Abstract
OBJECTIVES
This study sought to test the hypothesis that elimination of sites of abnormal repolarization, via epicardial RFA, suppresses the electrocardiographic and arrhythmic manifestations of BrS.
BACKGROUND
Brugada syndrome (BrS) is associated with ventricular tachycardia and ventricular fibrillation leading to sudden cardiac death. Nademanee et al. reported that radiofrequency ablation (RFA) of right ventricular outflow tract epicardium significantly reduced the electrocardiogram and arrhythmic manifestations of BrS. These authors concluded that low-voltage fractionated electrogram activity and late potentials are caused by conduction delay within the right ventricular outflow tract and that the ameliorative effect of RFA is caused by elimination of this substrate. Szel et al. recently demonstrated that the abnormal electrogram activity is associated with repolarization defects rather than depolarization or conduction defects.
METHODS
Action potentials (AP), electrograms, and pseudoelectrocardiogram were simultaneously recorded from coronary-perfused canine right ventricular wedge preparations. Two pharmacological models were used to mimic BrS genotype: combination of INa blocker ajmaline (1 to 10 μM) and IK-ATP agonist pinacidil (1 to 5 μM); or combination of Ito agonist NS5806 (4 to 10 μM) and ICa blocker verapamil (0.5 to 2 μM). After stable induction of abnormal electrograms and arrhythmic activity, the preparation was mapped and epicardial RFA was applied.
RESULTS
Fractionated low-voltage electrical activity was observed in right ventricular epicardium but not endocardium as a consequence of heterogeneities in the appearance of the second upstroke of the epicardial AP. Discrete late potentials developed as a result of delay of the second upstroke of the AP and of concealed phase 2 re-entry. Epicardial RFA of these abnormalities normalized Brugada pattern and abolished arrhythmic activity, regardless of the pharmacological model used.
CONCLUSIONS
Our results suggest that epicardial RFA exerts its ameliorative effect in the setting of BrS by destroying the cells with the most prominent AP notch, thus eliminating sites of abnormal repolarization and the substrate for ventricular tachycardia ventricular fibrillation.
Keywords: ECG, electrophysiology, J-wave syndrome, sudden cardiac death, ventricular arrhythmias
Brugada syndrome (BrS) is an inherited cardiac arrhythmia syndrome associated with increased vulnerability for ventricular tachycardia (VT) and fibrillation (VF) leading to sudden arrhythmic death, without the manifestation of structural heart disease. BrS is characterized by distinctive J-wave or ST-segment elevation in the right precordial electrocardiogram (ECG) leads. Three types of ECG morphology are generally recognized, but type I (coved type) is the only one diagnostic for the syndrome. The electrographic manifestations are often dynamic or concealed and can be unmasked using potent sodium channel blockers. The most effective and most commonly used agents are ajmaline, procainamide, and pilsicainide. The pathophysiology of the disease has long been a subject of debate.
The 2 principal hypotheses are the repolarization hypothesis and the depolarization hypothesis. In the repolarization hypothesis, an outward shift in the balance of current in the right ventricular outflow tract (RVOT) epicardium, the region displaying a highest transient outward current (Ito) density and action potential (AP) notch, leads to repolarization defects resulting in heterogeneous accentuation of the AP notch and loss of the dome. The resulting dispersion of repolarization is responsible for the development of phase 2 re-entry (P2R) and polymorphic VT. The transmural voltage-gradient created by the manifestation of a prominent AP notch in epicardium but not endocardium is responsible for inscription of the J-wave, which when broad and tall is often referred to as ST-segment elevation. In the depolarization hypothesis, conduction delay within the RVOT gives rise to the Brugada ECG and to the development of re-entrant arrhythmias.
The most compelling evidence in support of the depolarization hypothesis derives from the recent elegant work of Nademanee et al. (1). They recorded fractionated electrogram (EG) activity and late potentials from the anterior RVOT epicardium in patients with BrS and showed that radiofrequency ablation (RFA) of these epicardial (Epi) sites produced an ameliorative effect by reducing the manifestation of the BrS ECG and by suppressing the induction of VT/VF. Because fragmented EG activity is traditionally attributed to conduction abnormalities, the authors concluded that the underlying electrophysiological mechanism in patients with BrS is delayed depolarization over the anterior aspect of the RVOT epicardium (1). Similar results and conclusions were arrived at by Brugada et al. (2), who also demonstrated the ability of flecainide to identify the substrate for ablation.
Szel and Antzelevitch (3) recently tested this hypothesis and provided evidence in support of an alternative pathophysiologic basis for the EG abnormalities described by Nademanee et al. (1), showing that fractionated EG activity and late potentials can arise as a consequence of repolarization defects. The present study was designed to test the hypothesis that RFA suppresses arrhythmogenesis in the setting of BrS by abolishing the substrate responsible for these repolarization abnormalities.
METHODS
ARTERIALLY PERFUSED RIGHT VENTRICULAR WEDGE PREPARATION
All experiments were performed using arterially perfused canine right ventricular (RV) wedge preparations. Methodological details are as previously described (4). Transmembrane APs were simultaneously recorded from 2 Epi and 1 endocardial (Endo) or midmyocardial sites together with bipolar EG and a pseudo-ECG positioned along the transmural axis. Bipolar EGs were obtained using a quadripolar catheter (Livewire 7-F with 4-mm tip and 2-5-2-mm spacing, St. Jude Medical) and Teflon-insulated silver electrodes.
