Key Points
AG-348 is a small-molecule allosteric activator of WT red cell pyruvate kinase as well as mutant enzymes associated with hemolytic anemia.
Activity in vitro, in mice, and in red blood cells suggests it may address the underlying molecular pathology in PK deficiency patients.
Abstract
Pyruvate kinase (PK) deficiency is a rare genetic disease that causes chronic hemolytic anemia. There are currently no targeted therapies for PK deficiency. Here, we describe the identification and characterization of AG-348, an allosteric activator of PK that is currently in clinical trials for the treatment of PK deficiency. We demonstrate that AG-348 can increase the activity of wild-type and mutant PK enzymes in biochemical assays and in patient red blood cells treated ex vivo. These data illustrate the potential for AG-348 to restore the glycolytic pathway activity in patients with PK deficiency and ultimately lead to clinical benefit.
Introduction
Pyruvate kinase (PK) deficiency is a glycolytic enzymopathy first described 50 years ago1-4 that is characterized by a life-long chronic hemolytic anemia with severe comorbidities. Treatment is generally supportive with common interventions including splenectomy and blood transfusions.5,6 Thus, there is an unmet medical need for novel therapies that address the underlying basis for PK deficiency.
Mammals have 2 PK genes, encoding the PK muscle (PKM) and PK liver and red blood cell (PKLR) forms.7 PKLR controls the expression of the red blood cell (PK-R) or liver (PKL) isoforms from tissue-specific promoters. Loss-of-function mutations in the PKLR gene cause PK deficiency, with clinical symptoms apparently confined to red blood cells (RBCs), although the vast majority of mutations associated with PK deficiency affect both PK-R and PKL. PK-R catalyzes the final and irreversible step in glycolysis, converting phosphoenolpyruvate (PEP) to pyruvate, with concomitant formation of the energy carrier molecule adenosine triphosphate (ATP; Figure 1A). The importance of glycolysis in RBCs is highlighted by the fact that mutations in nearly every glycolytic enzyme result in hemolytic anemia.8 This is because mature RBCs lack mitochondria and rely almost exclusively on glycolysis to generate ATP.
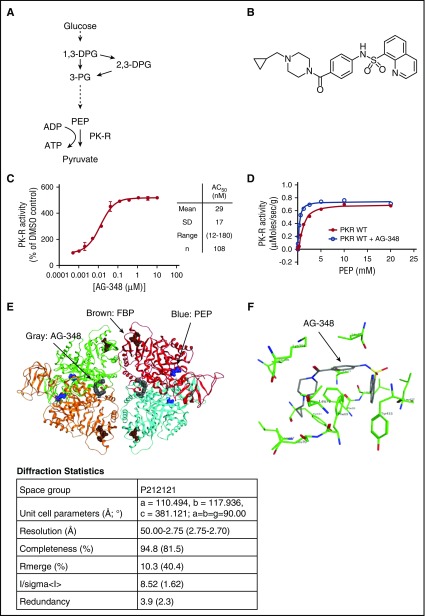
Figure 1.
Structure and function of PK-R and AG-348. (A) Simplified schematic of glycolysis. (B) Chemical structure of AG-348. (C) Activity of recombinant PK-R enzyme incubated with indicated concentrations of AG-348 (PEP = 0.065 mM). Mean, standard deviation, range, and number of experimental replicates are indicated in the column on right-hand side of panel. (D) Plot of activity of recombinant WT PK-R enzyme stimulated with PEP with or without preincubation by AG-348 (5 μM); the average of 3 technical replicates is shown. (E) Ribbon diagram of the cocomplex crystal structure of PK-R tetramer bound to AG-348 (shown as space-filling model in gray). PEP (blue) and FBP (brown) also shown as space-filling models. Diffraction statistics are shown below the diagram. (F) Model of AG-348 in PK-R allosteric- binding pocket illustrating key interactions. All error bars are standard deviations. ADP, adenosine diphosphate; PG, phosphoglyceric acid.
PK-R is a tetrameric enzyme that exists in equilibrium between a less active T-state and a more active R-state that can be induced by binding to the glycolytic intermediate fructose bisphosphate (FBP).9 Hundreds of mutations, most of which are single nucleotide missense mutations, have been described, which can have deleterious effects on PK-R catalytic activity, protein stability, or protein expression.6 For example, the R486W mutation, present in ∼25% of patients with PK deficiency in southern Europe,10 is thought to affect the catalytic efficiency of the enzyme, and the R510Q mutation, found in ∼40% of northern European patients with PK deficiency, significantly destabilizes the active tetrameric species.11 PK deficiency is an autosomal recessive disease, and patients are either homozygous, or more commonly, compound heterozygous for 2 mutant PK-R (mtPK-R) alleles.12
A variety of experimental therapies, including gene therapy,13-15 have been proposed for PK deficiency5,16,17; however, there are no approved drugs that directly target mutated PK-R.
We sought to address the underlying cause of PK deficiency by identifying a molecule that could induce and stabilize the active R-state of the PK-R tetramer, thus directly restoring PK activity to the mutant enzyme(s). Although PK-R is already subject to endogenous activation by FBP,9 this is insufficient to overcome the deleterious effects of mutations in patients with PK deficiency. We and others have previously described small-molecule allosteric activators of the PKM2 isoform of PK that bind in a pocket distinct from the FBP binding site.18,19 We asked whether we could identify a small-molecule agonist that targeted the analogous pocket in PK-R.
