Summary
Calorie restriction, without malnutrition, has been shown to increase lifespan and is associated with a shift away from glycolysis toward beta-oxidation. The objective of this study was to mimic this metabolic shift using low-carbohydrate diets and to determine the influence of these diets on longevity and healthspan in mice. C57BL/6 mice were assigned to a ketogenic, low-carbohydrate, or control diet at 12 months of age and were either allowed to live their natural lifespan or tested for physiological function after 1 or 14 months of dietary intervention. The ketogenic diet (KD) significantly increased median lifespan and survival compared to controls. In aged mice, only those consuming a KD displayed preservation of physiological function. The KD increased protein acetylation levels and regulated mTORC1 signaling in a tissue-dependent manner. This study demonstrates that a KD extends longevity and healthspan in mice.
Keywords: Ketogenic diet, longevity, healthspan, low-carbohydrate diet
In brief (eTOC blurb)
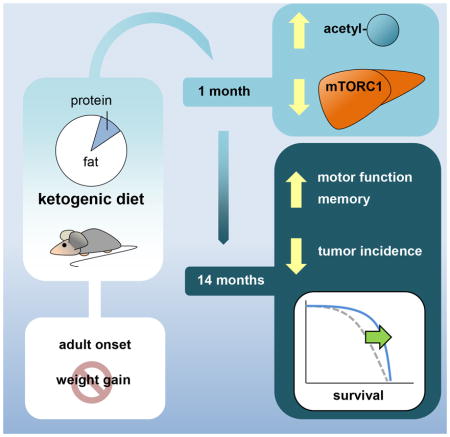
Roberts et al. show that a ketogenic diet extends longevity in adult, male mice and preserve motor function, memory and muscle mass in aged mice. The ketogenic diet increased protein acetylation levels and regulated mTORC1 signaling in a tissue-dependent manner. See related paper by Newman etal.
Introduction
Caloric restriction (CR) extends longevity and delays age-related diseases across numerous animal models (Speakman and Mitchell, 2011). While the exact mechanisms contributing to increased longevity in CR animals are still subject to debate, CR induces a shift from carbohydrate to fat metabolism (Bruss et al., 2010). It remains to be determined whether the increased fatty acid oxidation and ketogenesis that occurs with CR contributes to lifespan extension.
Low-carbohydrate diets (LCDs) also induce a shift in metabolism away from carbohydrates toward fatty acid oxidation. The most extreme LCD, the ketogenic diet (KD), has been shown to promote an anti-inflammatory metabolic state and to increase levels of ketone bodies in mice, resembling key features of CR (Meidenbauer et al., 2014). Despite these similarities, there is a dearth of information on lifespan and healthspan outcomes of animals maintained long-term on these diets. To date, only one study has shown that mice fed a lifelong, ad libitum KD demonstrate no significant difference in longevity compared to mice fed a standard chow diet (Douris et al., 2015). However, the control and KD C57BL/6 mice used by Douris and colleagues were short lived for this mouse strain and therefore the effect of a KD on aging is still equivocal, especially in animals that are not obese or fed ad libitum.
In the current study, adult mice were fed isocaloric amounts of a control diet, LCD, or KD. The objective of this study was to determine the influence of a LCD or KD on longevity and healthspan in mice.
Results
A Ketogenic Diet Increases Longevity and Healthspan in Lean Mice
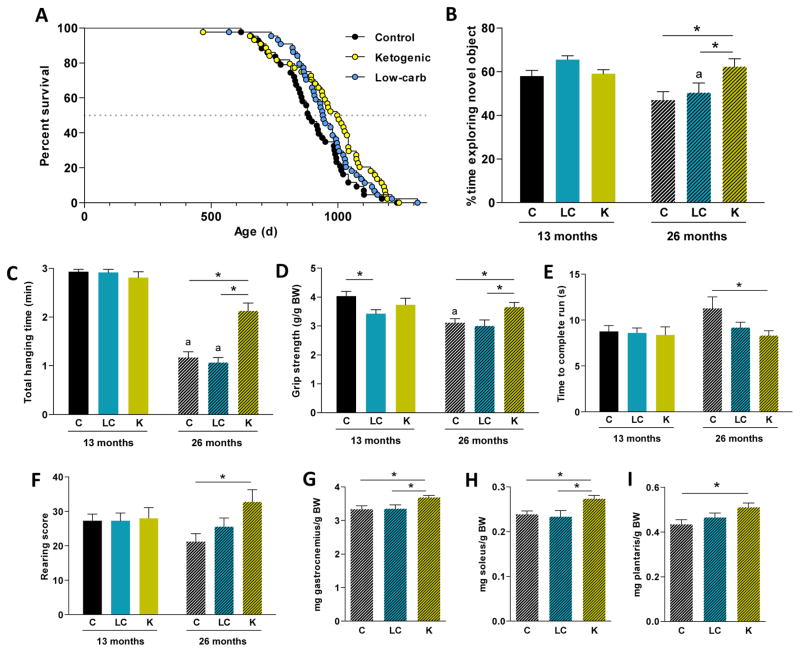
To study the effects of LCDs on longevity in adult male mice, we compared a LCD (70% kcal from fat) and a KD (89% kcal from fat) with a control diet (65% kcal from carbohydrate). Diets were fed in isocaloric amounts starting at 12 months of age. Lifespan was significantly increased in the KD compared to control group (Figure 1A), with the ketogenic group showing a 13.6% increase in median lifespan versus the control mice. The LCD group had a lifespan intermediate to the KD and control groups, and was not significantly different from either group. Median lifespans were 886, 943, and 1003 days for the control, LCD, and KD groups, respectively. Maximum lifespan (90th percentile) was 1064, 1123 and 1175 days, respectively. Median, but not maximum (p = 0.16), lifespan was significantly increased in the KD versus control group. Of specific interest, incidence of tumors at time of death, particularly histiocytic sarcoma, was decreased with a KD (Table S1).
