Abstract
Multifunctional factor progranulin (PGRN) plays an important role in lysosomes, and its mutations and insufficiency are associated with lysosomal storage diseases, including neuronal ceroid lipofuscinosis and Gaucher disease (GD). The first breakthrough in understanding the molecular mechanisms of PGRN as regulator of lysosomal storage diseases came unexpectedly while investigating the role of PGRN in inflammation. Challenged PGRN null mice displayed typical features of GD. In addition, GRN gene variants were identified in GD patients and the serum levels of PGRN were significantly lower in GD patients. PGRN directly binds to and functions as a chaperone of the lysosomal enzyme β-glucocerebrosidase (GCaase), whose mutations cause GD. In addition, its C-terminus containing granulin E domain, termed Pcgin (PGRN C-terminus for GCase Interaction), is required for the association between PGRN and GCase. The concept that PGRN acts as a chaperone of lysosomal enzymes was further supported and extended by a recent article showing that PGRN acts as a chaperone molecule of lysosomal enzyme cathepsin D (CSTD), and the association between PGRN and CSTD is also mediated by PGRN's C-terminal granulin E domain. Collectively, these reports suggest that PGRN may act as a shared chaperone and regulates multiple lysosomal enzymes.
Keywords: β-glucocerebrosidase, Cathepsin D, Chaperone, Lysosomal storage diseases, Lysosomal trafficking, Progranulin
PGRN is an important molecule involved in various pathophysiological conditions, including embryonic development, autoimmunity and inflammation, neurodegeneration, and tumorgenesis.1, 2 Emerging evidences have shown that PGRN also plays important roles in lysosome function. Homozygous mutations in GRN (the gene encoding PGRN) are known to cause neuronal ceroid lipofuscinosis (NCL),3, 4 a rare lysosome storage disease pathologically characterized by lysosomal aggregation of lipopigments and predominately presenting through neurodegenerative effects on cognition and sensorimotor ability; however, the mechanisms underlying PGRN insufficiency-associated lysosomal storage and neurodegenerative diseases remain largely unclear.
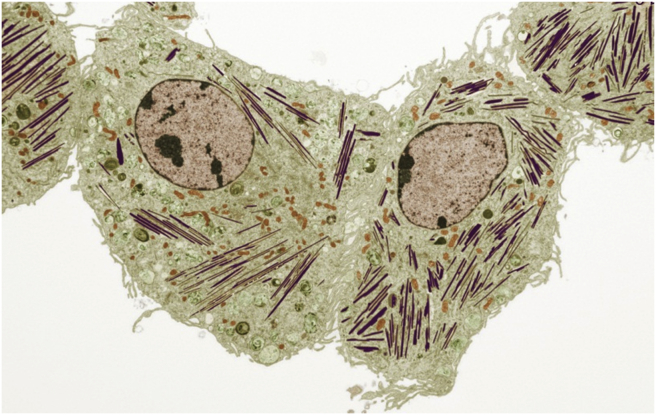
We reported previously that PGRN binds to and acts as a chaperone of lysosomal enzyme β-glucocerebrosidase (GCaase), mutations of which are causative of Gaucher Disease (GD), the most common lysosome storage disease. In an effort to determine the role of PGRN in the lung inflammation5 we unexpectedly found that ovabumin-challenged and aged PGRN null mice develop a GD phenotype, including typical Gaucher cells, β-glucocerebroside accumulation, and classical tubular like-structural transformation of lysosomes as examined under electronic microscope (Fig. 1).6 In line with the findings from mouse models, we found the serum levels of PGRN were significantly reduced in GD patients, and several GRN gene variants were identified in GD patients.6 Mechanistic studies demonstrated that PGRN functions as a chaperone molecule of GCase, and recruits HSP70 to form a ternary complex required for lysosomal appearance of GCase.7 A series of C-terminal and N-terminal deletion mutants identified the C-terminus of PGRN containing the granulin E domain is necessary for the association between PGRN and GCase. In addition, the C-terminus of PGRN was shown to effectively ameliorate GD phenotypes in vitro and in vivo.7
Figure 1.
Transmission electronic microscope assays of lung tissues from Ovabumin-challenged PGRN knockout mice. Macrophage lysosome from ovabumin-challenged PGRN KO mice became tubular-like instead of a regular round shape. Other organelles, such as mitochondria appeared normal. Tubular-like lysosomes in “giant” macrophages (i.e. Gaucher cells) are shown in purple, and mitochondria is colored in orange (Thanks to Chris Petzold and Kristen Dancel at NYU Medical School OCS Microscopy Core for creating this colored image from the original black and white electronic microscope image).
The finding that PGRN acts as a lysosomal enzyme chaperone6, 7 was extended by a very recent article by Beel, et al.8 This paper shows that PGRN acts as a chaperone molecule of another lysosomal enzyme, cathepsin D (CSTD), and the association between PGRN and CSTD is also mediated by PGRN's C-terminal granulin E domain.8 Interestingly, both PGRN and CSTD are among the 14 as-of-yet identified genes implicated in neuronal ceroid lipofuscinosis (NCL).9 Beel, et al paper provides physical and functional evidences linking PGRN and CSTD together. PGRN directly binds to CSTD and is important for maintaining the enzyme activity of CTSD.8 In addition, the C-terminal granulin E domain was identified as the functional domain mediating binding of PGRN to CSTD and PGRN's activity as a chaperone molecule.
In brief, the recent publication from Beel's group further strengthens the concept originally reported in EBiomedicine that PGRN acts as a chaperone of lysosomal enzymes and that the PGRN C-terminus is responsible for these associations.6, 7, 10 It is conceivable that PGRN could have a more general function, beyond its associations with GCase and CSTD, as a lysosomal protein chaperone. Demonstration of associations of PGRN with additional lysosome enzymes forthcoming, application of this chaperone molecule or its derivatives may lead to innovative therapeutics for a host of neurodegenerative and lysosome storage diseases.
Conflicts of interest
The authors declare no conflict of interest and competing financial interests.
Acknowledgments
We apologize to the colleagues whose publications are not included due to the space limitation. This work was supported partly by NIH research grants R01AR062207, R01AR061484, and a DOD research grant W81XWH-16-1-0482.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Jian J., Konopka J., Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman A., Bennett H.P. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–1254. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- 3.Smith K.R., Damiano J., Franceschetti S. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotzl J.K., Mori K., Damme M. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014;127:845–860. doi: 10.1007/s00401-014-1262-6. [DOI] [PubMed] [Google Scholar]
- 5.Tang W., Lu Y., Tian Q.Y. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jian J., Zhao S., Tian Q.Y. Association between progranulin and gaucher disease. EBioMedicine. 2016;11:127–137. doi: 10.1016/j.ebiom.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jian J., Tian Q.Y., Hettinghouse A. Progranulin recruits HSP70 to beta-glucocerebrosidase and is therapeutic against gaucher disease. EBioMedicine. 2016;13:212–224. doi: 10.1016/j.ebiom.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beel S., Moisse M., Damme M. Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Hum Mol Genet. 2017 doi: 10.1093/hmg/ddx162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marotta D., Tinelli E., Mole S.E. NCLs and ER: a stressful relationship. Biochim Biophys Acta. 2017;1863:1273–1281. doi: 10.1016/j.bbadis.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy F.Y., Christensen C.L. Progranulin as a novel factor in gaucher disease. EBioMedicine. 2016;13:13–14. doi: 10.1016/j.ebiom.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]