Here, we describe the mechanism of action and cellular effects of AG-348, a first-in-class small-molecule allosteric activator of PK-R. We demonstrate that AG-348 is a potent activator of wild-type (WT) PK-R in biochemical assays, in RBCs, and in vivo in mice. Moreover, as the genetic heterogeneity of PK deficiency dictates that a small-molecule therapy should enhance the activity of a spectrum of mtPK-R enzymes to maximize potential clinical benefit, we show that AG-348 is a pan-activator of several mtPK-R enzymes associated with PK deficiency and that ex vivo treatment of RBCs from patients with PK deficiency results in increased PK-R activity and changes in metabolism consistent with enhanced glycolysis. These data support the hypothesis that treatment of patients with PK deficiency with AG-348 may correct the underlying pathology of this disease.
Methods
Biochemistry
WT and mtPK-R proteins were expressed in the E. coli strain BL21 (Invitrogen, Carlsbad, CA) as an N-terminal His6-fusion, purified after refolding, and active fractions were snap-frozen in liquid nitrogen and stored at −80°C. PK-R activity was detected spectrophotometrically using a coupled enzyme system with lactate dehydrogenase. Reaction conditions were optimized for each individual mtPK-R to have the largest difference in activity following stimulation with FBP (see supplemental Methods, available on the Blood Web site). Compounds were dissolved in relevant vehicle (typically dimethyl sulfoxide [DMSO]) and were preincubated with PK-R proteins for 30 min before reaction initiation. Fold-change was defined as the ratio of activity between AG-348 versus DMSO treatment under the specific assay conditions. For thermostability studies, following compound incubation at room temperature, reactions were shifted to 53°C and aliquots taken over time to assess residual activity. For off-rate measurements, compounds were incubated at 10 × AC50 (concentration at which activity reaches 50% of maximum) for 30 min before dilution into activity buffer and assessment of residual activity over time.
Cell assays
Peripheral blood was obtained from a commercial source (Research Blood Components, Brighton, MA) or from patients following each institution’s relevant institutional review board protocol (Boston Children’s Hospital, Boston, MA; Tufts Medical Center, Boston, MA; and St. Jude Children’s Research Hospital, Memphis, TN). RBCs were isolated via Percoll centrifugation and resuspended in phosphate-buffered saline containing 1% glucose, 170 mg/L adenine, and 5.25 g/L mannitol. For activity and ATP assays, following addition of AG-348 (0.1% final DMSO concentration), cells were incubated at 37°C overnight. ATP was measured using CellTiterGlo (Promega, Madison, WI), and PK-R activity was assessed from the lysate using the coupled enzyme assay described above. For some experiments, PK-R activity was measured using direct detection of pyruvate using mass spectrometry. PK-R protein levels were quantitated using a MesoScale assay (antibodies from Abcam, Cambridge, UK: ab89071; and from Aviva, London, UK: OAGA00912).
For additional details and methods, please see the full supplemental Methods section.
Results
AG-348 is a quinolone sulfonamide (N-(4-(4-(cyclopropylmethyl)piperazine-1-carbonyl)phenyl)quinoline-8-sulfonamide; Figure 1B) related to a family of activators of PKM2 that we have previously described.19 We identified AG-348 through a series of medicinal chemistry efforts optimizing both compound activity against PK-R and molecular properties consistent with an investigational medicine for use in humans.
We recombinantly expressed and purified WT PK-R enzyme and evaluated its activity upon AG-348 treatment. AG-348 is a potent activator of PK-R, stimulating activity to ∼5-fold of DMSO control with a concentration that increases activity by 50% (AC50) of 29 ± 17 nM (n = 108; Figure 1C). Examination of key kinetic parameters reveals that AG-348 increases the catalytic efficiency (kcat/KM) of WT PK-R, primarily by increasing the affinity for the substrate PEP, as reflected in the change of KM for PEP following AG-348 treatment (Figure 1D; Table 1). AG-348 also is an activator of PKM2 and PKL (supplemental Figure 1).
Table 1.
Kinetic parameters from recombinant PK-R enzymes measured with or without treatment with AG-348
| Enzyme | No compound | 5 μM AG-348 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vmax, μmol/s/mg | PEP KM, mM | kcat, 1/s | kcat/KM | Vmax, μmol/s/mg | PEP KM, mM | kcat, 1/s | kcat/KM | % of WT PK-R kcat/KM | |
| WT | 0.69 | 1.13 | 42.5 | 37.6 | 0.74 | 0.32 | 45.7 | 142.0 | 378 |
| R532W | 0.22 | 1.73 | 13.6 | 7.9 | 0.21 | 0.42 | 12.9 | 30.4 | 81 |
| R510Q | 1.60 | 1.45 | 98.8 | 68.1 | 1.64 | 0.32 | 101.5 | 317.3 | 844 |
| R479H | 0.50 | 1.65 | 30.9 | 18.7 | 0.53 | 0.55 | 32.4 | 58.5 | 156 |
| R486W | 0.34 | 1.45 | 21.2 | 14.6 | 0.39 | 0.25 | 24.2 | 98.4 | 262 |
| G332S | 0.14 | 2.73 | 8.3 | 3.0 | 0.15 | 1.11 | 9.4 | 8.5 | 23 |
| R490W | 0.37 | 1.52 | 22.8 | 15.0 | 0.39 | 0.51 | 24.2 | 47.6 | 127 |
| G364D | 0.16 | 2.14 | 10.1 | 4.7 | 0.22 | 0.55 | 13.5 | 24.7 | 65 |
| T384M | 0.11 | 1.42 | 6.9 | 4.8 | 0.12 | 0.37 | 7.2 | 19.3 | 51 |
kcat, first-order rate constant; kcat/KM, measure of enzymatic efficiency39; KM, substrate concentration to achieve half maximal velocity; Vmax, maximum velocity.