Figure 1. Effect of low-carbohydrate diets on longevity and healthspan in male mice.
(A) Survival curves comparing mice fed either a KD, a LCD, or the control diet (n = 43–44). (B) Novel object recognition test: proportion of time spent exploring the novel object. (C) Hanging wire test: total hanging time before falling ten times from the wire. (D) Grip strength test: relative force exerted on the force meter. (E) Locotronic run test: time to complete a run after one round of training. (F) Rearing score: number of rearing behavior events in 5 minutes. n = 14–18.
Relative muscle mass of gastrocnemius (G), soleus (H) and plantaris (I) after 14 months of diet.
Diets: C = control, LC = low-carbohydrate, K = ketogenic.
* p < 0.05 between diets.
ap < 0.05 between 13-mo and 26-mo for the same diet.
See also Figure S1.
To assess the effects of these diets on healthspan, a battery of physical and behavioral tests was conducted after either 1 or 14 months of the dietary intervention (13 or 26 months of age). The results of the novel object recognition test (Leger et al., 2013) (Figure 1B) indicate that memory was preserved in old mice fed a KD compared to those fed a control or LCD. Coordination, strength and endurance were assessed with the hanging wire and grip strength tests. Male mice fed a KD for 14 months were more resistant to falling from the hanging wire (Figure 1C) and had greater forelimb grip strength (Figure 1D) compared to age-matched controls. Old ketogenic mice were also faster in the Locotronic speed test (Rousselet et al., 2003) (Figure 1E) and more active during the rearing test (Figure 1F) compared to controls, suggesting better preservation of motor coordination. In most cases, LCD group performance was intermediate to the control and the ketogenic groups. Noteworthy, and consistent with increased motor function, the relative mass of gastrocnemius and other hind limb muscles was higher in the old KD mice (Figure 1G–I and S1). Altogether, these tests suggest that the KD is able to extend both lifespan and healthspan in adult mice.
Low-Carbohydrate Diets Alter Physiology and Metabolism
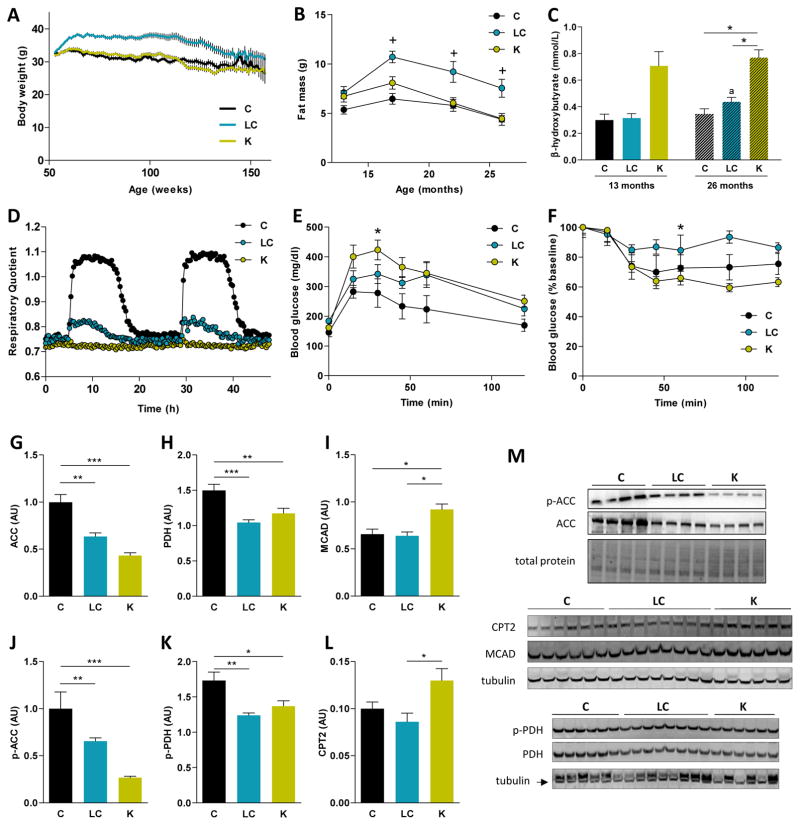
To investigate the physiologic and metabolic changes induced by these diets, we measured body weight (BW) and composition, a panel of serum biomarkers, energy expenditure, and physical activity. Despite being fed the same amount of calories, mice fed a LCD were heavier throughout the study than mice fed either a control or KD (Figure 2A). Body composition analysis showed that lean mass increased with age in the control and LCD mice, and was significantly lower in the KD mice at 26 months of age (Figure S1). LCD mice had significantly more fat mass compared to control or ketogenic mice (Figure 2B). In all diet groups, total fat mass peaked at 17 months of age.
Figure 2. Metabolic adaptations of male mice to low-carbohydrate diets.
(A) Body weights along the longevity study (n = 43–44).
(B) Fat mass from 1 to 14 months of dietary interventions (n = 15); + = fat mass is greater (p < 0.05) for the LCD versus the other groups.
(C) Circulating levels of β-hydroxybutyrate, 3 hours postprandrial.
