Abstract
Background
Treatment patterns for metastatic castration-resistant prostate cancer (mCRPC) have changed substantially in the last few years. In trial COU-AA-302 (chemotherapy-naïve men with mCRPC), abiraterone acetate plus prednisone (AA) significantly improved radiographic progression-free survival and overall survival (OS) when compared to placebo plus prednisone (P).
Objective
This post hoc analysis investigated clinical responses to docetaxel as first subsequent therapy (FST) among patients who progressed following protocol-specified treatment with AA, and characterized subsequent treatment patterns among older (≥75 yr) and younger (<75 yr) patient subgroups.
Design, setting, and participants
Data were collected at the final OS analysis (96% of expected death events). Subsequent therapy data were prospectively collected, while response and discontinuation data were collected retrospectively following discontinuation of the study drug.
Intervention
At the discretion of the investigator, 67% (365/546) of patients from the AA arm received subsequent treatment with one or more agents approved for mCRPC.
Outcome measurements and statistical analysis
Efficacy analysis was performed for patients for whom baseline and at least one post-baseline prostate-specific antigen (PSA) values were available.
Results and limitations
Baseline and at least one post-baseline PSA values were available for 100 AA patients who received docetaxel as FST. While acknowledging the limitations of post hoc analyses, 40% (40/100) of these patients had an unconfirmed ≥50% PSA decline with first subsequent docetaxel therapy, and 27% (27/100) had a confirmed ≥50% PSA decline. The median docetaxel treatment duration among these 100 patients was 4.2 mo. Docetaxel was the most common FST among older and younger patients from each treatment arm. However, 43% (79/185) of older patients who progressed on AA received no subsequent therapy for mCRPC, compared with 17% (60/361) of younger patients.
Conclusions
Patients with mCRPC who progress with AA treatment may still derive benefit from subsequent docetaxel therapy. These data support further assessment of treatment patterns following AA treatment for mCRPC, particularly among older patients.
Trial registration
ClinicalTrials.gov NCT00887198.
Patient summary
Treatment patterns for advanced prostate cancer have changed substantially in the last few years. This additional analysis provides evidence of clinical benefit for subsequent chemotherapy in men with advanced prostate cancer whose disease progressed after treatment with abiraterone acetate. Older patients were less likely to be treated with subsequent therapy.
Keywords: Abiraterone acetate, Docetaxel, Elderly, Metastatic castration-resistant, prostate cancer, Subsequent therapy, Treatment patterns
1. Introduction
Prostate cancer is the most common cancer among men in industrialized countries and represents one of the leading causes of cancer deaths [1,2]. The mainstay for treatment of metastatic castration-resistant prostate cancer (mCRPC) in the past was docetaxel in combination with androgen deprivation therapy [3–5]. However, treatment patterns for patients with mCRPC have changed substantially in the last few years following the approval of five new agents for mCRPC, including androgen signaling–directed therapy, immunotherapy, and radiopharmaceutical products [6–8].
Abiraterone acetate (AA) is a prodrug of abiraterone, a potent and specific inhibitor of the enzyme 17α-hydroxylase/C17,20-lyase that blocks extragonadal and testicular androgen biosynthesis [9]. AA (1 g daily) plus prednisone or prednisolone (5 mg twice daily) is approved for the treatment of patients with mCRPC on the basis of results for two pivotal phase 3 trials [10,11]. In patients with mCRPC who had received prior docetaxel chemotherapy, treatment with AA improved overall survival (OS) by 4.6 mo (hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.64–0.86; p < 0.0001) compared to placebo and prednisone or prednisolone 5 mg twice daily (hereafter referred to as P) [10,12]. In COU-AA-302, asymptomatic or mildly symptomatic men with chemotherapy-naïve mCRPC had significantly better radiographic progression-free survival (rPFS; HR 0.52; p < 0.0001) and OS (34.7 vs 30.3 mo; HR 0.81, 95% CI 0.70–0.93; p = 0.0033) with AA compared to P [11,13].