To understand the molecular basis of PK-R activation by AG-348, we solved the crystal structure of AG-348 bound to PK-R to 2.75 Å resolution using the molecular replacement method. The overall cocomplex structure is in the active R-state also observed with FBP-bound PK-R9,20; however, AG-348 is bound in a pocket at the dimer–dimer interface (Figure 1E), distinct from the FBP-binding domain. The AG-348 binding pocket is deeply buried within the PK-R tetramer and occluded from the solvent; this could suggest a postbinding conformational change to the final R-state.
Although the coverage of electron density obtained for the compound was incomplete, it was sufficient to allow us to model 1 copy of the ligand in 1 pocket and to model 2 copies in the other site. With the symmetric nature of the binding pocket, each ligand establishes the same interactions (Figure 1F). The quinoline moiety sits in a flat and mainly apolar surface defined by residues Phe69, Leu70, and Leu73 from chain A and Phe69, Tyr433, and Leu437 from chain B. One of the 2 oxygen atoms of the sulfonamide accepts a hydrogen bond from the backbone oxygen of Tyr433. The nitrogen of the sulfonamide establishes a hydrogen bond with the backbone oxygen of Leu396, but this interaction is likely to be weak because closer analysis of the electron density does not allow a clear placement for this atom. The cyclopropyl ring of AG-348 sits in the other copy of the quinoline pocket. The electron density does not allow an easy placement of the 2 remaining rings; therefore, they were modeled in the pocket using low-energy conformations. There are no known mutations associated with PK deficiency that affect the amino acids listed above that make contact with AG-348.21
Next, we evaluated the effects of AG-348 on PK activity in intact human RBCs. We purified RBCs from whole blood from healthy donors and incubated them with AG-348 overnight. RBCs were then spun down, washed, and lysed by freeze thawing, and the lysate was evaluated for PK-R activity. AG-348 treatment increases PK-R activity in a dose-dependent manner to ∼2.5-fold of DMSO control (average 275% ± 56%) with an AC50 of 62 ± 35 nM (Figure 2A). The observation of increased PK-R activity even after washing and lysing the RBCs implies that AG-348 binds with slow-off kinetics, consistent with the binding mode observed in the crystal structure.
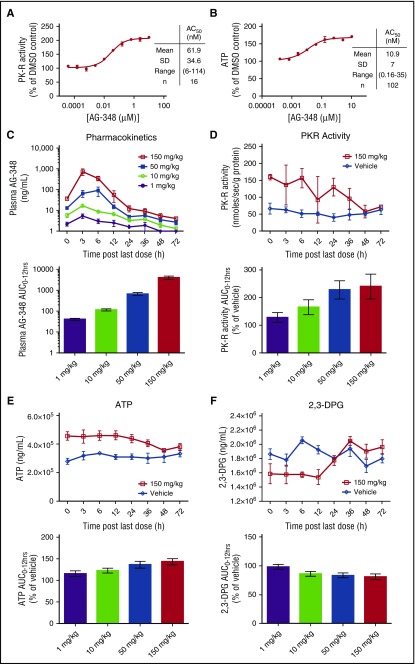
Figure 2.
AG-348 activates WT PK-R in RBCs from healthy donors and in mice. (A) WT PK-R activity from RBCs from healthy donors incubated overnight with indicated concentrations of AG-348 (PEP = 0.1 mM). Mean, standard deviation, range, and number of experimental replicates are indicated in the column on right-hand side of panel. (B) ATP levels of healthy- donor RBCs incubated overnight with indicated concentrations of AG-348. (C) Concentration of AG-348 measured in plasma of mice dosed with AG-348 twice daily for 7 days at indicated dose levels. The bar graph depicts the calculated AUC0-12 hours at each dose level. (D) PK-R activity in RBCs from mice dosed with AG-348 as described in panel C (PEP = 0.1 mM). For clarity, only the 150 mg/kg dose level is shown here. See supplemental Figure 2 for full data plots. The bar graph depicts the calculated AUC0-12 hours at each dose level. (E) ATP levels and calculated AUC0-12 hours in whole blood from mice dosed with AG-348 as described in panel D. (F) 2,3-DPG levels and calculated AUC0-12 hours in whole blood from mice dosed with AG-348 as described in panel D. For clarity, only the 150 mg/kg dose level is shown in panels E and F. All error bars are standard deviations.
Although the PK-R activity assay demonstrates that AG-348 can enter the RBC and bind to the PK-R enzyme, the rate of metabolism through the PK step in RBCs is highly regulated and potentially influenced by a host of factors, including FBP and PEP concentrations.22 For example, if the intracellular concentration of PEP was maintained at millimolar concentrations, rather than the actual physiological concentration of ∼10 μM,23,24 PK-R would be maximally activated, and there would be no further effect of AG-348 in the cell setting (Figure 1D). Thus, we tested whether activation of PK-R by AG-348 is capable of promoting glycolytic metabolism in intact RBCs, both in vitro and in vivo, where these factors would be fully accounted for. First, we measured the levels of ATP in DMSO control– or AG-348–treated RBCs. In RBCs isolated from different donors, AG-348 consistently increased ATP levels in a dose-dependent manner by an average of 60% over DMSO control with an AC50 of 10.9 ± 7 nM (n = 102; Figure 2B).