For the physiological tests (D–F) and protein levels in liver (G–K), animals were on the diets for 1 month. (D) RER during two 24 hour cycles for each dietary group (n = 7). (E) GTT after a 16-hour fast, the areas under the curve (AUC) differ between the KD and the control (n = 6). (F) ITT after a 4-hour fast, AUC differ between the LCD and the KD (n = 6).
Quantification of (G) ACC, (H) PDH, (I) MCAD, (J) p-ACC, (K) p-PDH and (L) CPT2 protein levels in liver by western blot. (M) Representative blots are shown for each protein. A representative loading control is shown for each case (n = 4–8).
Diets: C = control, LC = low-carbohydrate, K = ketogenic.
* p < 0.05 between diets.
ap < 0.05 between 13-mo and 26-mo for the same diet.
See also Figures S1 and S2.
Circulating β-hydroxybutyrate (βHB) levels were measured 3 hours postprandial (Figure 2C). In both age groups, blood ketones were significantly elevated in KD mice compared to control or LCD mice. We also analyzed other serum biomarkers after 1 and 14 months of dietary intervention (Table S2). No changes were detected between diets at 13 months of age. At 26 months of age, the only observed difference was that free fatty acid concentration was higher in the LCD compared to the other groups.
Respiratory quotient (RQ), energy expenditure (EE), and physical activity were measured in mice at 13 and 26 months of age. At both ages, average RQ was decreased by a LCD or a KD compared to a control diet (Figure 2D and Table S3). No significant differences between diets were observed in 24-hour EE, however aging decreased EE, unadjusted or adjusted for either BW or lean mass, in all diet groups (Table S3). Overall, spontaneous physical activity did not differ with age or between diet groups at either 13 or 26 months of age (Table S3).
Glucose and insulin tolerance tests (GTT, ITT) were conducted in mice after 1 month of dietary intervention. Mice on a KD displayed impaired glucose tolerance compared to those on a control diet (Figure 2E). The LCD group did not differ in glucose disposal compared to either the control or KD groups. Although no differences were observed between the control mice and the other groups, insulin sensitivity after a 4-hour fast was enhanced by a KD if compared to the LCD (Figure 2F), indicating that insulin signaling is functioning normally in mice fed a KD. Interestingly, hepatic levels of phosphorylated AS-160 (Akt substrate of 160 kDa), a key mediator of insulin sensitivity, were increased in ketogenic mice compared to the other diets (Figure S2). Circulating levels of fibroblast growth factor 21 (FGF21) were not significantly altered by any of the dietary interventions (Table S2).
To further characterize the metabolic shift on these diets, we analyzed protein content of several enzymes linked to fatty acid metabolism in the liver of these mice. A KD decreased hepatic levels of phosphorylated and total acetyl-CoA carboxylase (ACC), while increasing those of carnitine palmitoyltransferase 2 (CPT2) and medium-chain acyl-CoA dehydrogenase (MCAD) (Figures 2G–L). Phosphorylated and total pyruvate dehydrogenase (PDH) protein levels were also reduced by both LCD and KD (Figure 2H and 2K).
A Ketogenic Diet Increases Protein Acetylation and Modulates mTORC1 Signaling in a Tissue-Specific Manner
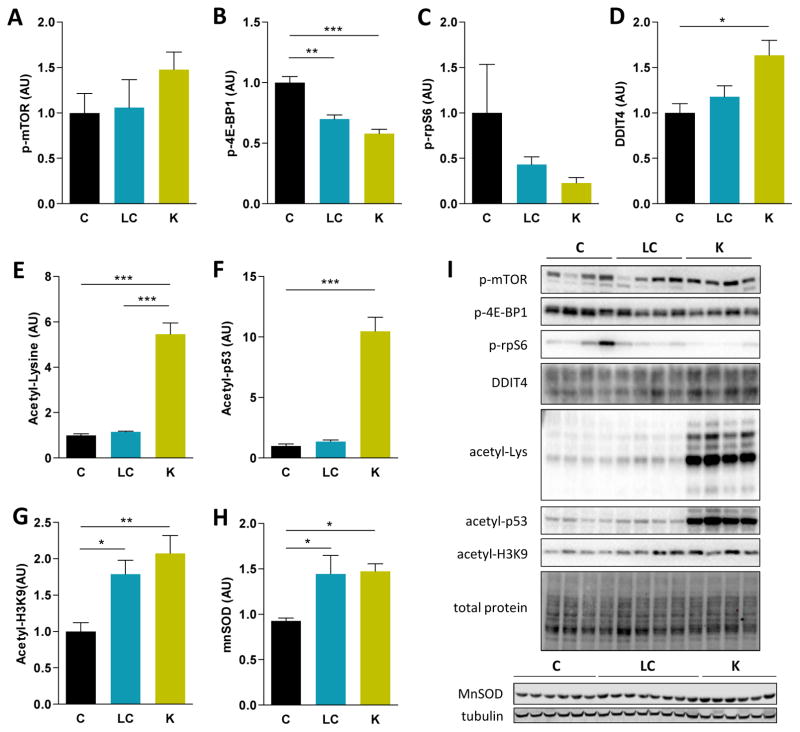
βHB can inhibit histone deacetylase (HDAC) activity in vivo (Shimazu et al., 2013). After 1 month on the KD, total acetyl-Lys levels were increased 5-fold in the liver of KD mice compared to control and LCD mice (Figure 3E). A 2.5-fold increase was detected in the skeletal muscle of the same mice (Figure S3). The level of acetylated p53, a key tumor suppressor protein, was 10-fold higher in liver after 1 month on a KD (Figure 3F). Interestingly, acetylation levels of histone 3 at Lys9 (H3K9) were increased in the liver after 1 month on either a KD or a LCD (Figure 3G). HDAC inhibition and H3K9 acetylation by βHB have also been shown to upregulate gene expression of FoxO3a and some of its target genes involved in antioxidant responses (Shimazu et al., 2013), including manganese superoxide dismutase (MnSOD). FoxO3a and MnSOD protein levels were increased in liver after 1 month of either a LCD or KD compared to a control diet (Figure 3H and S2).