BrS MODELS
BrS was pharmacologically mimicked by targeting ion-channel currents affected by mutations associated with BrS (5). Two distinct models were created using a combination of agents that inhibit inward (depolarizing) currents and that increase outward (repolarizing) currents. The first model was designed to mimic a gain of function of the transient outward potassium current (Ito) using the Ito agonist NS5806 (4 to 10 μM) and a loss of function of calcium channel current (ICa) using the ICa blocker verapamil (0.5 to 2 μM). The second model was designed to mimic a gain of function of the adenosine triphosphate–sensitive potassium current (IK-ATP) using the IK-ATP agonist pinacidil (1 to 5 μM) and a loss of function of fast sodium channel current (INa) using the class IA INa blocker ajmaline (2 to 10 μM). The concentration of these compounds was increased until the development of fractionated EG activity, late potentials, closely coupled premature ventricular complexes, and polymorphic tachycardia (VT) and/or fibrillation (VF), either spontaneously or following programmed electrical stimulation.
ARRHYTHMOGENIC SUBSTRATES AND ABLATION PROCESS
After stable induction of arrhythmogenesis, the preparations were mapped using the quadripolar catheter to identify the arrhythmic substrates. Abnormal EGs were defined as potentials with prolonged duration (≥80 ms), fractionated potentials (consisting of 2 or more distinct components separated by ≥20 ms isoelectric segment), or late potentials (discrete high-frequency potentials appearing after the end of the QRS complex). RFA was performed using the previously described catheter and Biosense Webster–Stockert 70-F radiofrequency generator system at 30 W/65°C maximal parameters, avoiding coronary arteries. The vasculature was further protected by increased coronary perfusion rate at the time of ablation. The vascular resistance was continuously monitored by perfusion pressure. At the time of ablation, the preparation was turned sideways so that the epicardium was on top, and was covered with a thin (1 to 2 mm) layer of Tyrode solution. Deeper immersion of the ablation catheter results in distribution of RFA energy into the bath solution instead of the tissue surface. Effective convection was controlled by monitoring RFA parameters, using EP-Win software. The endpoint of ablation process was the complete elimination of abnormal EGs.
Immediately following ablation, we washed out the provocative agents to prevent desensitization or homogeneous loss of the AP dome throughout epicardium and subepicardium, thus abolishing Epi dispersion of repolarization (EDR) responsible for the vulnerable window that permitted the development of arrhythmogenesis.
After an average of 1 h of recovery, the provocative agents were reintroduced to the perfusate in the same concentration, as before ablation. Recordings were obtained for an additional 2 h. As a control, we performed the same ablation protocol on the endocardium instead of epicardium. In previous studies, we have performed time controls demonstrating the viability of these preparations over a 4- to 5-h period (4,6–8).
The methods for measurement of electrophysiological and electrocardiographic parameters are provided in the online appendix. Online Figure 1 schematically illustrates the parameters measured.
STATISTICAL ANALYSIS
Parameters of noninducible and inducible experiments were compared using Student t test (2-tailed p values are shown at each parameter). For multiple comparisons, 1-way repeated measures analysis of variance was used followed by all-pairwise comparisons using the Bonferroni method (Figures 6 and 7, Table 1). Data are shown as mean ± SEM throughout the study.
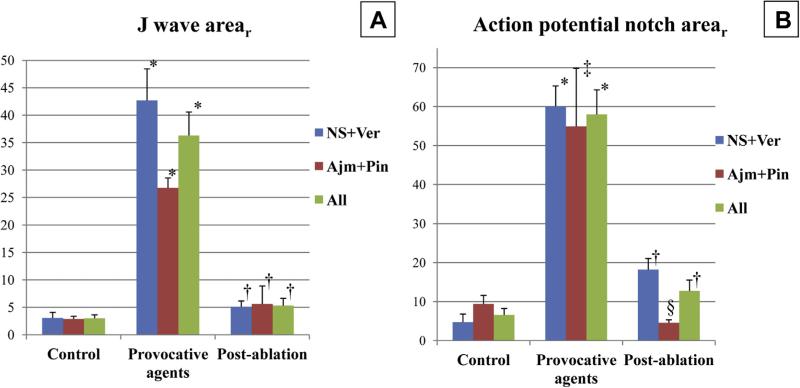
FIGURE 6. J-Wave and Maximal Action Potential Notch Area Recorded at Each Step of the Epicardial Ablation Experiments in the 2 Models.
Addition of the provocative agents, NS5806 + verapamil (NS + Ver; n + 6) or ajmaline + pinacidil (Ajm + Pin; n + 4), significantly increased, whereas epicardial ablation significantly decreased J-wave area (A) and maximal action potential notch area (B). *p < 0.001 versus control. †p < 0.001 versus pre-ablation. ‡p = 0.027 versus control. §p = 0.017 versus pre-ablation.
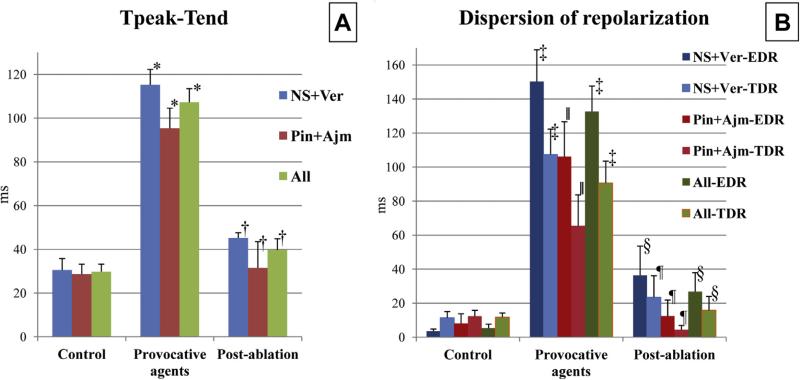
FIGURE 7. Tpeak-Tend Interval and Dispersion of Repolarization Recorded at Each Step of Epicardial Ablation Experiments in the 2 Models.