Next, we characterized the effects of AG-348 treatment on RBC metabolism in vivo in mice. C57/BL6 mice were dosed twice daily by oral gavage with AG-348 (1 mg/kg, 10 mg/kg, 50 mg/kg, and 150 mg/kg) for 7 days. At time points up to 72 h following the last dose, groups of mice (n = 4) were sacrificed, and plasma and whole blood samples were collected to evaluate drug exposure and pharmacodynamic markers, including levels of ATP, 2,3-diphosphoglycerate (2,3-DPG), and PK-R activity. Analysis of AG-348 exposure in the plasma showed dose-proportional increases in maximal concentration (Cmax) and the area under the curve (AUC) during each dosing period (AUC0-12 hrs; Figure 2C). Treatment with AG-348 was associated with dose-dependent changes in all 3 pharmacodynamic markers (Figure 2D-F; supplemental Figure 2). PK-R activity levels were increased up to ∼2-fold over vehicle with residual activity detectable up to 36 h after the last dose at the 150 mg/kg dose level (Figure 2D). There was a concomitant increase in ATP levels, with an ∼40% increase in the AUC0-12 hrs (Figure 2E). The upstream glycolytic intermediate 2,3-DPG was reduced accordingly by ∼20% (Figure 2F). The changes in levels of ATP and 2,3-DPG are substantial, given that these metabolites are among the most abundant intracellular metabolites in RBCs, present at millimolar concentrations.22 These data confirm that AG-348 can activate PK-R in vivo and that pharmacological activation of PK-R results in dramatic changes in RBC metabolism.
Valentini et al previously described the properties of PK-R mutants that they noted spanned distinct subdomains of the enzyme.20 We recombinantly expressed and purified several of these (G332S, G364D, T384M, R479H, R486W, R532W) as well as additional mtPK-Rs, including the commonly found R510Q enzyme (Figure 3A). Kinetic analyses (Table 1) reveal deficiencies in both the affinity for PEP substrate (KM) and the maximal velocity (Vmax) in comparison with the WT enzyme. This leads to overall reduced enzyme efficiency (kcat/KM) for the panel of mutants analyzed (range, 8% to 50% of WT kcat/KM; Table 1), except for R510Q (181% of WT kcat/KM) where the mutational defect is known to be in protein stability.11 Physiological PEP concentration23,24 (supplemental Figure 3) in both normal (∼10 µM) and PK-deficient (∼30 µM) RBCs is far below KM values (>1 mM) for WT or mtPK-R enzymes, so the PK-R enzyme reaction should not approach Vmax in the cellular setting. Therefore, in kinetic terms, increasing enzyme efficiency (kcat/KM) with respect to PEP substrate is predicted to be the most effective mode of action to compensate for glycolytic deficiency. Indeed, in this panel of mutants, if kcat/KM of the most severe mutant G322S could be increased by 6-fold, the result should theoretically be equivalent to 50% of WT PK-R activity (the approximate level of activity found in asymptomatic carriers of PK deficiency).
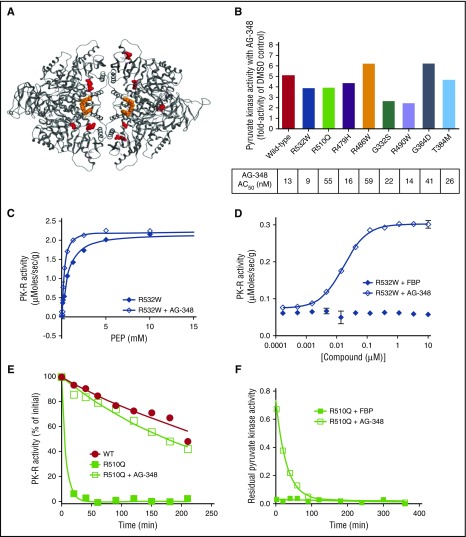
Figure 3.
AG-348 activates a spectrum of recombinant mtPK-R enzymes. (A) PK-R tetramer with sites of mtPK-R enzymes tested in Figure 3B highlighted in red. For clarity, each mutation site is only shown in a single monomer. (B) Fold activation and AC50 values of AG-348 treatment of mtPK-R enzymes (AG-348 = 10 μM, PEP, as listed in the supplemental table). (C) Activity of recombinant R532W mtPK-R enzyme stimulated with PEP with or without preincubation with AG-348 (AG-348 = 5 μM). (D) Activity of recombinant R532W mtPK-R enzyme incubated with indicated concentration of FBP or AG-348 (PEP = 0.05 mM). Panels C and D show the average of 3 technical replicates. (E) Residual activity over time of WT or R510Q recombinant enzymes following incubation at 53°C (AG-348 = 5 μM, PEP = 2 mM). (F) Off-rate measurement of AG-348 or FBP (both at 5 μM final assay concentration, PEP = 2 mM) from recombinant R510Q enzyme. All error bars are standard deviations.
We preincubated each mtPK-R enzyme with AG-348 for 30 min and repeated the same kinetic characterization. We observed enhanced activity of all mutant enzymes tested with a ≥2-fold change in activity in comparison with baseline (Figure 3B). With the exception of G332S, kcat/KM of the activated mtPK-R exceeded 50% of baseline WT enzyme efficiency (Table 1). In each case, the greatest contributor to the enhanced activity came from an increased affinity to PEP and not a change in Vmax (Table 1; representative graphs shown in Figure 3C and supplemental Figure 4A).
Activation of mtPK-R enzymes by AG-348 was dose dependent (representative data shown in Figure 3D and supplemental Figure 4B), with AC50 values ranging from 9 to 59 nM (Figure 3B). A compelling example of AG-348’s unique mechanism of action is illustrated by the R532W mutation, which renders PK-R insensitive to stimulation by FBP (Figure 3D). In contrast, AG-348 is able to activate the enzyme ≥4-fold with an AC50 of 9 nM, consistent with the fact that it occupies a distinct allosteric-binding pocket from that of FBP.