Figure 3. Alterations in protein acetylation and mTOR signaling in the liver of male mice after 1 month of diet.
Quantification of (A) p-mTOR, (B) p-4E-BP1, (C) p-rpS6, (D) DDIT4, (E) total acetyl-Lysine, (F) p-53, (G) acetyl-H3K9 and (H) MnSOD protein levels in liver after analysis by western blot (n = 4–8). (I) Representative blots are shown for each of the quantified proteins. For total lysine acetylation, a fraction of the membrane is shown. A representative loading control is shown for those blots with n = 4, corresponding to the acetyl-p53 gel.
Diets: C = control, LC = low-carbohydrate, K = ketogenic.
* p < 0.05 between diets.
See also Figure S2 and S3.
To further elucidate the mechanisms underlying the beneficial effects of a KD on longevity and healthspan, we examined the levels and activation state of several factors linked to the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) signaling pathway, which has been suggested to modulate aging in response to dietary interventions (McDaniel et al., 2011, Klement and Champ, 2014, Solon-Biet et al., 2014). Total and phosphorylated levels of mTOR were not altered in liver by 1 month of LCD or KD (Figure 3A and S2). However, lower levels of phosphorylated 4E binding protein 1 (4E-BP1) and a similar trend for phosphorylated S6 ribosomal protein (rpS6) were detected, suggesting decreased mTORC1 signaling (Figure 3B and 3C) in liver. In contrast to liver, p-4E-BP1 levels were increased in the skeletal muscle of ketogenic mice after 1 month of diet (Figure S3). We also analyzed several signaling cascades modulating hepatic nutrient sensing by mTORC1. No changes were detected in phosphorylated AMPK, p-Akt or p-Erk1/2 between control and KD mice. Tuberous sclerosis complex 2 (TSC2) phosphorylation at S939 or S1387, as well as p-Raptor levels, were also unaltered by the KD (Figure S2). In contrast, levels of DNA damage-inducible transcript protein 4 (DDIT4, also known as Regulated In Development and DNA Damage or REDD1), a negative regulator of mTORC1, were significantly increased in the KD mice (Figure 3D).
Discussion
The objective of this study was to mimic the metabolic changes accompanying CR by manipulating dietary macronutrient composition and to determine whether these changes in diet composition increase longevity and healthspan in mice. The results clearly demonstrate that lifespan is increased in mice consuming a KD compared to a standard control diet. However, feeding strategies and husbandry issues may play a role in determining the influence of ketogenic diets on aging. This hypothesis is supported by the fact that a previous study reported that ketogenic diets do not alter survival curves in C57BL/6 mice (Douris et al., 2015). It is important to note that the lifespan of the control group reported by Douris and colleagues was shorter than would be expected for this strain (Yuan et al., 2009). Level of energy intake and prevention of weight gain may be particularly important for positive lifespan effects with a KD, and the results of the present study suggest that longevity is increased when a feeding strategy is followed that mitigates weight gain in adult mice.
Similar to ketogenic diets, very little is known about the impact of LCDs on longevity in animals that are not allowed ad libitum access to food. It is often assumed that high-fat diets shorten lifespan since they have been shown to induce weight gain and obesity when fed ad libitum to C57BL/6 mice (Surwit et al., 1995). However, our results indicate that a caloriecontrolled LCD started in middle-aged mice does not have a negative impact on aging.
There has been considerable interest in the impact of dietary macronutrient composition on longevity with several studies focusing on protein or methionine restriction as an approach to increase lifespan (Miller et al., 2005, Orentreich et al., 1993). In our study, lifespan did not significantly differ between the LCD and KD groups despite higher protein intake in LCD animals compared to the KD animals. Furthermore, no increase in lifespan was observed in rats fed diets in which protein content was decreased to levels, and in a proportion, comparable to those of our study (Nakagawa and Masana, 1971, Ross and Bras, 1973). Another study found that survival was not increased in rats consuming a 12% versus 20% protein diet (Davis et al., 1983). Thus, available evidence does not support the idea that level of protein is primarily responsible for the increased longevity in our KD mice. It has also been proposed that a low dietary protein to carbohydrate ratio drives longevity (Solon-Biet et al., 2014). However, the results of the present study are not consistent with this hypothesis. Nonetheless, additional studies are needed to determine if dietary protein contributes to improved physiological function and longevity in KD mice. It is also possible that the optimal dietary macronutrient composition may differ between an animal that is fed ad libitum and one that is not.
Our results show that a KD slows cognitive decline and preserves motor function in aging mice. It should be noted that although the LCD did not significantly differ from the ketogenic group in longevity, the two diets differed in their ability to preserve physiological function with age. This suggests that ketones may be necessary to elicit an extension of healthspan.
The novel object recognition test has been previously used to study memory in models of aging and its associated diseases (Fahlstrom et al., 2012, Stover et al., 2015). Our results support the notion that ketones may play an important role as neuro-protective signaling molecules (Newman and Verdin, 2014). Further support for this hypothesis comes from the fact that a diet that mimics fasting also increases ketone production and improves memory in mice (Brandhorst et al., 2015). The present study along with the literature supports the notion that a KD promotes long-term cognitive health.