Although the use of sequential therapy is common and its efficacy is of great interest to clinicians, there is limited information about subsequent therapy for mCRPC following treatment with AA. We conducted a post hoc analysis of COU-AA-302 to evaluate the clinical outcome for docetaxel as first subsequent therapy (FST) among patients in the AA treatment arm who experienced disease progression after protocol-specified treatment with AA and to characterize subsequent treatment patterns among older (≥75 yr) and younger (<75 yr) patient subgroups.
2. Patients and methods
COU-AA-302 (ClinicalTrials.gov NCT00887198) is a phase 3, multinational, randomized, double-blind, placebo-controlled study conducted at 151 sites in 12 countries [14]. Patients were enrolled from April 2009 to June 2010. Patients aged ≥18 yr with asymptomatic or mildly symptomatic mCRPC were chemotherapy-naïve and had received previous anti-androgen therapy. Additional inclusion criteria included ongoing androgen deprivation with serum testosterone <0.50 ng/ml and life expectancy of ≥6 mo. Patients were medically or surgically castrated, and had tumor progression. Patients with visceral metastases or patients who had received previous therapy with ketoconazole for >7 d were excluded.
2.1. Study design
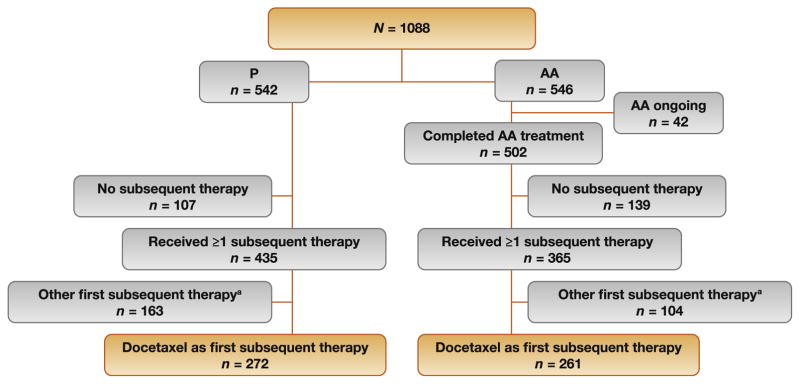
A total of 1088 patients were stratified by Eastern Cooperative Oncology Group performance status (0 vs 1) and randomized 1:1 to AA (1000 mg QD plus P 5 mg BID; n = 546) or placebo plus P (n = 542). All study personnel were blinded to the patient treatment assignments, and patient treatment assignments remained blinded at the time of disease progression. The co-primary end points were rPFS and OS. The primary and secondary end point results obtained at the time of this analysis have been described in detail previously [11,13].
As of March 2014, 365 (67%) patients in the AA treatment arm and 435 (80%) in the P arm received subsequent treatment with one or more agents approved for mCRPC at the discretion of the investigator after protocol-specified treatment (Fig. 1) [11]. At the time of data collection, 8% (42/546) of patients continued on AA. The use of a specific subsequent therapy for mCRPC was not proscribed in the study, but these data were collected prospectively, while response and discontinuation data on subsequent therapy were collected retrospectively after patients discontinued the study drug. Data that could be accessed for these patients were included in the current analysis. Efficacy analysis was performed among patients from the AA treatment arm with available baseline prostate-specific antigen (PSA) within 30 d before the first dose of docetaxel and at least one post-baseline PSA value. As recommended by the Prostate Cancer Clinical Trials Working Group (PCWG2) [15], PSA response was defined as a ≥50% PSA decline from baseline with at least two available PSA values measured 3–4 wk apart. Unconfirmed ≥50% PSA declines were defined as a ≥50% PSA decline from baseline with at least one available PSA value. Reasons for discontinuation were investigator reported without specific criteria. Efficacy data for subsequent therapy were not collected for patients from the P arm.
Fig. 1.
CONSORT diagram. a Abiraterone acetate, cabazitaxel, enzalutamide, ketoconazole, or sipuleucel-T.
