes G.L., Kostenuik P.J., Gerstenfeld L.C., Einhorn T.A. Growth factor regulation of fracture repair. J Bone Min Res. 1999;14(11):1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 234.Lieberman J.R., Daluiski A., Stevenson S. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Jt Surg Am. 1999;81(7):905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 235.Park J., Ries J., Gelse K. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10(13):1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 236.Govender S., Csimma C., Genant H.K. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Jt Surg Am. 2002;84-A(12):2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 237.Boden S.D., Kang J., Sandhu H., Heller J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 238.Friedlaender G.E., Perry C.R., Cole J.D. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Jt Surg Am. 2001;83-A(Suppl 1(Pt 2)):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 239.Johnsson R., Strömqvist B., Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27(23):2654–2661. doi: 10.1097/00007632-200212010-00004. [DOI] [PubMed] [Google Scholar]
- 240.Dayoub H., Dumont R.J., Li J.Z. Human mesenchymal stem cells transduced with recombinant bone morphogenetic protein-9 adenovirus promote osteogenesis in rodents. Tissue Eng. 2003;9(2):347–356. doi: 10.1089/107632703764664819. [DOI] [PubMed] [Google Scholar]
- 241.Varady P., Li J.Z., Cunningham M. Morphologic analysis of BMP-9 gene therapy-induced osteogenesis. Hum Gene Ther. 2001;12(6):697–710. doi: 10.1089/104303401300057423. [DOI] [PubMed] [Google Scholar]
- 242.Paul R., Haydon R.C., Cheng H. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976) 2003;28(8):755–763. [PMC free article] [PubMed] [Google Scholar]
- 243.Mehta V., Kang Q., Luo J., He T.C., Haydon R.C., Mass D.P. Characterization of adenovirus-mediated gene transfer in rabbit flexor tendons. J Hand Surg Am. 2005;30(1):136–141. doi: 10.1016/j.jhsa.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 244.Bolt P., Clerk A.N., Luu H.H. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J Bone Jt Surg Am. 2007;89(6):1315–1320. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- 245.Xiong W., Goverdhana S., Sciascia S.A., Candolfi M., Zirger J.M., Barcia C. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80(1):27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xiong W., Candolfi M., Kroeger K.M. Immunization against the transgene but not the TetReduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16(2):343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shi C.X., Graham F.L., Hitt M.M. A convenient plasmid system for construction of helper-dependent adenoviral vectors and its application for analysis of the breast-cancer-specific mammaglobin promoter. J Gene Med. 2006;8(4):442–451. doi: 10.1002/jgm.867. [DOI] [PubMed] [Google Scholar]
- 248.Gonzalez-Aparicio M., Mauleon I., Alzuguren P. Self-inactivating helper virus for the production of high-capacity adenoviral vectors. Gene Ther. 2011;18(11):1025–1033. doi: 10.1038/gt.2011.58. [DOI] [PubMed] [Google Scholar]
- 249.Candolfi M., Curtin J., Xiong W., Kroeger K., Liu C., Rentsendorj A. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol Ther. 2006;14(3):371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sternberg S.H., Doudna J.A. Expanding the biologist's toolkit with CRISPR-Cas9. Mol Cell. 2015;58(4):568–574. doi: 10.1016/j.molcel.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 252.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13(5):273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Figueroa J.A., Reidy A., Mirandola L. Chimeric antigen receptor engineering: a right step in the evolution of adoptive cellular immunotherapy. Int Rev Immunol. 2015;34(2):154–187. doi: 10.3109/08830185.2015.1018419. [DOI] [PubMed] [Google Scholar]