Motor function was evaluated with approaches previously used to detect age-related deficits in muscle strength and function (Fahlstrom et al., 2012, Justice et al., 2014). The KD mice did not show the age-related decrease in grip strength observed in the control mice and 26-month old KD mice outperformed the other diet groups in the hanging wire test. This suggests that the KD maximizes and preserves forelimb grip strength with age. There is evidence that the ketone body acetoacetate plays an important role as a signaling molecule in muscle cells independent of its metabolic effects (Zou et al., 2016). Acetoacetate has been shown to improve muscular dystrophy outcomes and accelerate muscle regeneration, suggesting that it may be an important player in attenuating age-related decline in muscle function. The results of our tests and the work of others suggest that ketones positively impact muscle homeostasis. However, more work is needed to elucidate the exact mechanisms underlying this protective effect.
According to our results, extension of both longevity and healthspan appear to be unique to the KD. Interestingly, there is evidence of interaction between ketone bodies and pathways proposed to modulate aging.
The ketone body βHB is a direct inhibitor of histone deacetylases (HDACs), a process that has been shown to occur in vivo (Shimazu et al., 2013, Newman and Verdin, 2014). HDAC inhibitors extend lifespan in models from yeast to flies, through mechanisms not yet elucidated, but associated with hyperacetylation of histones and a large number of other proteins. In our study, a KD resulted in a dramatic increase in total levels of acetylated lysine. Interestingly, in both the LCD and the KD mice, we observed an increase in acetyl-H3K9, concomitant with increased FoxO3a and MnSOD in liver, an effect previously described in the literature as a contribution of ketone bodies to stress response pathways and potentially longevity (Shimokawa et al., 2015, Shimazu et al., 2013). However, since the effect on acetyl-H3K9, FoxO3a, and MnSOD were similar in the LCD and KD groups our data suggest that these changes do not underlie the lifespan and healthspan of the diet.
Many dietary interventions known to extend or modulate lifespan have been shown to be mediated, at least partially, by decreased mTORC1 activity (Houtkooper et al., 2010), an effect that has been previously reported to occur with ketogenic diets (McDaniel et al., 2011). The decreased protein content in the KD likely contributes to a lower mTORC1 activity, eliciting a response analogous to that of protein or methionine restriction (Pissios et al., 2013). Our experimental design attempted to minimize this effect by using a higher protein content (10% of calories) than previous laboratory rodent studies of ketogenic diets and protein restriction (Douris et al., 2015, Levine et al., 2014).
We detected a tissue-dependent modulation of mTORC1 signaling. In skeletal muscle, the KD increased p-4E-BP1 levels. Similarly, a recent study in rats fed a relatively high protein (~20% kcal) ketogenic diet detected no change in p-rpS6 and a trend towards an increase in p-4E-BP1 (Roberts et al., 2016). Thus, protein level may have a major influence on mTORC1 signal in response to a KD. There is a lack of sufficient understanding of the trade-off between prolongevity mTOR modulation and skeletal muscle homeostasis (Sharples et al., 2015), and further research is needed to fully understand the mechanisms responsible for the preservation of muscle mass with aging in our KD mice. In liver, however, we have shown that mTORC1 signaling is inhibited by the KD. Interestingly, it has been reported that p53 hyperacetylation inhibits mTORC1 in response to fasting by increasing the expression of DDIT4, a negative regulator of mTORC1 (Schupp et al., 2013). Moreover, this same pathway seems to mediate the effects of metformin (Ben Sahra et al., 2011). Our results, including increased p53 acetylation and DDIT4 levels and decreased mTORC1 downstream signaling, only in the ketogenic group are in accordance with this model. Of note, p53 hyperacetylation and stabilization may also be contributing to the marked decrease in cancer incidence in the KD mice. Cross-talk between HDAC inhibition and liver mTORC1 signaling is therefore a potential mechanism contributing to the longevity extension with a KD.
A goal of the present study was to determine if a KD could mimic the changes in healthspan and longevity induced by CR. The KD did induce some of the same changes reported with CR. In particular, overall lifespan is increased by both CR (Speakman and Mitchell, 2011) and the KD. Fatty acid β-oxidation and βHB production is stimulated (Mahoney et al., 2006), and protein acetylation is increased as described in previous CR studies (Schwer et al., 2009). Signaling downstream of mTORC1 is also downregulated in liver with both CR (Miller et al., 2013) and a KD.
The KD, however, also showed several differences from CR. Unlike CR, the KD mice in the present work were glucose intolerant compared to controls, in contrast with previous reports of enhanced glucose tolerance in ad libitum fed KD (Douris et al., 2015). Additionally, the level of intake of the KD in the present study did not produce the decrease in body weight observed with a CR diet.
This study demonstrates that energy-controlled high fat, LCDs are not detrimental to health but rather a KD extends lifespan and slows age-related decline in physiological function in mice. Future studies are warranted to further investigate the mechanisms through which this diet works and to optimize diet composition and feeding approaches to further extend healthspan.
STAR Methods
Contact for Reagent and Source Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact (jjramsey@ucdavis.edu).