2.2. Statistical analyses
All data for the current analyses were collected at the final OS analysis (96% of expected death events). On the basis of the aggregate efficacy and safety data at the second interim analysis (clinical cutoff December 2011), the independent data-monitoring committee unanimously recommended unblinding in February 2012, 20 mo after the last patient was enrolled. To characterize subsequent therapy and treatment patterns by age subgroup, patients were dichotomized by age at 75 yr. This is the same cutoff used for age subgroup analysis for COU-AA-302 [16] and COU-AA-301 [17] and is consistent with US regulatory guidance to define a geriatric population in clinical trials [18]. Clinical progression data were obtained from investigator reports, and data on responses and subsequent therapy for mCRPC were collected by trial monitors during site visits. The data were then source-verified and entered into the database. PSA response rates and post-treatment PSA declines were summarized using frequency and percentages. The time to PSA progression (TTPP) was estimated using PCWG2 criteria and included censored patients. Median TTPP with 95% CI was estimated using the Kaplan-Meier method.
3. Results
Baseline characteristics for patients who progressed on AA and received docetaxel as FST were similar to the full COU-AA-302 intention-to-treat (ITT) population (Table 1). Among patients in the AA arm, 36% (194/546) and 15% (83/ 546) had two or more and three or more subsequent therapies, respectively (Table 2). Among those in the P arm, 45% (243/542) and 22% (121/542) had two or more and three or more subsequent therapies, respectively.
Table 1.
Baseline characteristics of the ITT population and patients who received docetaxel as FST
| COU-AA-302 AA treatment arm | ||
|---|---|---|
| Docetaxel as FST | ITT population | |
| Patients (N) | 261 | 546 |
| Median age, yr (range) [n] | 69 (44–93) [261] | 71 (44–95) |
| Median time from ID to FD, yr (range) [n] | 4.4 (<1–28) [261] | 5.5 (<1–28) [542] |
| Median PSA at ID, ng/ml (range) [n] | 23 (2–5036) [236] | 22 (0.4–5036) [470] |
| Gleason score ≥8 at ID, n/N (%) | 129/244 (53) | 263/488 (54) |
| Extent of disease, n/N (%) | ||
| Bone only | 132/261 (51) | 274/542 (51) |
| Soft tissue a or node | 128/261 (49) | 267/542 (49) |
| Other | 1/261 (<1) | 4/542 (<1) |
| ECOG PS, n/N (%) | ||
| 0 | 206/261 (79) | 423/546 (76) |
| 1 | 55/261 (21) | 133/546 (24) |
| Prior prostate cancer therapy, n/N (%) | ||
| Surgery | 125/261 (48) | 256/544 (47) |
| Radiotherapy | 138/261 (53) | 283/544 (52) |
| Hormonal | 261/261 (100) | 544/544 (100) |
| Other | 39/261 (15) | 82/544 (15) |
| Median baseline PSA, ng/ml (range) [n] | 48 (1–3266) [261] | 42 (0–3927) [546] |
| Median baseline LDH, IU/l (range) [n] | 189 (60–871) [261] | 187 (60–871) [543] |
| Median baseline ALP, IU/l (range) [n] | 103 (32–1927) [261] | 93 (32–1927) [546] |
ITT = intention to treat; FST = first subsequent therapy; AA = abiraterone acetate plus prednisone; ID = initial diagnosis; FD = first dose; ECOG PS = Eastern Cooperative Oncology Group performance status; PSA = prostate-specific antigen; LDH = lactate dehydrogenase; ALP = alkaline phosphatase.
Excludes visceral metastases.
Table 2.
