Abstract
Background & Purpose
Ambient particulate matter has been shown to be associated with declining human health, although the association between fine particulate matter (PM2.5) and stroke is uncertain.
Methods
We utilized satellite-derived measures of PM2.5 to examine the association between exposure and stroke in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. We used a time-stratified case cross-over design, with exposure lags of 1-day, 2-days and 3-days. We examined all strokes, as well as ischemic and hemorrhagic separately.
Results
Among 30,239 participants in the REGARDS study, 746 incident events were observed: 72 hemorrhagic, 617 ischemic, and 57 of unknown type. Participants exposed to higher levels of PM2.5 more often resided in urban areas compared to rural, and in the southeastern US. After adjustment for temperature and relative humidity, no association was observed between PM2.5 exposure and stroke, regardless of the lag (1-day lag OR=0.99, 95% CI: 0.83–1.19; 2-day lag OR=0.95, 95% CI: 0.80–1.14; 3-day lag OR=0.95, 95% CI=0.79–1.13). Similar results were observed for the stroke subtypes.
Conclusions
In this large cohort of African Americans and whites, no association was observed between PM2.5 and stroke. The ability to examine this association with a large number of outcomes and by stroke subtype helps to fill a gap in the literature examining the association between fine particulate matter and stroke.
Keywords: stroke, epidemiology, air pollution, REGARDS
Introduction
Ambient particulate matter has been shown to be associated with declining human health1–3, and recent work has indicated that fine particulate matter, characterized as particles less than 2.5 μm in diameter (PM2.5), may impact cardiovascular 4, 5. In fact, a recent AHA Scientific health Statement has suggested that the relationship between PM2.5 and cardiovascular morbidity and mortality is causal6. The proposed mechanisms through which PM2.5 exposure is thought to impact cardiovascular and cerebrovascular health include systematic inflammation mediated by oxidative stress and endothelial dysfunction7, 8, which would suggest that the impact of PM2.5 on stroke would be cumulative. However, direct effects can occur through increases in heart rate and blood pressure9, 10, arguing for the plausibility of shorter term associations. Several studies have examined both long- and short-term exposure to PM2.5 and risk of stroke, and results have been conflicting.11, 12 Most of the studies have examined small samples and have used sparse ground-level measures for exposure estimates, or have meta-analyzed several studies with varying approaches to measure exposure.
One promising method for characterizing PM2.5 exposure for public health practice and epidemiologic research is integration of remote sensing satellite systems data with air monitoring network data.13–18 Remotely-sensed data have been used to detect and track particulate matter plumes from major events such as dust storms, volcanic emissions, and fires. The aerosol optical properties measured by space-borne sensors can also be useful in filling the temporal and spatial gaps found with ground-level monitor data. Satellite data cover large geographic areas at moderate spatial resolution for multiple years and with reliable repeated measurements. Research has shown that aerosol optical depth (AOD) is indirectly related to ground-level PM2.5, with the correlation between the two being strongest on days with low cloud cover, low relative humidity, and good vertical mixing within the atmospheric column. 14, 19–23
In this paper, we used measures of PM2.5 that have been estimated using a national version of the algorithm of Al-Hamdan et al. (2009), which combines data from remotely-sensed satellite data and EPA ground-level data to determine the impact of PM2.5 exposure on stroke in participants from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, using a time-stratified case-crossover design.24, 25 We hypothesized that among REGARDS participants who have suffered a stroke, the level of PM2.5 exposure will be higher in the 24-hour period prior to their stroke, than on other similar days. We further hypothesized that these results will be similar among REGARDS participants suffering from either ischemic or hemorrhagic strokes.
Methods
Study procedures
This analysis used a subset of the REGARDS cohort who have experienced an incident stroke. REGARDS is a prospective cohort study of black and white participants of both sexes, in order to study geographic and racial differences in stroke incidence and mortality, the details of which can be found elsewhere.26 In brief, participants were recruited by mail from across the continental US, with oversampling in the Stroke Belt (North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Louisiana, and Arkansas) and Stroke Buckle (coastal plains of North Carolina, South Carolina, and Georgia), as well as oversampling of black residents. At baseline (between 2003–207), participants completed a computer-assisted telephone interview (CATI), followed by an in-home visit by a trained health professional, during which time blood and urine samples were collected, as well as several anthropomorphic traits (e.g., height and weight). Blood pressure was measured during the in-home visit by taking the average of two blood pressure measurements after the participant had been seated for five minutes. Participants were followed by CATI every six months to ascertain suspected stroke events. All participants provided written informed consent, and IRB approval was obtained by all participating institutions.