(A) Addition of the provocative agents, either NS5806 + verapamil (NS + Ver; n + 6) or pinacidil + ajmaline (Pin + Ajm; n = 4), significantly increased, whereas radiofrequency ablation of epicardium significantly reduced Tpeak-Tend intervals.*p < 0.01 versus control, †p < 0.01 versus pre-ablation. (B) Addition of the provocative agents significantly increased but epicardial ablation significantly reduced both TDR and EDR. Dark colors represent EDR, pale colors represent TDR. ‡p < 0.001 versus control. §p < 0.001 versus pre-ablation. ∥p ≤ 0.015 versus control. ¶p ≤ 0.028 versus pre-ablation. The highest Tpeak-Tend values were associated with the most pronounced delay of the second action potential upstroke giving rise to negative T waves and appeared just before the start of arrhythmic activity. EDR = epicardial dispersion of repolarization; TDR = transmural dispersion of repolarization; Tpeak-Tend = interval between the peak and the end of the T wave.
TABLE 1.
Electrocardiogram and AP Parameters in Epicardial Ablation Experiments
| Control | Provocative Agents | Epicardial Ablation | |
|---|---|---|---|
| NS5806 + verapamil* | |||
| J-wave arear | 3.07 ± 1.04 | 42.68 ± 5.77† | 5.11 ± 1.06‡ |
| Tpeak-Tend, ms | 30.53 ± 5.27 | 115.27 ± 7.05† | 45.18 ± 2.43‡ |
| AP notch arear | 4.74 ± 2.10 | 60.05 ± 5.24† | 18.19 ± 2.88‡ |
| EDR, ms | 3.6 ± 1.24 | 150.25 ± 18.74† | 36.38 ± 17.18‡ |
| TDR, ms | 11.38 ± 3.76 | 107.4 ± 14.91† | 23.4 ± 12.82‡ |
| Pinacidil + ajmaline§ | |||
| J-wave arear | 2.90 ± 0.48 | 26.75 ± 1.83† | 5.61 ± 3.28‡ |
| Tpeak-Tend, ms | 28.65 ± 4.61 | 95.38 ± 9.21† | 31.55 ± 11.99‡ |
| AP notch arear | 9.38 ± 2.25 | 54.90 ± 14.93† | 4.56 ± 0.90‡ |
| EDR, ms | 8.1 ± 5.64 | 106.2 ± 20.49† | 12.4 ± 9.5‡ |
| TDR, ms | 12.33 ± 3.44 | 65.55 ± 18.17† | 4.43 ± 2.49‡ |
| All∥ | |||
| J-wave arear | 3.0 ± 0.63 | 36.31 ± 4.28† | 5.31 ± 1.35‡ |
| Tpeak-Tend, ms | 29.78 ± 3.48 | 107.31 ± 6.2† | 39.73 ± 5.11‡ |
| AP notch arear | 6.6 ± 1.65 | 57.99 ± 6.29† | 12.74 ± 2.79‡ |
| EDR, ms | 5.4 ± 2.3 | 132.63 ± 14.99† | 26.79 ± 11.21‡ |
| TDR, ms | 11.76 ± 2.51 | 90.66 ± 12.84† | 15.81 ± 8.07‡ |
Values are s mean ± SEM.
n = 6.
p < 0.05 versus control.
p < 0.05 versus pre-ablation.
n = 4.
n = 10.
AP = action potential; EDR = epicardial dispersion of repolarization; TDR = transmural dispersion of repolarization; Tpeak-Tend = T wave peak-to-end duration.
RESULTS
INDUCTION OF THE ELECTROCARDIOGRAPHIC AND ARRHYTHMIC MANIFESTATIONS OF BrS. Pinacidil + ajmaline BrS model
After the addition of pinacidil (1 to 5 μM) + ajmaline (2 to 10 μM) to the coronary perfusate, we observed a significant increase in maximal J-wave area, AP notch area, EDR, TDR, and interval between the peak and the end of the T-wave (Figures 1A, 2B, 2C, 6, and 7, Table 1).
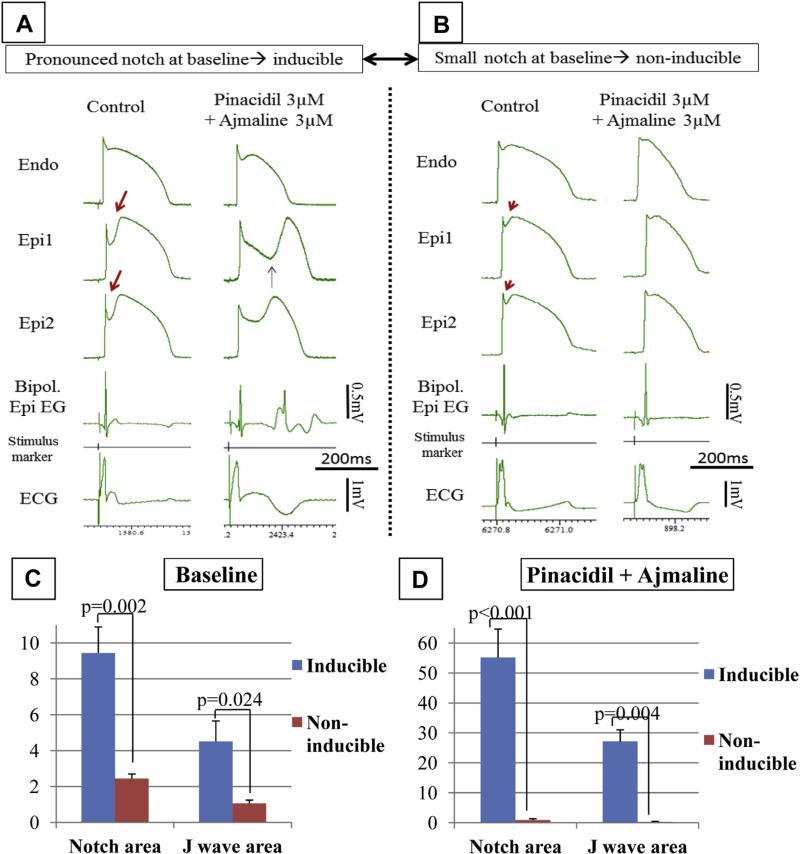
FIGURE 1. Effect of a Combination of Pinacidil and Ajmaline to Induce Arrhythmogenesis and Late Potentials Depends Exclusively on the Magnitude of the Epicardial Action Potential Notch and Consequentional J-Wave at Baseline.