Subsequent therapy for metastatic castration-resistant prostate cancer following discontinuation of protocol-specified study drug
| AA | P | |
|---|---|---|
| Patients | 546 | 542 |
| Any subsequent therapy | 365 (67.0) | 435 (80.3) |
| Two or more subsequent therapies | 194 (36.0) | 243 (45.0) |
| Three or more subsequent therapies | 83 (15.2) | 121 (22.3) |
| No subsequent therapy | 139 (25.4) | 107 (19.7) |
| Protocol-specified treatment ongoing | 42 (7.7) | 0 |
AA = abiraterone acetate plus prednisone; P = placebo plus prednisone. Data are presented as n (%).
3.1. FST with docetaxel
FST included taxane chemotherapy, androgen signaling–directed therapy, and immunotherapy (Table 3). Overall, there was low prevalence of cabazitaxel and enzalutamide as FST. Docetaxel was by far the most common FST in the AA arm (48%, 261/546) and in the P arm (50%, 272/542). The median duration of docetaxel therapy following AA was 3.0 mo (interquartile range [IQR] 0.95–5.7; Table 4). The reason most commonly reported for discontinuation of docetaxel as FST was PSA progression, although more than one reason was reported for 39 patients. Toxicity appeared to be a fairly infrequent reason for docetaxel discontinuation, even though these patients had advanced disease and previous medical therapy for mCRPC.
Table 3.
First subsequent therapy for metastatic castration-resistant prostate cancer
| AA | P | |
|---|---|---|
| Patients | 546 | 542 |
| Taxane chemotherapy | ||
| Docetaxel | 261 (48.0) | 272 (50.2) |
| Cabazitaxel | 4 (<1) | 3 (<1) |
| Androgen synthesis inhibitor | ||
| Abiraterone acetate | 13 (2.4) | 80 (14.8) |
| Ketoconazole | 36 (6.6) | 56 (10.3) |
| Androgen receptor antagonist (enzalutamide) | 20 (3.7) | 4 (<1) |
| Immunotherapy (sipuleucel-T) | 31 (5.7) | 20 (3.7) |
AA = abiraterone acetate plus prednisone; P = placebo plus prednisone. Data are presented as n (%).
Table 4.
Treatment duration and discontinuation reasons for 261 patients who received FST with docetaxel
| Median duration of docetaxel as FST, mo (IQR) a | 3.02 (0.95–5.72) |
| Reason for discontinuation per investigator, n (%) b | |
| Clinical progression | 38 (15) |
| Radiographic progression | 36 (14) |
| Prostate-specific antigen progression | 75 (29) |
| Adverse event | 41 (16) |
| Therapy ongoing | 11 (4) |
| Other | 73 (28) |
FST = first subsequent therapy; IQR = interquartile range.
Start and end dates for docetaxel therapy were known for 235 patients. Among 100 patients for whom baseline and at least one post-baseline prostate-specific antigen values were available, the median duration was 4.17 mo (IQR 2.79–6.37).
During first subsequent therapy with docetaxel. Reasons were based on investigator judgment without specific criteria; more than one reason was selected for 39 patients.
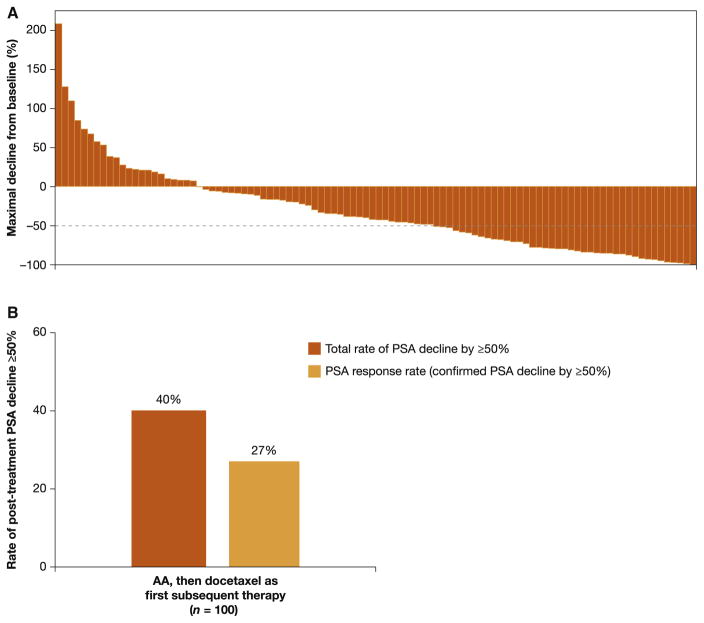
A total of 100 AA patients who received docetaxel as FST had post-trial baseline and post-baseline PSA values available. Among these 100 patients the median duration of docetaxel therapy was 4.2 mo (IQR: 2.8–6.4). However, data on the median number of docetaxel courses administered were not available. The rate of post-treatment PSA decline ≥50% was 40% (40/100), including the 27 patients with a confirmed response (PSA response rate 27%; Fig. 2). TTPP was estimated based on 29 events and 71 censored patients. The median TTPP for these 100 patients was 7.6 mo (95% CI 5.0 to not estimable; Supplementary Fig. 1). The major reasons for censoring were the proportion of patients who did not have PSA progression and those who had PSA progression but did not have complete PSA data available because of retrospective data collection.