Individual variables
Age (years), race (black or white), gender, income, and education were all self-reported during the CATI. Region and urbanicity were assigned using the participant’s geocoded residential address at baseline. Total cholesterol (mg/dL) was measured from a blood sample collected during the in-home visit. Participants were determined to have hypertension if their systolic blood pressure was ≥ 140 mmHg, diastolic blood pressure was ≥90 mmHg, or they self-reported taking medication to control blood pressure. Participants were determined to have dyslipidemia if their total cholesterol was ≥240 mg/dL, their low density lipoprotein cholesterol (LDLC) was ≥160 mg/dL, their high density lipoprotein cholesterol (HDLC) was ≤40 mg/dL, or they self-reported taking lipid-lowering medications. Participants were determined to have diabetes if their fasting glucose was ≥126 mg/dL, their non-fasting glucose was ≥240 mg/dL, or they self-reported using diabetes medication or insulin. Smoking status (current, past, never) was self-reported.
Environmental variables
Ambient PM2.5 concentration was the exposure of interest. Previous studies have used Environmental Protection Agency (EPA) ground-level monitoring stations to estimate the concentrations of PM2.5 for participants.27 In order for measurements from these monitoring stations to accurately reflect the exposure of the participants, the study participants are usually restricted to those living in metropolitan statistical areas (MSAs). Since approximately 20% of REGARDS participants do not live in urban areas, and excluding them might have biased our sample, we used an estimated PM2.5 concentration that integrated ground-level measurements and satellite measurements of the total amount of aerosols within the air column, called aerosol optical depth (AOD), from the Moderate Resolution Imaging Spectroradiometer (MODIS) instrument on the NASA Aqua satellite.13, 14 Briefly, regression equations for each combination of EPA region of the US and season were used to estimate ground-level PM2.5 from AOD measurements,28 then these measurements were integrated with ground-level monitoring station measurements and spatially smoothed using a b-spline to a 10 km × 10 km resolution across the continental US. Daily estimates of PM2.5 concentration were created for REGARDS participants from 2003 to 2011, which was based upon the 10 km by 10 km grid cell of the PM2.5 surfaces containing the participant’s geocoded residential address.
Ambient temperature and relative humidity were included as potential confounders of the relationship between PM2.5 and incident stroke. Mean daily temperatures were computed from the North American Land Data Assimilation System (NLDAS) hourly air temperature dataset on a 12 km × 12 km grid across the continental US13, 29. Relative humidity was derived according to Iribarne and Godson (1981)30 using NLDAS air temperature, atmospheric pressure and specific humidity on the NLDAS grid. Mean daily temperatures and relative humidity were assigned for REGARDS participants based upon which 12 km by 12 km NLDAS grid cell contained the participant’s residence location.
Stroke Ascertainment
Potential events were ascertained during the follow-up calls, and those reported as possible stroke, transient ischemic attack (TIA), death, hospitalization or emergency department visit for brain aneurysm, brain hemorrhage, stroke symptoms, or unknown reason prompted a request for medical records. Initial review by a stroke nurse excluded obvious non-strokes; the remaining medical records were centrally adjudicated by physicians. Death certificates were examined and adjudicated for deaths without a medical record, and proxy interviews undertaken to ascertain potential events. Stroke events were defined following the World Health Organization (WHO) definition.31 Those events not meeting the WHO definition but with symptoms lasting >24 hours and neuroimaging consistent with acute ischemia or hemorrhage were classified as clinical strokes, and were included in analyses.