(A and B) Each column shows action potentials simultaneously recorded from an endocardial (Endo) and 2 epicardial (Epi1 and Epi2) sites together with a bipolar epicardial electrogram (Bipol. Epi EG) and an electrocardiogram (ECG) recorded accross the bath. (A) Preparation exhibiting a large spike and dome action potential morphology at baseline (control). The provocative agents induce a Brugada syndrome ECG and concealed phase 2 re-entry giving rise to distinct late potentials (Bipol. Epi EG). (B) Preparation exhibiting a relatively small spike and dome action potential morphology at baseline. The provocative agents do not induce a Brugada syndrome ECG, but diminish the J-wave. (C and D) Comparison of epicardial action potential notch arear and J-wave arear of preparations vulnerable (inducible) and nonvulnerable (noninducible) to the induction of Brugada syndrome pattern and arrhythmias. n = 6 for inducible and n = 5 for noninducible preparations. (C) Parameters at baseline. Inducible preparations showed an average 3.9-fold higher action potential notch and 4.3-fold higher J-wave area at baseline, compared with the noninducible ones (inducible vs. noninducible, p = 0.002 and p = 0.024 for notch arear and J-wave arear, respectively). (D) After the addition of provocative agents, inducible preparations showed a pronounced increase (p = 0.004 vs. baseline), whereas noninducible preparations showed a significant decrease (p = 0.017 vs. baseline) in both J-wave and action potential notch area. The provocative agents produced an average 60.5-fold higher notch area and 88.7-fold higher J-wave area in inducible compared with noninducible preparations (inducible vs. noninducible, p < 0.001 and p = 0.004 for notch area and J-wave arear, respectively).
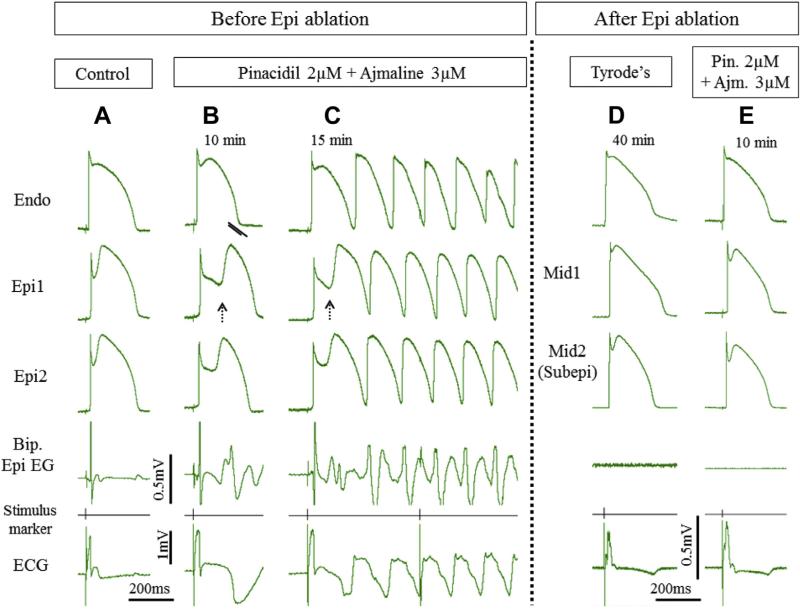
FIGURE 2. Radiofrequency Ablation of Epi Suppresses the Electrocardiographic and Arrhythmic Manifestations of Brugada Syndrome in Coronary-Perfused Canine Right Ventricular Wedge Model Generated Using a Combination of Pinacidil + Ajmaline.
Traces are as described in Figure 1. (A) Epi displays pronounced action potential notch at baseline. (B) Addition of pinacidil (2 μM) and ajmaline (3 μM) to the coronary perfusate induces the typical Brugada syndrome phenotype. The bipolar epicardial electrogram (Bip. Epi EG) shows fractionated electrogram activity and a late potential due to the development of concealed phase 2 re-entry. (C) Successful conduction of phase 2 re-entrant beat gives rise to ventricular tachycardia. (D) Recorded 40 min after Epi ablation and withdrawal of the provocative agents. Action potential recordings were obtained from midmyocardial (Mid) and subepicardial (Subepi) layers due to ablation of the epicardial cells. (E) Recorded 10 min after reintroduction of the provocative agents to the perfusate (in the same concentration as before). After Epi ablation, Brugada syndrome phenotype and arrhythmias were no longer inducible. Abbreviations as in Figure 1.
Abnormal EGs, P2R activity, and VT/VF developed in 6 of 11, 6 of 11, and 4 of 11 preparations, respectively (Figures 1A, 2B, 2C, Online Table 1). EGs displaying fractionated low-voltage activity and discrete late potentials developed exclusively in the presence of accentuated spike-and-dome morphology and concealed P2Rs, respectively (Figures 1A and 2B). Preparations that failed to develop the Brugada pattern and arrhythmic activity (“noninducible”), either spontaneously or in response to programmed electrical stimulation, displayed significantly lower J-wave and AP notch area at baseline and after the addition of the provocative agents, than those in which the provocative agents were successful in inducing the ECG and arrhythmic manifestations of BrS (“inducible”) (Figure 1B). At baseline, inducible versus noninducible values were 4.5 ± 1.1 versus 1.1 ± 0.2 (p = 0.0238) for J-wave area, and 9.4 ± 1.5 versus 2.4 ± 0.3 (p = 0.002) for AP notch area (Figures 1C and 1D).