Next, we examined whether binding to AG-348 could confer heightened stability on mtPK-R enzymes. We examined the thermostability of WT and R510Q enzymes by subjecting them to incubation at 53°C for up to 4 h, with and without AG-348 pretreatment. The WT enzyme retains ∼50% residual activity at the conclusion of the assay, whereas the R510Q enzyme loses ≥90% of its activity within 10 min, with a calculated half-life (t1/2) ∼2% of that of the WT enzyme (Figure 3E). After preincubation of R510Q with AG-348 for 30 min at room temperature, the enzyme demonstrated markedly enhanced stability, with a t1/2 ∼70% of the WT enzyme (t1/2 (WT) = 258 min, t1/2 (R510Q) = 5 min, t1/2 (R510Q+AG-348) = 177 min; Figure 3E). We further evaluated the binding characteristics of AG-348 with the R510Q enzyme by measuring the off-rate of the compound, in comparison with the endogenous activator FBP. The off-rate of FBP is too fast to be measurable, whereas AG-348 has a significantly slower off-rate, with a t1/2 of ∼20 min (Figure 3F).
Collectively, these experiments demonstrate that AG-348 enhances the activity of a spectrum of mtPK-R enzymes associated with PK deficiency. AG-348 has the potential to act both by enhancing the efficiency of the residual enzyme that is present and by increasing the t1/2 of unstable mtPK-R tetramers.
Next, we evaluated the effect of AG-348 on RBCs from PK-deficient patients. Patient peripheral blood samples were obtained from local hospitals (samples A, B, C, D, and G, usually within several hours post–blood draw) or shipped via overnight courier (samples E and F). Minimal data were provided with these deidentified patient samples, except that it was understood that the patients had not received recent transfusions. We sequenced the PKLR gene in each patient sample and confirmed the presence of 2 mtPK-R alleles (Figure 4A). We also used antibody-based capture to quantitate the total levels of PK-R protein in the blood. A range of PK-R protein levels was observed (Figure 4B), from close to WT control to nearly undetectable (samples F and G).
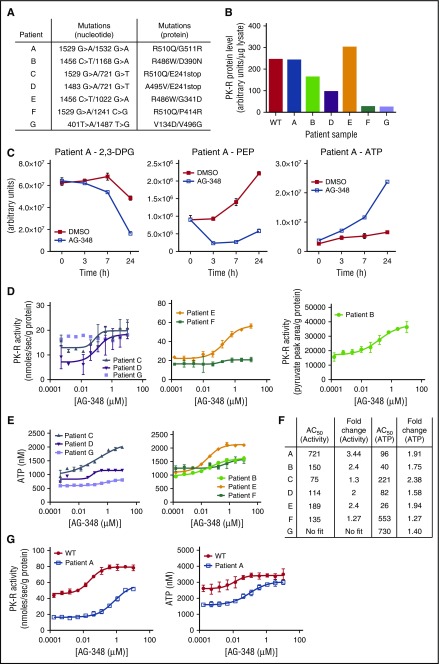
Figure 4.
AG-348 activation of mtPK-R in RBCs from patients with PK deficiency. (A) Genotypes of patients with PK deficiency described in these studies. (B) PK-R protein levels measured in patients with PK deficiency or WT controls. Data were not available from this assay for patient C, but a Western blot showing approximate protein levels in this patient is shown in supplemental Figure 6C. (C) Levels of 2,3-DPG, PEP, and ATP in RBCs from patient A at indicated times following treatment with DMSO or AG-348. (D) PK-R activity in PK-deficient RBCs incubated with AG-348 for 24 h (PEP = 0.5 mM). All PK-R activity measurements used the coupled enzyme spectrometric assay, except in the case of patient B, for whom activity was assessed by direct measurement of pyruvate formation using liquid chromatography followed by tandem mass spectrometry as described in the supplemental Methods. (E) ATP levels in PK-deficient RBCs incubated with AG-348 for 24 h. For panels D and E, please note that patient samples E and F were received via overnight courier post–blood draw, whereas other patient samples were received on the same day as blood draw, but potentially up to 8 h post–blood draw (supplemental Figure 5B-C). (F) Table showing AG-348 AC50 and fold-change observed in PK-R activity and ATP levels from indicated patient samples. AC50 values shown are in nanomolar. Fold-change (in relation to DMSO control) PK-R activity and ATP graphs are shown in supplemental Figure 6B. (G) PK-R activity (left) and ATP levels (right) in RBCs from patient A or a WT control (both prepared ∼30 min post–blood draw), incubated with AG-348 for 24 h. Panels D, E, and G show the average of 3 technical replicates. All error bars are standard deviations.
PK-deficient RBCs are characterized by changes in metabolism associated with defective glycolysis, including a build-up of the upstream glycolytic intermediate 2,3-DPG and PK-R substrate PEP and deficiency in the PK-R product ATP.23 We extracted metabolites from the blood of patient A as well as that of WT controls and observed differences supporting the presence of a constriction of glycolysis at the PK step (supplemental Figure 5A). In particular, ATP levels were reduced, and 2,3-DPG levels were elevated in patient A. PEP levels were found to be elevated ∼3-fold (estimated at ∼30 µM) in whole blood from patient A and separately quantitated to be ∼37 µM in patient G (supplemental Figure 3), in agreement with previously reported results,23 although metabolic comparisons between blood samples have a number of potential confounding factors, including differences in sample handling (supplemental Figure 5B-C) and the composition of blood (eg, the presence of reticulocytes and immature RBCs in PK-deficient blood).25
To determine the effect of AG-348 on PK-R activity and glycolysis metabolites, we purified RBCs from peripheral blood of patients with PK deficiency and incubated them with AG-348. In comparison with DMSO control, time-dependent decreases in relative levels of 2,3-DPG and PEP were seen in RBCs from patients A and B (Figure 4C; supplemental Figure 6A). Exposure of patient RBCs encompassing a range of genotypes to AG-348 led to dose-dependent increases in PK activity and ATP levels (Figure 4D-E; supplemental Figure 6B) at potencies similar to those observed in WT cells (Figure 4F). The effects seen in RBCs from patients F and G were less than those in the other samples (Figure 4F). Because AG-348 depends on physically binding to the PK-R protein to activate it and effect changes in cellular metabolism, these results are consistent with and likely due to the extremely low levels of residual PK-R protein present (Figure 4B).