Experimental Model and Subject Details
Animal Husbandry
Male C57BL/6 mice were obtained at 11 months of age from the NIA Aged Rodent Colony and housed in polycarbonate cages on racks in a room with controlled temperature (22–24°C) and humidity (40– 60%). Mice were individually housed in a HEPA filtered room maintained on a 12-hour light-dark cycle. Health checks were conducted on all mice at least once daily. Animals that showed dermatitis had nails clipped by a trained technician and were treated topically with 2% chlorohexidine daily until the lesion resolved. Mice were considered moribund if they could not reach food or water, were unresponsive to external stimuli, or presented with an ulcerated tumor. Sentinel mice were housed in the same room and exposed to bedding from the study mice on a weekly basis. Health screens were completed on sentinel mice every three months. Tests included aerobic cultures and serology (MHV, MPV, MVM, M. pul., TMEV [GDVII], Ectro, EDIM, MAD1, MAD2, LCM, Reo-3). All tests were negative throughout the study. All animal protocols were approved by the UC Davis Institutional Animal Care and Use Committee and were in accordance with the NIH guidelines for the Care and Use of Laboratory Animals.
Dietary Interventions
Upon arrival to the UC Davis facilities, mice were singly housed and provided ad libitum access to a chow diet LabDiet 5001 (LabDiet, Saint Louis, MO) prior to the start of the study. During this one month period, food intake was measured to determine the daily food intake required by these animals. At 12 months of age, mice were randomly placed on one of three diets: control, low-carbohydrate diet (LCD), or ketogenic diet (KD). The control diet contained (% of total kcal) 18% protein, 65% carbohydrate, and 17% fat. The LCD contained 20% protein, 10% carbohydrate, and 70% fat. The KD contained 10% protein, <1% carbohydrate, and 89% fat. For the longevity study, food intake was set at 11.9 kcal/day, and decreased to 11.2 kcal/day after weight gain was observed during the first weeks of the study. Cross-sectional mice were always maintained on 11.2 kcal/day. The following table provides detailed description of the diet composition:
| Ingredient (g/kg diet) | Control | Low-carbohydrate | Ketogenic |
|---|---|---|---|
| Protein, of which | 203 | 316.7 | 183.7 |
| Casein | 200 | 312 | 181 |
| L-cystein | 3 | 4.7 | - |
| D-methionine | - | - | 2.7 |
|
| |||
| Carbohydrates, of which | 630 | 112 | - |
| Corn starch | 398 | - | - |
| Maltodextrin | 132 | - | - |
| Sucrose | 100 | 112 | - |
|
| |||
| Fat, of which | 70 | 423 | 631 |
| Soybean oil | 70 | 70 | 70 |
| Lard | - | 353 | 561 |
|
| |||
| Choline bitartrate | 2.5 | 2.5 | ‡ |
| Cellulose (73.5 mg/day) | 50 | 71 | 85 |
| Tert-butylhydroquinone | 0.014 | 0.085 | 0.126 |
| Mineral mix | 35 | 60 | 60 |
| Vitamin mix | 10 | 15 | 13 |
| Other minerals | - | - | 27.5 |
Included in the vitamin mix
For the control and the LC diets, the Envigo (Indianapolis, IN) mineral mix TD94046 and the vitamin mix TD94047 were used. The KD included mixes TD79055 (mineral mix) and TD40060 (vitamin mix) instead, because of their lower content in carbohydrates. As a consequence, calcium phosphate (19.3 g/kg diet) and calcium carbonate (8.2 g/kg diet) supplementation was required. The vitamin mix added to the KD included choline (choline dihydrogen citrate) for a final concentration of 4.5 g/kg of diet.
For the lifespan study, 43–44 mice were randomly assigned to each dietary group. Mice were allowed to live out their natural lifespan and were euthanized only if moribund. Additionally, for each diet, 15 mice were tested at 13 months of age and a separate group of 20 mice at 26 months of age. Subsets of the mice were either euthanized by cervical dislocation for tissue collection or by CO2 overdose.
Method Details
Body Weight and Composition
Body weight was measured weekly. Body composition was assessed using NMR relaxometry (EchoMRI-100H, EchoMRI LLC, Houston, TX) at 13, 17, 22, and 26 months of age.
Behavior Tests
Novel Object Recognition Test
The novel object recognition test was conducted in a clear plastic box (16″x16″x16″) wrapped in white paper. Day 1 consisted of a 5-minute environmental habituation. Day 2 consisted of a 10-minute familiarization period with two identical objects (plastic boxes or bottles) and a 10-minute test with one known and one novel object (one box and one bottle). A video camera was used to record the tests on day 2. The familiarization period and novel object test were performed 6 hours apart. The time spent at both the novel and known objects were recorded. The test was finished when the total time of exploration reached 20 seconds or the mouse reached the cut-off time of 10 minutes.
Grip Strength
Forelimb grip strength was measured using the Imada (PS-500N, Northbrook, IL) push-pull force scale and a single metal bar. Mice were positioned to grasp the bar with forelimbs only and then pulled horizontally until letting go. The test was performed in fasted mice using two rounds of three trials each. Between trials, mice were given minimal rest (30 seconds); between rounds, mice were returned to their home cage to rest (45 minutes). The maximum grip strength of the 6 total trials was recorded.
Hanging Wire Test
Endurance and coordination were tested using the hanging wire test. A wire bar (3 mm in diameter) was suspended from the top of a clear plastic box (16″x16″x16″) lined with padding. Mice were positioned at the center of the wire, hanging from their forelimbs only. The initial “falling score” was recorded as 10. A fall was recorded when the mouse fell from the wire to the padding below. With each fall, the falling score decreased by one. When the mouse fell by its hind limbs, the fall was considered voluntary and was not counted. After a fall or whenever the mouse reached either end of the wire, it was immediately placed back at the original position at the center of the wire. The procedure was repeated until the falling score reached zero or the total time hanging reached 3 minutes.