Interestingly, in preparations displaying small basal AP notch (Figure 1B), ajmaline decreased the J-wave and AP notch area, presumably because of the multiple effects on other ion currents, including Ito and widening of the QRS engulfing the J-wave (Figures 1 and 3B) (9). These observations are consistent with clinical studies reporting improvement in the ECG manifestation of early repolarization pattern following ajmaline infusion (Online Figure 2) (10,11). To confirm these findings, we compared the effect of high-dose ajmaline (10 μM) in the absence and presence of the Ito agonist NS5806, in the same preparation. The results supported our conclusion that the effect of ajmaline is dependent on the magnitude of AP notch before introduction of ajmaline. This also provides an explanation for the RVOT predominance of the disease, because this region of the heart displays the most prominent AP notch (12). As illustrated in Figure 3, ajmaline (10 μM), when applied alone, reduced the size of the J-wave and AP notch (Figure 2B). Ajmaline, in this setting, produced little effect on the AP dome, ST segment, or Epi EG despite prolongation of QRS and slowing of transmural conduction. The effect was reversible on washout. When the Epi AP notch was accentuated by pre-treatment with the Ito agonist NS 5806 (7 μM) (Figure 3D), addition of ajmaline (10 μM) led to marked accentuation of Epi AP notch, leading to the development of abnormal EG activity, type I ST-segment elevation, and concealed P2R. The appearance of abnormal EG activity was secondary to the inhomogeneous accentuation of the AP notch and loss of the AP dome (Figures 3E and 3F). This association is further supported by the observation that at the maximal effect of high-dose ajmaline, loss of the Epi AP dome throughout the preparation resulted in loss of the fractionated EG activity, despite the further prolongation of QRS and further slowing of transmural conduction (Figure 3G).
FIGURE 3. The Opposite Effects of Ajmaline to Mask or Accentuate the J-Wave (Jw) Depend on the Basal Level of Ito-Mediated Action Potential Notch.
Traces are as described in Figure 1. The bipolar electrograms (Bip.-EG-Epi) were recorded from the epicardium using 3 different low cut filter settings (10 Hz, 30 Hz, and 100 Hz) and 25 0Hz “high cut” filter. When action potential notch was small (A), 10-μM ajmaline produced a decrease in J-wave and action potential notch area (B). The effect was reversible on wash-out (C). However, when the action potential notch was amplified using the Ito agonist NS5806 (D), the same concentration of ajmaline caused a marked accentuation of the JW appearing as an ST-segment elevation (E to G). Fragmented electrogram activity developed progressively as the repolarization defects became more pronounced and heterogeneous (D to F). Pronounced action potential notch (without re-entry) produced delayed potentials in a lower frequency range (D), whereas phase 2 re-entry depicted as “high-frequency” spike (E and F). After 15 min of ajmaline, loss of the action potential dome occurred throughout the preparation, which led to disappearance of the late potentials (G). Subendo/Mid = action potential from the subendocardium/midmyocardium; other abbreviations as in Figure 1.
Inducibility of the BrS phenotype was independent of conduction-dependent parameters. There was no difference in QRS duration and transmural conduction time between inducible and noninducible preparations (inducible vs. noninducible at baseline, p = 0.242 and p = 0.822 for QRS and conduction time, respectively).
NS5806 + verapamil BrS model
The addition of NS5806 (4 to 10 μM) + verapamil (0.5 to 2 μM) to the coronary perfusate significantly increased maximal J-wave area (3.1 ± 1.0 ms vs. 42.7 ± 5.8 ms), AP notch area (4.7 ± 2.1 ms vs. 60.1 ± 5.2 ms), EDR (3.6 ± 1.2 msvs. 150.3 ± 18.7 ms), TDR (11.4 ± 3.8 ms vs. 107.4 ± 14.9 ms), and interval between the peak and the end of the T wave (30.53 ± 5.27 ns vs. 115.27 ± 7.05 ms) when compared with control (Figures 6 and 7, Table 1). Fractionated EG and/or late potentials, P2R activity, and VT/VF developed in 9 of 9 experiments (Figure 4, Online Table 1).
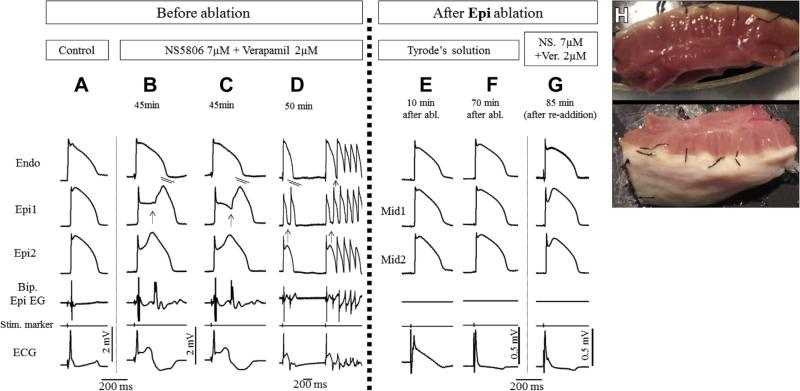
FIGURE 4. Radiofrequency Ablation of Epi Suppresses the Electrocardiographic and Arrhythmic Manifestations of Brugada Syndrome in Coronary-Perfused Canine Right Ventricle Wedge Model Generated Using a Combination of NS5806 + Verapamil.