As RBC metabolite levels can change significantly post–blood draw,26 we arranged for rapid transport of blood from patient A to our laboratory (within 30 min), along with a time-matched WT control. AG-348 treatment increased PK-R activity and ATP levels to within the range of baseline levels in WT control cells (Figure 4G), although WT cells had further increased ATP and PK-R activity after AG-348 exposure. Collectively, these data demonstrate enhanced glycolytic pathway activity induced by AG-348 in PK-deficient RBCs that contain a variety of mutations.
Discussion
The exact mechanism of extravascular hemolysis in PK deficiency is unknown, but it is likely to be related to the unique metabolic requirements of RBCs as they traverse the circulatory system. During passage through the hypoxic tissue in the spleen, mature erythrocytes and even mitochondria-containing reticulocytes depend on PK-R to generate ATP. Nathan et al27 elegantly demonstrated that PK-deficient reticulocytes had reduced lifespan through selective destruction in the spleen using radiolabeling of cell populations enriched by age. Experiments using agents such as cyanide and salicyate that can affect oxygen-dependent mitochondrial metabolism suggest that ATP depletion in PK-deficient cells leads to increased RBC dehydration through potassium efflux driven by increased activity of the Gardos channel.28-30 Given the genetic heterogeneity and broad spectrum of clinical presentation found in PK deficiency, it is likely that multiple factors influence the nature of hemolysis within individual patients. For instance, direct evidence for ineffective erythropoiesis has been found in splenic tissue from patients with PK deficiency.31 Nonetheless, the common feature found in patients with PK deficiency are genetic defects in the PKLR gene.
Here we have described the discovery and detailed biochemical, biophysical, cellular, and pharmacological characterization of AG-348, a novel small-molecule allosteric activator of both WT PK-R as well as mtPK-R enzymes associated with PK deficiency. We show that AG-348 binding induces the active R-state of the PK-R tetramer and that even in the absence of any deficiency of PK activity, activation of PK-R by AG-348 has profound effects on RBC metabolism in vivo in mice. These experiments show that AG-348 can increase PK activity in RBCs beyond the level achievable from the native effector molecule FBP and support its potential to correct defective metabolism in PK deficiency.
The relationship between activation of mtPK-R by AG-348 and physiological response (eg, increased red cell life span) is under investigation and will have to be determined with clinical studies. In our best estimation using recombinant enzymes and patient RBCs, AG-348’s ability to increase kcat/KM of mtPK-R enzymes to approximately that of the unstimulated WT activity is anticipated to result in a biological response. Ultimately, efficacious activation levels could be further dependent on a number of factors including PK-R protein concentration. It is also conceivable that PK-deficient cells might have adapted to their low PK-R activity levels and are thus poised to benefit from any additional stimulation. It is unlikely that full WT activity would have to be restored to see clinical benefit, because PK deficiency carriers have PK-R enzyme activity and ATP levels that are intermediate between patients with PK deficiency and WT controls and are asymptomatic.32
Our ex vivo experiments in PK-deficient RBCs demonstrate that AG-348 increases ATP levels and PK activity and thus may help address the energy deficiency that leads to the reduced lifespan of these cells (Figure 4D-E) or RBC precursors such as reticulocytes. Combined with the observation that glycolytic intermediates upstream of PK-R are reduced (Figure 4C), these results suggest that AG-348 treatment can induce a metabolic state more similar to WT RBCs than to baseline.
Importantly, AG-348’s activity in cells from patients A, B, and E (each being compound heterozygous for 2 different mtPK-R enzymes) demonstrate that the molecule is able to activate the mix of mtPK-R homotetrameric and heterotetrameric species presumably present in those RBCs. It may be sufficient to activate 1 of the 2 mutant alleles found in patients, as is illustrated in the case of patients C and D, in which 1 of the alleles is a severe truncation that is unlikely to contribute to the residual enzyme activity (Figure 4A). Given the genetic complexity of PK deficiency, it is probable that some mutant enzymes that do not respond to AG-348 will be found, perhaps in residues that govern the allosteric action mediated by AG-348.
AG-348 shows maximal activity in cells in which there are adequate levels of PK-R (Figure 4B,F; supplemental Figure 6C). In the ongoing natural history study for PK deficiency (ClinicalTrials.gov NCT02053480), ∼20% of non-Amish patients are estimated to have a genotype that predicts near-complete loss of functional PK-R protein expression, which would likely preclude activity of AG-348.33 For patients with low levels of PK-R caused by decreased protein stability (eg, R510Q), AG-348 may not effectively extend the lifespan of RBCs in the peripheral blood. However, our biochemical and structural analyses suggest the possibility that AG-348 might act on a precursor RBC to stabilize and extend the t1/2 of the newly synthesized mtPK-R enzyme. Further studies to explore this concept using ex vivo models of erythropoiesis34 are ongoing.