Statistical analysis
The analysis used a case-crossover design, where the PM2.5 exposure 1, 2, or 3 days previous to the stroke was compared with the exposure on three other days in the same month as the stroke. We chose the three other days to be the same days of the week as the lagged day before the stroke.24 Conditional logistic regression models were used to determine whether short-term PM2.5 exposure was associated with stroke. Thus, each participant served as their own control, removing the need to control for potential confounders that did not vary during the month, such as age, race, or sex. PM2.5 exposure was dichotomized as “good” (≤12 μg/m3) or “moderate/unhealthy” (12 – 150.4 μg/m3), which reflects 2012 EPA guidelines for 24-hour exposure.32 We included mean temperature and relative humidity as potential confounders, since these covariates did vary within a month. We also investigated potential interactions between category of PM2.5 concentration and urbanicity (rural, mixed, or urban) and between category of PM2.5 concentration and region (Stroke Belt, Stroke Buckle, or rest of US). Sensitivity analyses included using PM2.5 concentration as a continuous variable, as well as stratifying by stroke subtype (ischemic or hemorrhagic).
Results
Of the 30,239 participants in the REGARDS sample, 1,101 had strokes between January 1, 2003 and December 31, 2011. Of these participants, 227 were excluded due to self-reported history of stroke at baseline, 128 were excluded due to low confidence in their geocoded residential location, leaving a final sample of 746 participants with incident stroke for analysis (72 hemorrhagic, 617 ischemic, and 57 of unknown type). Among the 746 incident strokes, 403 (54%) occurred following a day classified as “good”, whereas the remaining 343 (46%) occurred following a day classified as “moderate” or “unhealthy”.
Table 1 presents the demographic characteristics of the cohort (n=746) by level of PM2.5 concentration on the day prior to the event, as well as data on co-morbidities. Few differences in demographics exist, although participants who live in the stroke belt or buckle and participants who live in urban areas are more often exposed to moderate levels of PM2.5, compared to those who live in the remainder of the US. Further, those exposed to higher PM2.5 levels more frequently have hypertension and are slightly more frequently current smokers.
Table 1.
Demographics and comorbidities of cohort, by category of PM2.5 exposure on day before stroke. Values are n (%) unless otherwise noted.
| PM2.5 concentration on day before stroke | ||
|---|---|---|
| Variable | Good (0 – 12 μg/m3) (n=403) | Moderate or unhealthy (12.1 – 150.4 μg/m3) (n=343) |
| Age*, years | 70(9) | 70(9) |
| Black race | 186(46) | 155(45) |
| Female | 206(51) | 159(46) |
| Income | ||
| < $20,000 | 98(24) | 66(19) |
| $20,000 – $34,000 | 128(32) | 105(31) |
| $35,000 – $74,000 | 98(24) | 93(27) |
| $75,000 and above | 31(8) | 31(9) |
| Refused | 48(12) | 48(14) |
| Education | ||
| Less than high school | 68(17) | 58(17) |
| High school graduate | 130(32) | 98(29) |
| Some college | 85(21) | 88(26) |
| College graduate and above | 120(30) | 98(29) |
| Region | ||
| Stroke Belt | 134(33) | 126(37) |
| Stroke Buckle | 67(17) | 81(24) |
| Rest of US | 202(50) | 136(40) |
| Total cholesterol*, mg/dL | 191(43) | 189(41) |
| Hypertension | 289(72) | 248(72) |
| Dyslipidemia | 245(61) | 217(63) |
| Diabetes | 107(27) | 98(29) |
| Smoking status at baseline | ||
| Current | 71(18) | 63(18) |
| Past | 160(40) | 148(43) |
| Never | 171(42) | 128(37) |
| Mean temperature*, Celsius | 16(9) | 19(9) |
| Relative humidity*, % | 59(19) | 55(17) |
mean(standard deviation)
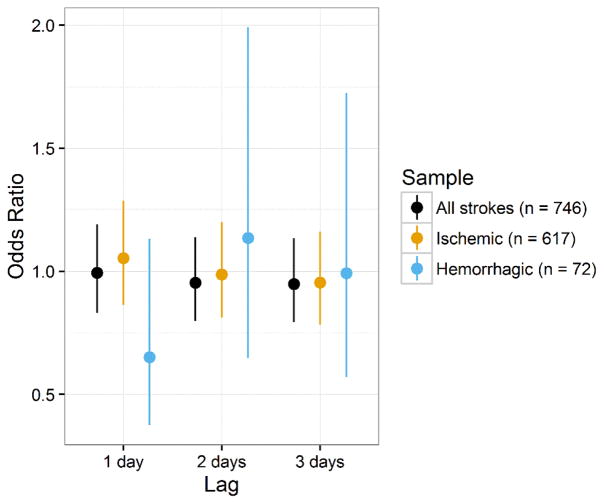
The odds ratios and 95% confidence intervals for moderate compared to good PM2.5 exposure for lags of 1, 2, and 3 days for the entire cohort, as well as the stratified by stroke subtype, are presented in Table 2 and Figure 1. These odds ratios were adjusted for mean temperature and relative humidity. PM2.5 exposure was not associated with incident stroke or ischemic stroke, regardless of lag. We did not observe a significant association between PM2.5 exposure and stroke, regardless of lag and regardless of stroke subtype. A sensitivity analysis using PM2.5 concentration as a continuous variable did not change these inferences. Interaction terms for a 1-day lag between urbanicity and PM2.5, and region and PM2.5 were not significant (p = 0.70 and p = 0.14, respectively). These results indicate that PM2.5 concentration is not significantly associated with incident stroke in the REGARDS cohort.