Traces are as described in Figure 1. (A) Control. (B to D) The addition of NS5806 7 μM and verapamil 2 μM to the perfusate induced pronounced J-waves and phase 2 re-entry depicting as abnormal electrogram activity when concealed, and giving rise to ventricular fibrillation when it succeeds in propagating out. (E and F) Recovery period of the preparation after epicardial ablation. Note the normalization of ST-segment elevation after 70 min. Action potential recordings were obtained from midmyocardial (Mid) and subepicardial layers due to inactivation of the epicardium. (G) With superficially ablated epicardium, the readministration of the provocative agents (in the same concentration as before) did not produce pronounced J-waves or arrhythmic activity. (H) Photograph of wedge preparation after epicardial ablation to a depth of 1 to 2 mm, taken at the end of the experiment. Abbreviations as in Figure 1.
Fractionated electrical activity was observed in RV epicardium but not endocardium as a consequence of heterogeneities in the appearance of the second upstroke of the Epi APs (Figures 4B and 4C). Discrete late potentials developed as a result of major delays in the appearance of the second AP upstroke and a result of concealed P2R (Figures 4B and 4C).
Both pharmacological models of BrS exhibited abnormal EGs diffusely dispersed throughout much of the epicardium, as observed by Nademanee et al. (1) in the RVOT of hearts of human victims of BrS. Late potentials associated with delayed second upstroke of the RV Epi AP and with concealed P2R appeared with a delay of up to 209 ms after the QRS, averaging 103.4 ± 32.4 ms (n = 926), very similar to the delays reported by Nademanee et al. (1).
EFFECT OF RFA
Epi ablation prevented the development of VT/VF in 10 of 10 and P2R in 9 of 10 preparations, irrespective of the pharmacological model used (Figures 2 to 4, Online Table 1), whereas Endo ablation failed to suppress arrhythmogenesis in all preparations tested (0 of 5) (Figure 5F, Online Table 1). Epi ablation significantly reduced J-wave arear (36.31 ± 4.28 vs. 5.31 ± 1.35; p < 0.001), AP notch arear (57.99 ± 6.29 vs. 12.74 ± 2.79; p < 0.001), EDR (132.63 ± 14.99 ms vs. 26.79 ± 11.21 ms; p < 0.001), TDR (90.66 ± 12.84 ms vs. 15.81 ± 8.07 ms; p < 0.001), and interval between the peak and the end of the T wave (107.31 ± 6.2 ms vs. 39.73 ± 5.11 ms; p < 0.001) compared with pre-ablation, in all preparations tested (Figures 4G, 2E, 6, and 7). In contrast, Endo ablation did not alter these parameters.
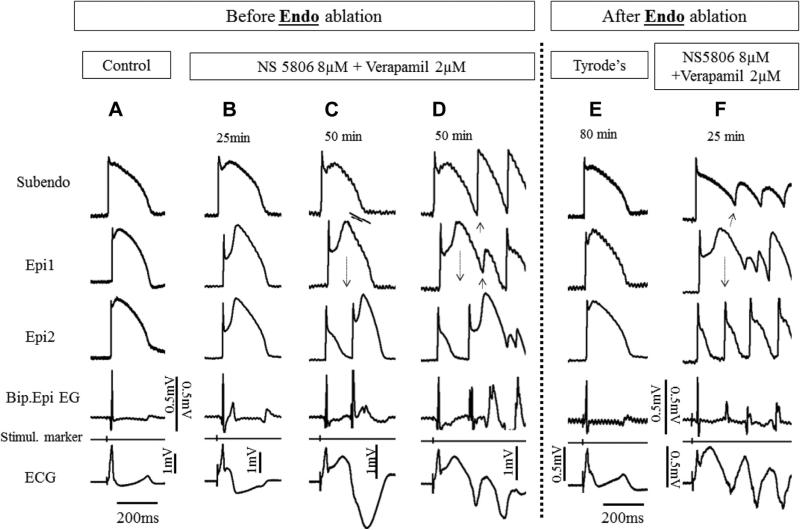
FIGURE 5. Radiofrequency Ablation of Endo Fails to Suppress Brugada Syndrome Phenotype.
Traces are as described in Figure 1. (A) Control. (B) Recorded 25 min after addition of 8 mM NS5806 and 2 μM verapamil to the coronary perfusate. Homogeneous delay of the second upstroke of the epicardial action potentials gives rise to a late potential on the bipolar electrogram (EG). (C) Recorded 50 min after addition of provocative agents. Concealed phase 2 re-entry gives rise to a high-frequency late potential in the bipolar electrogram. (D) Recorded 10 seconds later. Successful propagation of phase 2 re-entrant extrasystole initiates polymorphic ventricular tachycardia. (E) Recorded 80 min after Endo ablation and withdrawal of the provocative agents. (F) Recorded 25 min after reintroduction of the provocative agents. Endo ablation failed to exert any beneficial effect: the reintroduction of provocative agents induced pronounced Brugada syndrome phenotype with sustained polymorphic tachycardia. Subendo = subendocardium; other abbreviations as in Figure 1.
As expected, Epi ablation caused temporary ST-segment elevation, whereas Endo ablation resulted in temporary ST-segment depression, as a result of the development of injury currents. Both dissipated over a period of an hour or more (but did not always normalize completely).
DISCUSSION
The seminal study of Nademanee et al. (1) showing an ameliorative effect of Epi ablation of regions of fractionated EG activity in the RVOT of patients with BrS was interpreted to suggest that ablation of regions of delayed conduction is responsible for suppression of arrhythmogenesis. The present study provides a test of an alternative hypothesis. In the 2 different models of BrS used in this study, the fractionated bipolar EG activity and late potentials were not caused by major conduction delays, but rather by repolarization defects created by an outward shift in the balance of current active during the early phases of the AP. This outward shift of current can be achieved either with mutations or agents that increase outward current or those that decrease inward current, or a combination of the 2.
We studied 2 experimental models of BrS using a combination of outward current channel agonists (pinacidil or NS5806) and inward current channel blockers (ajmaline or verapamil), thus mimicking the genetic defects and ion channel heterogeneities known to be associated with BrS (13).