Locotronic
The Locotronic (Intellibio Innovation, Karlsruhe, Germany) test was performed in fasted mice immediately after room transfer in the morning. Mice were placed into the Locotronic corridor at the starting zone. A ruler was held behind each animal to ensure it continued in the forward direction toward the arrival zone. Time was recorded for three rounds per mouse. These rounds included a training run, trial #1 with all ladder rungs in place, and trial #2 with rungs removed (traps set at positions 10, 20, and 50 on the ladder).
Rearing Test
Mice were placed into a large clear cylinder (15 cm diameter). The latency to climb and number of rears in 5 minutes were recorded using video camera analysis. A “rear” was defined as putting both forepaws on the side of the cylinder.
Blood Ketones
Blood ketone levels were measured 3 hours post-prandial using the Precision Xtra glucose and ketone monitoring system (Abbott) according to the manufacturer’s instructions.
Glucose and Insulin Tolerance Tests
For the GTT, mice were fasted overnight and injected intraperitoneally with glucose (1 g/kg BW). For the ITT, mice were fasted for 4 hours and injected IP with 0.75 U/kg BW of human insulin. Blood glucose was measured using the Easy Plus II glucometer at 0, 15, 30, 45, 60, and 120 minutes for both tests.
Indirect Respiration Calorimetry and Measurement of Physical Activity
Energy expenditure (EE) and respiratory quotient (RQ) were measured using whole-body indirect respiration calorimetry (Columbus Instruments, Columbus, OH). EE was calculated from O2 consumption and CO2 production and RQ was determined as the ratio of the volume of CO2 produced to the volume of O2 produced. Infrared beams were used to record activity according to the number of beam break events in the horizontal (x) and vertical (z) plane.
The Oxymax/CLAMS calorimetry system (Columbus Instruments, Columbus, OH) was housed in a room maintained on a 12 hour light/12 hour dark cycle at 22°C. The mice were placed in acclimation cages (calorimetry chambers not connected to the calorimeter) and housed in the Oxymax/CLAMS room for 24 hours. The mice were then transferred to calorimetry chambers contained in a 22°C incubator and allowed to acclimate for 24 hours prior to the start of the calorimetry measurements. Calorimetry measurements were then completed over a 48-hour period. Room air was drawn through the calorimetry chambers at 500 ml/min. Samples of dried room and chamber air were analyzed for oxygen and carbon dioxide content using the Oxymax system. Calorimeter calibration was performed daily prior to the beginning of each 24-hour measurement. A 0.50% CO2 and 20.50% O2 (balance nitrogen) calibration gas (Airgas, Sacramento, CA) and dry room air were used to calibrate the analyzers. At the start and end of the experiments, the performance of the entire calorimetry system was validated by bleeding a 20% CO2 (balance nitrogen) standard (Airgas, Sacramento) into each calorimetry chamber at a regulate rate using an OxyVal gas infusion system (Columbus Instruments, Columbus, OH) and measuring recovery of CO2 and O2 dilution in the chamber exhaust.
Serum Analysis
Blood collection was performed via cardiac puncture. Serum was obtained after clotting and centrifugation (1000 g, 10 minutes), and sent to the UC Davis Mouse Metabolic Phenotyping Center for analysis. The following serum assays were completed using kits according to the manufacturer’s instructions: free fatty acids (Wako Diagnostics, Richmond, VA), triglycerides (Fisher Diagnostics, Middletown, VA), and total cholesterol, HDL-cholesterol, LDL-cholesterol, and VLDL-cholesterol (Fisher Diagnostics, Middletown, VA) were measured using enzymatic colorimetric assays. IL-6, CXCL1 and TNF-α were determined by ELISA (Meso Scale Discovery, Rockville, MD).
Protein Immunodetection
Frozen livers were powdered and homogenized in sucrose lysis buffer (50 mM Tris pH 7.5, 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, 1% Triton X100, and protease inhibitors). The supernatant was collected following centrifugation at 10,000 g for 5 minutes. 20 μg of protein was subjected to SDS-PAGE on 4–20% Criterion TGX gels (Bio-Rad) and transferred to nitrocellulose membranes for 2 h. Membranes were blocked in 1% fish skin gelatin dissolved in Tris-buffered saline with 0.1% Tween-20 for 1 h and then probed with primary antibody overnight at 4°C. Membranes were then incubated with HRP conjugated secondary antibodies at 1:10,000 for 1 h at room temperature. Immobilon Western Chemiluminescent HRP substrate (Millipore) was then applied to the membranes for protein band visualization. Quantification was performed using the ChemiDoc™ MP System and Image Lab 5.0 software (Bio-Rad). Total protein staining of the membrane (via Ponceau) was used as the normalization control.
Pathology
Following death, mice in the lifespan study were placed in a 10% formalin solution after opening the abdominal, thoracic, and cranial cavities. A random selection of these mice from each diet was submitted to the UC Davis Comparative Pathology Lab for necropsy and histological examination. Tissues processed for histologic examination included liver, brain, lungs and upper respiratory tract, spleen, pancreas, reproductive tract, heart and great vessels, thymus, lymph nodes, gastrointestinal tract, urinary tract, haired skin, ears, eyes, and skeletal muscle. Additional tissues were examined if gross lesions were observed at necropsy. Tissues were processed and embedded in paraffin by routine methods. Tissues (5 μm sections) were stained with hematoxylin and eosin for histologic evaluation.