These data support the hypothesis that AG-348 may restore RBC glycolytic pathway activity in vivo and correct the underlying pathology of PK deficiency. Early data from a phase 2 study of AG-348 in patients with PK deficiency demonstrated that increases in hemoglobin are possible during treatment with AG-348.35 Additional analysis of these emerging data, and correlative studies with the experiments described in this article, should increase understanding of the mechanism of AG-348 and its potential to provide clinical benefit to patients. The ability of AG-348 to restore, at least in part, the function of mtPK-Rs offers the possibility that other enzymopathies might also be treated using a similar allosteric activator approach.
Several groups have found that ATP depletion leads to significant changes in RBC membrane structure and integrity in WT erythrocytes.36,37 Given that AG-348 can increase the activity of the WT PK-R enzyme, AG-348 could possibly be beneficial in RBC diseases that are characterized by increased energy stress, even if only secondary to the underlying basis of the disease. For instance, β-thalassemia is a hemoglobinopathy characterized by the presence of excess α-hemoglobin chains, which are cleared by ATP-dependent proteolytic mechanisms.38 It will be interesting to further investigate these possibilities in ex vivo studies on patient RBCs as well as in mouse models of disease.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Boston Children’s Hospital (especially Ellis Neufeld), Tufts Medical Center and the Floating Hospital for Children (especially Cathy Rosenfield and Furha Cossor), and St. Jude Children’s Research Hospital (especially Kerri Nottage) for assistance in obtaining patient samples for research purposes. Editorial assistance was provided by Christine Tomlins of Excel Scientific Solutions and was supported by Agios Pharmaceuticals.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.K. designed experiments, prepared figures, and wrote the paper; J.H., P.A.K., G.C., Y.C., G.H., C.H., S.G., B.D., Y.S., and K.J. designed and performed experiments and prepared figures; Z.L., Z.G., G.Y., H.T., C.F., Y.X., and X.L. performed experiments and analyzed data; F.S., J.P.-M., H.Y., S.-S.M.S., S.B., L.S., and L.D. guided the research; and all authors critically revised the manuscript and approved the final version.
Conflict-of-interest disclosure: C.K., J.H., P.A.K., G.C., G.H., Y.C., C.H., S.G., Y.S., K.J., B.D., S.B., S.-S.M.S., H.Y., J.P.-M., F.S., L.S., and L.D. are or were full-time employees of Agios Pharmaceuticals. Work conducted at Shanghai ChemPartner Ltd. was funded by Agios Pharmaceuticals.
The current affiliation for J.H. is KSQ Therapeutics, Cambridge, MA.
The current affiliation for C.H. is Berg Health, Framingham, MA.
The current affiliation for B.D. is The Consulting Biochemist, Arlington, MA.
The current affiliation for S.-S.M.S. and J.P.-M. is Decibel Therapeutics, Cambridge, MA.
The current affiliation for F.S. is Sage Therapeutics, Cambridge, MA.
Correspondence: Charles Kung, 88 Sidney St, Cambridge, MA 02139; e-mail: charles.kung@agios.com.
References
- 1.Germain D. Apropos of 3 new types of hemolytic anemia in children. Infantile pyknocytosis. Familial hemolytic anemia with erythrocytic inclusions and pigmenturia. Anemia caused by pyruvate-kinase deficiency [in French]. Pediatrie. 1962;17:323-326. [PubMed] [Google Scholar]
- 2.Tanaka KR, Valentine WN, Miwa S. Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood. 1962;19(3):267-295. [PubMed] [Google Scholar]
- 3.Oski FA, Diamond LK. Erythrocyte pyruvate kinase deficiency resulting in congenital nonspherocytic hemolytic anemia. N Engl J Med. 1963;269:763-770. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti P, Puxeddu A, Nenci G, Migliorini E. Haemolytic anaemia due to pyruvate-kinase deficiency. Lancet. 1963;282(7300):169-170. [DOI] [PubMed] [Google Scholar]
- 5.Grace RF, Zanella A, Neufeld EJ, et al. . Erythrocyte pyruvate kinase deficiency: 2015 status report. Am J Hematol. 2015;90(9):825-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanella A, Bianchi P. Red cell pyruvate kinase deficiency: from genetics to clinical manifestations. Best Pract Res Clin Haematol. 2000;13(1):57-81. [DOI] [PubMed] [Google Scholar]
- 7.Israelsen WJ, Vander Heiden MG. Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Wijk R, van Solinge WW. The energy-less red blood cell is lost: erythrocyte enzyme abnormalities of glycolysis. Blood. 2005;106(13):4034-4042. [DOI] [PubMed] [Google Scholar]
- 9.Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6(2):195-210. [DOI] [PubMed] [Google Scholar]
- 10.Zanella A, Bianchi P, Baronciani L, et al. . Molecular characterization of PK-LR gene in pyruvate kinase-deficient Italian patients. Blood. 1997;89(10):3847-3852. [PubMed] [Google Scholar]
- 11.Wang C, Chiarelli LR, Bianchi P, et al. . Human erythrocyte pyruvate kinase: characterization of the recombinant enzyme and a mutant form (R510Q) causing nonspherocytic hemolytic anemia. Blood. 2001;98(10):3113-3120. [DOI] [PubMed] [Google Scholar]
- 12.Zanella A, Fermo E, Bianchi P, Valentini G. Red cell pyruvate kinase deficiency: molecular and clinical aspects. Br J Haematol. 2005;130(1):11-25. [DOI] [PubMed] [Google Scholar]