Although late potentials and fractionated EG activity are traditionally ascribed to slow or delayed conduction, our results provide further evidence in support of the hypothesis that in the setting of BrS, abnormal EG activity can be a consequence of repolarization defects, consistent with the recent report of Szel and Antzelevitch (3). In addition to our previous findings pointing to concealed P2R as the cause of high-frequency late potentials (3), we here report that delay of the second upstroke of Epi APs can also manifest as discrete late potentials (“spikes”) (Figures 2B, 2C, 3D, and 5B, Online Figures 3 and 4). The magnitude and delay of these electrophysiological abnormalities depends critically on the characteristics of the second upstroke and notch of the Epi AP (Online Figure 3).
In both models, the extent of repolarization abnormalities induced by the provocative agents was directly proportional to the degree of phase 1 repolarization and AP notch observed under baseline conditions (Figure 1). It is noteworthy that homogeneous “loss of the second upstroke” of APs throughout the epicardium and subepicardium led to disappearance of these late potentials, as anticipated (Figure 3G).
The morphology and frequency range of these potentials are similar to those described by Nakagawa et al. (14) in early repolarization syndrome–related idiopathic VF (Online Figure 4). These observations may explain the lower frequency range of late potentials recorded in SAECG of patients with BrS when compared with late potentials associated with arrhythmogenic RV dysplasia, a disease associated with significant conduction delay secondary to structural defects caused by fibrofatty replacement of cardiomyocytes (15–17).
The fractionation of the Epi EG and appearance of late potentials following the addition of ajmaline is very similar to that recorded by Sacher et al. (18) in the epicardium of the RVOT of a BrS patient. Although the authors interpreted this phenomenon as a proof of depolarization abnormality, in our experiments, ajmaline exerted these effects via accentuation of the AP notch and induction of P2R, and not via a slowing of conduction (Figures 1 and 3).
In preparations exhibiting a relatively small AP notch, ajmaline failed to produce any sign of BrS, even at relatively high concentrations (Figures 1B and 3B, Online Figure 2). These observations explain why ajmaline produces ST-segment elevation in the right precordial ECG leads of BrS patients, but fails to provoke a Brugada pattern in other ECG leads or in healthy subjects. This observation may also explain the recent observations of Park et al. (19). These authors genetically engineered Yucatan minipigs to heterozygously express a nonsense mutation in SCN5A (E558X) originally identified in a child with BrS. Patch clamp analysis of atrial myocytes isolated from the SCN5AE558X/+ pigs showed a loss of function of INa. Conduction abnormalities consisting of prolongation of P-wave, QRS complex, and PR interval were observed, but a BrS phenotype was not observed, not even after the administration of flecainide. These observations are expected because of the lack of Ito and lack of an AP notch in the pig, which is a prerequisite for the development of the repolarization abnormalities associated with BrS. These findings collectively lend strong support for the repolarization hypothesis.
Zhang et al. (20) recently performed noninvasive ECG imaging on 25 BrS and 6 right bundle branch block patients. The authors reported the presence of slow discontinuous conduction and steep dispersion of repolarization in the RVOT of patients with BrS. In 6 BrS patients the response to an increase in rate was examined. Increasing rate increased fractionation of the EG but reduced ST-segment elevation (Brugada phenotype), indicating that the conduction impairment was not the principal cause of the BrS ECG.
If, as suggested by our findings, abnormal EG activity in the setting of BrS is not caused by major conduction delay, then why is ablation effective in normalizing the Brugada pattern and preventing the development of VT? Our results suggest that ablation is effective because it destroys the cells with the most prominent AP notch in ventricular epicardium, presumably the cells with the largest AP notch and highest Ito density, thus preventing the development of accentuated repolarization abnormalities that are responsible for causing a pronounced Epi and TDR, the substrates for the genesis of P2R and VT. Our results show that pronounced repolarization heterogeneities can recapitulate the electrographic manifestations of the BrS and the response to Epi RFA.
It should be emphasized, that our study is not aimed at proving the exclusivity of repolarization hypothesis. It does not deny the possible contribution of slow or delayed conduction to the development of arrhythmogenesis in the setting of BrS, but does provide support for the hypothesis that in experimental models that mimic the principal genetic factors and ion channel heterogeneities responsible for BrS, repolarization abnormalities alone are capable of causing the ECG and arrhythmic manifestations of the syndrome.
There is no doubt that depolarization abnormalities can contribute to arrhythmogenesis in BrS and that several factors can modulate both the degree of repolarization and depolarization abnormalities, including the degree of electrotonic coupling, transmural differences in tissue resistivity, differences in the expression of connexin 43, and transmural differences in the expression of other currents (7,21–24). A recent study by Nademanee et al. (25) suggests that microscopic fibrosis plays a role in the pathophysiology of BrS. Regardless of whether it is a late-stage by-product or the original primary cause of BrS, this can lead to conduction impairment. To the extent that propagation of an AP dome is similar to propagation of an early afterdepolarization, fibrosis has also been shown to markedly reduce the number of myocytes with abnormal repolarization required to overcome the source-sink effect and thus generate a propagated impulse. It is intriguing to speculate that the development of mild fibrosis in the RVOT of patients with BrS may increase arrhythmic risk by promoting (25) the propagation of otherwise silent triggers, as has been demonstrated in the case of early and delayed after-depolarizations (26,27). It seems reasonable to suggest that this facilitation can also apply to the propagation of the AP dome giving rise to P2R.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: RFA is an emerging therapy for Brugada syndrome, providing an adjunct or replacement to implantable cardioverter-defibrillator. Identification of substrates amenable to RFA may be helpful in identification of individuals who can benefit from this procedure.