Table 2.
Model results for likelihood of stroke for moderate/unhealthy to good PM2.5 exposure with 1- to 3-day lag, for all strokes and by stroke subtype.
| Outcome | Lag | Odds Ratio (95% CI) |
|---|---|---|
| All Strokes | 1 day | 0.99 (0.83, 1.19) |
| 2 day | 0.95 (0.80, 1.14) | |
| 3 day | 0.95 (0.79, 1.13) | |
| Ischemic Stroke | 1 day | 1.05 (0.86, 1.29) |
| 2 day | 0.99 (0.81, 1.20) | |
| 3 day | 0.95 (0.78, 1.16) | |
| Hemorrhagic Stroke | 1 day | 0.65 (0.37, 1.13) |
| 2 day | 1.14 (0.65, 1.99) | |
| 3 day | 0.99 (0.57, 1.73) |
Figure 1.
Odds ratios and 95% CIs for likelihood of stroke for moderate/unhealthy to good PM2.5 exposure with 1- to 3-day lag, for all strokes and by stroke subtype
Discussion
Evidence regarding the association between PM2.5 and stroke is mixed, and our conclusions did not support an association between short-term PM2.5 exposure and stroke. We were able to examine this association in a large, geographically and racially diverse sample of stroke, using a novel measure of PM2.5 exposure, and accounting for temperature and humidity. This research directly addressed gaps in the research calling for large studies that specifically examined ischemic and hemorrhagic stroke as well as used medical-record review for event ascertainment.33 We have provided strong evidence of a lack of association.
Recent reports describing relationships between longer-term PM2.5 exposure and stroke have drawn mixed conclusions. With respect to long-term exposures, a meta-analysis of 11 European Cohorts found that the risk of stroke increased by 19% for each 5 μg/m3 increase in mean annual PM2.5, although these results were not statistically significant.12 A global meta-analysis including 20 studies found long-term PM2.5 exposure to be associated with stroke, but that there was geographic variability in the association.11
Short-term exposure data have also been conflicting. Lisabeth et al. (2008) showed marginally significant associations between same-day PM2.5 and stroke in a community in Texas,34 while Wellenius et al. (2012) showed that the likelihood of stroke following a 24-hour period of moderate PM2.5 exposure (PM2.5 15–40 μg/m3), was 34% higher than for periods classified as good (PM2.5 ≤ 15 μg/m3).27 This research has indicated that PM2.5 exposure within ranges classified as “good” by the EPA still result in increased risk of stroke among stroke patients in the Boston area. Conversely, a recent meta-analysis of short-term changes in PM2.5 exposure and risk of hospital admissions for ischemic stroke including six studies found no significant association.33 However, these studies were based on EPA ground-level data, and many were limited to single communities. A larger study in Israel, also using satellite-derived measures of exposure, found no overall association between ischemic stroke and same-day PM2.5 exposure, but did find marginally significant association among those <55 years of age in stratified models.35
While our study has addressed gaps in the literature by including a large, geographically diverse US population, and with our ability to examine ischemic and hemorrhagic strokes separately, our research does suffer from some limitations. First, we used ambient PM2.5 exposure at the participants’ residences at the time of their stroke, and thus we may have misclassified exposure as the participant may have lived elsewhere during this time. Further, research considering PM2.5 exposure was limited by the inability to measure indoor PM2.5 exposure, and all PM2.5 research to date assumes that outdoor levels are reasonable surrogates for total exposure levels. Additionally, we did not have data about the components of the PM2.5 (the speciation of the fine particulate matter), and thus could not determine what comprised the PM2.5 exposure. It is known that speciation varies regionally, and thus we could have increased variability in our exposure measure by calling it all PM2.5.