- 13.Garate Z, Quintana-Bustamante O, Crane AM, et al. . Generation of a high number of healthy erythroid cells from gene-edited pyruvate kinase deficiency patient-specific induced pluripotent stem cells. Stem Cell Rep. 2015;5(6):1053-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Gomez M, Calabria A, Garcia-Bravo M, et al. . Safe and efficient gene therapy for pyruvate kinase deficiency. Mol Ther. 2016;24(7):1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meza NW, Alonso-Ferrero ME, Navarro S, et al. . Rescue of pyruvate kinase deficiency in mice by gene therapy using the human isoenzyme. Mol Ther. 2009;17(12):2000-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staal GE, van Berkel TJ, Nijessen JG, Koster JF. Normalisation of red blood cell pyruvate kinase in pyruvate kinase deficiency by riboflavin treatment. Clin Chim Acta. 1975;60(3):323-327. [DOI] [PubMed] [Google Scholar]
- 17.Blume KG, Arnold H, Hasslinger K, Löhr GW. Effect of riboflavin treatment on human red cell pyruvate kinase deficiency. Clin Chim Acta. 1976;71(2):331-334. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MJ, Brimacombe KR, Anastasiou D, et al. . ML265: a potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model. In: Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD: National Center for Biotechnology Information; 2010-2013. Available at: http://www.ncbi.nlm.nih.gov/books/NBK153222/. Accessed 24 November 2016. [PubMed] [Google Scholar]
- 19.Kung C, Hixon J, Choe S, et al. . Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem Biol. 2012;19(9):1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentini G, Chiarelli LR, Fortin R, et al. . Structure and function of human erythrocyte pyruvate kinase. Molecular basis of nonspherocytic hemolytic anemia. J Biol Chem. 2002;277(26):23807-23814. [DOI] [PubMed] [Google Scholar]
- 21.Canu G, De Bonis M, Minucci A, Capoluongo E. Red blood cell PK deficiency: an update of PK-LR gene mutation database. Blood Cells Mol Dis. 2016;57:100-109. [DOI] [PubMed] [Google Scholar]
- 22.Lakomek M, Winkler H, Pekrun A, et al. . Erythrocyte pyruvate kinase deficiency. The influence of physiologically important metabolites on the function of normal and defective enzymes. Enzyme Protein. 1994-1995;48(3):149-163. [PubMed] [Google Scholar]
- 23.Oski FA, Bowman H. A low Km phosphoenolpyruvate mutant in the Amish with red cell pyruvate kinase deficiency. Br J Haematol. 1969;17(3):289-297. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. New York, NY: Grune & Stratton; 1975 [Google Scholar]
- 25.Bartosz G, Grzelińska E, Wagner J. Aging of the erythrocyte. XIV. ATP content does decrease. Experientia. 1982;38(5):575. [DOI] [PubMed] [Google Scholar]
- 26.Guppy M, Attwood PV, Hansen IA, Sabaratnam R, Frisina J, Whisson ME. pH, temperature and lactate production in human red blood cells: implications for blood storage and glycolytic control. Vox Sang. 1992;62(2):70-75. [DOI] [PubMed] [Google Scholar]
- 27.Nathan DG, Oski FA, Miller DR, Gardner FH. Life-span and organ sequestration of the red cells in pyruvate kinase deficiency. N Engl J Med. 1968;278(2):73-81. [DOI] [PubMed] [Google Scholar]
- 28.Mentzer WC Jr, Baehner RL, Schmidt-Schönbein H, Robinson SH, Nathan DG. Selective reticulocyte destruction in erythrocyte pyruvate kinase deficiency. J Clin Invest. 1971;50(3):688-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glader BE. Salicylate-induced injury of pyruvate-kinase-deficient erythrocytes. N Engl J Med. 1976;294(17):916-918. [DOI] [PubMed] [Google Scholar]
- 30.Koller CA, Orringer EP, Parker JC. Quinine protects pyruvate-kinase deficient red cells from dehydration. Am J Hematol. 1979;7(3):193-199. [DOI] [PubMed] [Google Scholar]
- 31.Aizawa S, Kohdera U, Hiramoto M, et al. . Ineffective erythropoiesis in the spleen of a patient with pyruvate kinase deficiency. Am J Hematol. 2003;74(1):68-72. [DOI] [PubMed] [Google Scholar]
- 32.Ayi K, Liles WC, Gros P, Kain KC. Adenosine triphosphate depletion of erythrocytes simulates the phenotype associated with pyruvate kinase deficiency and confers protection against Plasmodium falciparum in vitro. J Infect Dis. 2009;200(8):1289-1299. [DOI] [PubMed] [Google Scholar]
- 33.Bianchi P, Fermo E, Lezon-Geyda K, et al. Molecular characterization of 140 patients in the pyruvate kinase deficiency (PKD) natural history study (NHS): report of 20 new variants. Poster presented at 57th Annual Meeting of the American Society of Hematology. 7 December 2015. Orlando, FL. [Google Scholar]
- 34.Giarratana MC, Kobari L, Lapillonne H, et al. . Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69-74. [DOI] [PubMed] [Google Scholar]
- 35.Grace RF, Rose C, Layton DM, et al. . Effects of AG-348, a pyruvate kinase activator, on anemia and hemolysis in patients with pyruvate kinase deficiency: early data from the DRIVE PK study [abstract]. Haematologica. 2016;101(s1). Abstract S466. [Google Scholar]
- 36.Park Y, Best CA, Auth T, et al. . Metabolic remodeling of the human red blood cell membrane. Proc Natl Acad Sci USA. 2010;107(4):1289-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weed RI, LaCelle PL, Merrill EW. Metabolic dependence of red cell deformability. J Clin Invest. 1969;48(5):795-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khandros E, Thom CS, D’Souza J, Weiss MJ. Integrated protein quality-control pathways regulate free α-globin in murine β-thalassemia. Blood. 2012;119(22):5265-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fersht A. Enzyme Structure and Mechanism. San Francisco, CA: W. H. Freeman; 1977. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.