TRANSLATIONAL OUTLOOK 1: Understanding the basis for low-voltage fractionated electrogram activity and high-frequency late potentials in the right ventricular outflow tract may be helpful in targeting RFA and adjunctive pharmacological therapy.
TRANSLATIONAL OUTLOOK 2: Demonstration that low-voltage fractionated electrogram activity and late potentials can be caused by defects in repolarization rather than depolarization or conduction, represents a paradigm shift in the understanding of such activity with wide implications for both diagnosis and treatment of cardiac disease.
ACKNOWLEDGEMENTS
The authors thank Biosense Webster for making the radiofrequency ablation equipment available for this study, and Robert Goodrow and Dr. José Di Diego for their kind technical support.
Dr. Antzelevitch is supported by National Institutes of Health grant HL47678 and the Wistar and Martha Morris Fund.
ABBREVIATIONS AND ACRONYMS
- AP
transmembrane action potential
- BrS
Brugada syndrome
- ECG
electrocardiogram
- EDR
epicardial dispersion of repolarization
- EG
electrogram recorded using bipolar electrodes
- Endo
endocardial
- Epi
epicardial
- P2R
phase 2 re-entry
- RFA
radiofrequency ablation
- RV
right ventricular
- RVOT
right ventricular outflow tract
- TDR
transmural dispersion of repolarization
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–9. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 2.Brugada J, Pappone C, Berruezo A, et al. Brugada syndrome phenotype elimination by epicar-dial substrate ablation. Circ Arrhythm Electrophysiol. 2015 doi: 10.1161/CIRCEP.115.003220. [DOI] [PubMed] [Google Scholar]
- 3.Szel T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J Am Coll Cardiol. 2014;63:2037–45. doi: 10.1016/j.jacc.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Diego JM, Sicouri S, Myles RC, Burton FL, Smith GL, Antzelevitch C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013;54:53–64. doi: 10.1016/j.yjmcc.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antzelevitch C, Yan GX. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm. 2015;12:1852–66. doi: 10.1016/j.hrthm.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 7.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially-perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921–7. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C, Sun ZQ, Zhang ZQ, Yan GX. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J Am Coll Cardiol. 1996;28:1836–48. doi: 10.1016/S0735-1097(96)00377-4. [DOI] [PubMed] [Google Scholar]
- 9.Wolpert C, Echternach C, Veltmann C, et al. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm. 2005;2:254–60. doi: 10.1016/j.hrthm.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastiaenen R, Raju H, Sharma S, et al. Characterization of early repolarization during ajmaline provocation and exercise tolerance testing. Heart Rhythm. 2013;10:247–4. doi: 10.1016/j.hrthm.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Roten L, Derval N, Sacher F, et al. Ajmaline attenuates electrocardiogram characteristics of inferolateral early repolarization. Heart Rhythm. 2012;9:232–9. doi: 10.1016/j.hrthm.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Lu YY, Chung FP, Chen YC, et al. Distinctive electrophysiological characteristics of right ventricular out-flow tract cardiomyocytes. J Cell Mol Med. 2014;18:1540–8. doi: 10.1111/jcmm.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antzelevitch C. J wave syndromes: molecular and cellular mechanisms. J Electrocardiol. 2013;46:510–8. doi: 10.1016/j.jelectrocard.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa K, Nagase S, Morita H, Ito H. Left ventricular epicardial electrogram recordings in idiopathic ventricular fibrillation with inferior and lateral early repolarization. Heart Rhythm. 2013;11:314–7. doi: 10.1016/j.hrthm.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 15.Furushima H, Chinushi M, Okamura K, et al. Comparison of conduction delay in the right ventricular outflow tract between Brugada syndrome and right ventricular cardiomyopathy: investigation of signal average ECG in the precordial leads. Europace. 2007;9:951–6. doi: 10.1093/europace/eum128. [DOI] [PubMed] [Google Scholar]
- 16.Yodogawa K, Morita N, Kobayashi Y, et al. High-frequency potentials developed in wavelet-transformed electrocardiogram as a novel indicator for detecting Brugada syndrome. Heart Rhythm. 2006;3:1436–44. doi: 10.1016/j.hrthm.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Yodogawa K, Morita N, Kobayashi Y, et al. A new approach for the comparison of conduction abnormality between arrhythmogenic right ventricular cardiomyopathy/dysplasia and Brugada syndrome. Ann Noninvasive Electrocardiol. 2011;16:263–9. doi: 10.1111/j.1542-474X.2011.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacher F, Jesel L, Jais P, Haissaguerre M. Insight into the mechanism of Brugada syndrome: epicardial substrate and modification during ajmaline testing. Heart Rhythm. 2014;11:732–4. doi: 10.1016/j.hrthm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Park DS, Cerrone M, Morley G, et al. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J Clin Invest. 2015;125:403–12. doi: 10.1172/JCI76919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Sacher F, Hoffmayer K, et al. Cardiac electrophysiological substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients. Circulation. 2015;131:1950–9. doi: 10.1161/CIRCULATIONAHA.114.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol. 1995;269:H571–82. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 22.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res. 1995;76:351–65. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 23.Poelzing S, Akar FG, Baron E, Rosenbaum DS. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am J Physiol Heart Circ Physiol. 2004;286:H2001–9. doi: 10.1152/ajpheart.00987.2003. [DOI] [PubMed] [Google Scholar]
- 24.Yamada KA, Kanter EM, Green KG, Saffitz JE. Transmural distribution of connexins in rodent hearts. J Cardiovasc Electrophysiol. 2004;15:710–5. doi: 10.1046/j.1540-8167.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 25.Nademanee K, Raju H, de Noronha SV, et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–86. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TP, Xie Y, Garfinkel A, Qu Z, Weiss JN. Arrhythmogenic consequences of myofibroblastmyocyte coupling. Cardiovasc Res. 2012;93:242–51. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–15. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.