Conclusions
In a racially and geographically diverse cohort of participants in the REGARDS study, including ischemic and hemorrhagic stroke events, we were unable to identify an association between short-term exposure to PM2.5 and stroke. Future work should consider other environmental pollutants, as well as particular components of PM2.5, and focus on longer term exposure.
Acknowledgments
Sources of Funding
NIH/NINDS: U01 NS041588
NASA: NNX09AV81G
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.2 The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Leslie A. McClure, Department of Epidemiology & Biostatistics, Drexel University.
Matthew S. Loop, Department of Epidemiology, University of Alabama at Birmingham.
William Crosson, NASA.
Dawn Kleindorfer, Department of Neurology, University of Cincinnati.
Brett Kissela, Department of Neurology, University of Cincinnati.
Mohammad Al-Hamdan, NASA.
References
- 1.Walker B, Jr, Mouton CP. Environmental influences on cardiovascular health. Journal of the National Medical Association. 2008;100:98–102. doi: 10.1016/s0027-9684(15)31182-2. [DOI] [PubMed] [Google Scholar]
- 2.Peng RD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among medicare patients. JAMA: the journal of the American Medical Association. 2008;299:2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larrieu S, Jusot JF, Blanchard M, Prouvost H, Declercq C, Fabre P, et al. Short term effects of air pollution on hospitalizations for cardiovascular diseases in eight french cities: The psas program. The Science of the total environment. 2007;387:105–112. doi: 10.1016/j.scitotenv.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA: the journal of the American Medical Association. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. American journal of epidemiology. 2008;167:667–675. doi: 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD. You are what you breathe: Evidence linking air pollution and blood pressure. Current hypertension reports. 2005;7:427–434. doi: 10.1007/s11906-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 8.Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, et al. Particulate air pollution is associated with an acute phase response in men; results from the monica-augsburg study. European heart journal. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 9.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environmental health perspectives. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environmental health perspectives. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-term exposure to particulate matter air pollution is a risk factor for stroke: Meta-analytical evidence. Stroke; a journal of cerebral circulation. 2015;46:3058–3066. doi: 10.1161/STROKEAHA.115.009913. [DOI] [PubMed] [Google Scholar]
- 12.Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, et al. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: Results from 11 european cohorts within the escape project. Environmental health perspectives. 2014;122:919–925. doi: 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hamdan MZ, Crosson WL, Economou SA, Estes MG, Jr, Estes SM, Hemmings SN, et al. Environmental public health applications using remotely sensed data. Geocarto international. 2014;29:85–98. doi: 10.1080/10106049.2012.715209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hamdan MZ, Crosson WL, Limaye AS, Rickman DL, Quattrochi DA, Estes MG, Jr, et al. Methods for characterizing fine particulate matter using ground observations and remotely sensed data: Potential use for environmental public health surveillance. Journal of the Air & Waste Management Association. 2009;59:865–881. doi: 10.3155/1047-3289.59.7.865. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Franklin M, Kahn R, Koutrakis P. Using aerosol optical thickness to predict ground-level pm2.5 concentrations in the st. Louis area: A comprison between misr and modis. Remote Sensing of Environment. 2007;107:33–44. [Google Scholar]
- 16.Koelemeijder RBA, Homan CD, Matthijsen J. Comparison of spatial and temporal variations of aerosol optical thickness and particulate matter over europe. Atmospheric Environment. 2006;40:5304–5315. [Google Scholar]