Quantification and Statistical Analysis
All data are expressed as mean ± SEM unless otherwise indicated. Differences between diet groups and ages were evaluated using a two-way analysis of variance. Post-hoc analysis was carried out using a Tukey’s honest significant difference test to determine which diet and age groups differed significantly. For energy expenditure data, analysis of covariance was used with either BW or lean mass as a covariate in the model. Survival curves for each diet group were estimated using the Kaplan-Meier method and differences in survival among the diet groups were tested using log rank tests and a Tukey’s test to maintain the family-wise Type I error rate at 0.05. The Fisher’s exact tests were used to determine associations between diet and histology for pathology data. Significance was determined as a two-sided p<0.05. All statistical analyses were conducted in SAS version 9.4.
Supplementary Material
Highlights.
A low-carbohydrate, ketogenic diet extends longevity in adult, male mice.
Motor function, memory and muscle mass are preserved in aged ketogenic mice.
Protein acetylation is increased in the liver and skeletal muscle of ketogenic mice.
Acknowledgments
This work was supported by a Program Project Grant (2P01AG025532) from the NIH (USA) and the UC Davis mouse metabolic phenotyping center (U2CDK092993).
Footnotes
Author Contributions
JJR, JAL and KH conceived the study; JJR, JAL and MNR designed the experiments; JAL, MNR, DT, ZZ and EG and performed the physiological and behavioral tests; MAW, GRM and AT assessed protein levels; SK performed the ITT; DT and GP oversaw the lifespan colony; KK and TAK performed the statistical analyses; SMG and DI performed the histopathological analysis; KB, GAC and FGH contributed to the discussion and data interpretation; MNR, JAL and JJR wrote the article with contributions from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau l, Yap LP, Park R, Vinciguerra M, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298:E108–116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Bales CW, Beauchene RE. Differential effects of dietary caloric and protein restriction in the aging rat. Exp Gerontol. 1983;18:427–435. doi: 10.1016/0531-5565(83)90021-9. [DOI] [PubMed] [Google Scholar]
- Douris N, Melman T, Pecherer JM, Pissios P, Flier JS, Cantley LC, Locasale JW, Maratos-Flier E. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim Biophys Acta. 2015;1852:2056–2065. doi: 10.1016/j.bbadis.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlstrom A, Zeberg H, Ulfhake B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age (Dordr) 2012;34:1435–1452. doi: 10.1007/s11357-011-9320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142:9–14. doi: 10.1016/j.cell.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, Seals DR. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr) 2014;36:583–592. doi: 10.1007/s11357-013-9589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement RJ, Champ CE. Calories, carbohydrates, and cancer therapy with radiation: exploiting the five R’s through dietary manipulation. Cancer Metastasis Rev. 2014;33:217–229. doi: 10.1007/s10555-014-9495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13. doi: 10.1186/1476-511X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidenbauer JJ, Ta N, Seyfried TN. Influence of a ketogenic diet, fish-oil, and calorie restriction on plasma metabolites and lipids in C57BL/6J mice. Nutr Metab (Lond) 2014;11:23. doi: 10.1186/1743-7075-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Robinson MM, Reuland DJ, Drake JC, Peelor FF, 3rd, Bruss MD, Hellerstein MK, Hamilton KL. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci Med Sci. 2013;68:530–538. doi: 10.1093/gerona/gls219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Masana Y. Effect of protein nutrition on growth and life span in the rat. J Nutr. 1971;101:6136–6120. doi: 10.1093/jn/101.5.613. [DOI] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, Defelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Pissios P, Hong S, Kennedy AR, Prasad D, Liu FF, Maratos-Flier E. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol Metab. 2013;2:306–313. doi: 10.1016/j.molmet.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Holland AM, Kephart WC, Mobley CB, Mumford PW, Lowery RP, Fox CD, McCloskey AE, Shake JJ, Mesquita P, et al. A putative low-carbohydrate ketogenic diet elicits mild nutritional ketosis but does not impair the acute or chronic hypertrophic responses to resistance exercise in rodents. J Appl Physiol (1985) 2016;120:1173–1185. doi: 10.1152/japplphysiol.00837.2015. [DOI] [PubMed] [Google Scholar]
- Ross MH, Bras G. Influence of protein under- and overnutrition on spontaneous tumor prevalence in the rat. J Nutr. 1973;103:944–963. doi: 10.1093/jn/103.7.944. [DOI] [PubMed] [Google Scholar]
- Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, Launay JM, Cohen-Salmon C, Hirsch EC. Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol Dis. 2003;14:218–228. doi: 10.1016/s0969-9961(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Schupp M, Chen F, Briggs ER, Rao S, Pelzmann HJ, Pessentheiner AR, Bogner-Strauss JG, Lazar MA, Baldwin D, Prokesch A. Metabolite and transcriptome analysis during fasting suggest a role for the p53-Ddit4 axis in major metabolic tissues. BMC Genomics. 2013;14:758. doi: 10.1186/1471-2164-14-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, Giallourakis C, Comb MJ, Alt FW, Lombard DB. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604–606. doi: 10.1111/j.1474-9726.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015;14:511–523. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, Hayashi H, Yamaza H, Chiba T, Mori R. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell. 2015;14:707–709. doi: 10.1111/acel.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stover KR, Campbell MA, Van Winssen CM, Brown RE. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav Brain Res. 2015;289:29–38. doi: 10.1016/j.bbr.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Meng J, Li L, Han W, Li C, Zhong R, Miao X, Cai J, Zhang Y, Zhu D. Acetoacetate accelerates muscle regeneration and ameliorates muscular dystrophy in mice. J Biol Chem. 2016;291:2181–2195. doi: 10.1074/jbc.M115.676510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.