- 17.Al-Saadi J, Szykman J, Pierce RB, Kittaka C, Neil D, Chu DA, et al. Improving national air quality forecases with satellite aerosol observations. Bulletin of the American Meterological Society. 2005;86:1249–1261. [Google Scholar]
- 18.Engel-Cox JA, Holloman C, Coutant B, Hoff R. Qualitative and quantitative evaluation of modis satellite sensor data for regional and urban scale air quality. Atmospheric Environment. 2004;38:2495–2509. [Google Scholar]
- 19.Gupta P, Christopher SA, Box MA, Box GP. Multi year satellite remote sensing of particulate matter air quality over sydney, australia. International Journal of Remote Sensing. 2007;28:4483–4498. [Google Scholar]
- 20.Gupta P, Christopher S, Wang J, Gehrig R, Lee Y, Kumar R. Satellite remote sensing of particulate matter and air quality over global cities. Atmospheric Environment. 2006;40:5880–5892. [Google Scholar]
- 21.Rush A, Dougherty J, Engel-Cox JA. Correlating seasonal averaged in situ monitoring of fine pm with satellite remote sensing data using geographic information system (gis). 49th Annual Meeting of the International Society for Optical Engineering; 2004; Denver, Colorado. [Google Scholar]
- 22.Wang J, Christopher SA. Intercomparison between satellite-derived aerosol optical thickness and pm2.5 mass: Implications for air quality studies. Geophysical Research Letters. 2003;30:2095. [Google Scholar]
- 23.Chu A, Kaufman YJ, Zibordi G, Chern JD, Mao J, Li C, et al. Global monitoring of air pollution over land from the earth observing system-terra moderate resolution imaging spectroradiometer (modis) Journal of Geophysical Resarch. 2003;108:D21. [Google Scholar]
- 24.Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology. 2005;16:717–726. doi: 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- 25.Lumley T, Levy D. Bias in the case-crossover design: Implications for studies of air pollution. Environmetrics. 2000;11:689–704. [Google Scholar]
- 26.Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: The reasons for geographic and racial differences in stroke study. Stroke; a journal of cerebral circulation. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 27.Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, et al. Ambient air pollution and the risk of acute ischemic stroke. Archives of internal medicine. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Hoff RM, Engel-Cox JA. The relation between moderate resolution imaging spectroradiometer (modis) aerosol optical depth and pm2.5 over the united states: A geographical comparison by u.S. Environmental protection agency regions. Journal of the Air & Waste Management Association. 2009;59:1358–1369. doi: 10.3155/1047-3289.59.11.1358. [DOI] [PubMed] [Google Scholar]
- 29.Cosgrove BA, Lohmann D, Mitchell KE, Houser PR, Wood ER, Schaake JC, et al. Real-time and retrospective forcing in the north american land data assimilation system (nldas) project. Journal of Geophysical Resarch. 2003;108:8842. [Google Scholar]
- 30.Iribarne JV, Goddson WL. Atmospheric thermodynamics. London: D. Reidel Publishing Compnay; 1981. [Google Scholar]
- 31.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke; a journal of cerebral circulation. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 32.Environmental Protection Agency. [Accessed June 1, 2016];Revised air quality standards for particle pollution and updates to the air quality index (aqi) https://www3.epa.gov/airquality/particlepollution/2012/decfsstandards.pdf.
- 33.Wang Y, Eliot MN, Wellenius GA. Short-term changes in ambient particulate matter and risk of stroke: A systematic review and meta-analysis. Journal of the American Heart Association. 2014;3(4) doi: 10.1161/JAHA.114.000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Annals of neurology. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yitshak Sade M, Novack V, Ifergane G, Horev A, Kloog I. Air pollution and ischemic stroke among young adults. Stroke; a journal of cerebral circulation. 2015;46:3348–3353. doi: 10.1161/STROKEAHA.115.010992. [DOI] [PubMed] [Google Scholar]