Fig. 2.
Unconfirmed PSA declines among patients treated with abiraterone acetate who received docetaxel as first subsequent therapy. (A) Maximum PSA decline from baseline. (B) Total and confirmed post-treatment PSA decline. Waterfall plot with maximum PSA change and PSA response rate for patients with available baseline PSA within 30 d of subsequent docetaxel therapy and at least one post-baseline PSA value. PSA = prostate-specific antigen; AA = abiraterone acetate plus prednisone.
3.2. Treatment patterns by age subgroup
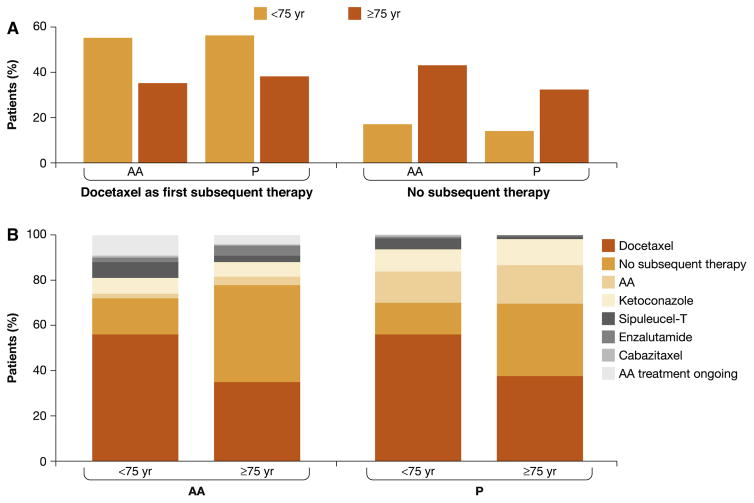
The treatment patterns by age subgroup are shown in Fig. 3 and Supplementary Tables 1 and 2. In the overall ITT population, 15% (114/738) of younger patients received no subsequent therapy, compared with 38% (132/350) of older patients (Supplementary Table 1). The proportion of patients who died without receiving subsequent therapy followed the same pattern (Supplementary Table 1). Moreover, 43% (79/185) of older patients with progression on AA did not receive subsequent therapy for mCRPC following discontinuation of the protocol-specified study drug. Docetaxel was the most common FST among older and younger patients in each treatment arm. More than half of younger patients from both treatment arms received docetaxel as FST: 55% (197/361) in the AA arm and 56% (210/377) in the P arm. By contrast, 35% (64/185) and 38% (62/165) of older patients from the AA and P arms, respectively, received docetaxel as FST. Similar trends were observed when treatment patterns were assessed according to the mCRPC drugs used in any sequence (Supplementary Table 2). For both younger and older patients in the P arm, the subsequent therapy most commonly used was docetaxel and AA. Cabazitaxel was more commonly used as subsequent therapy among younger compared to older patients.
4. Discussion
This post hoc analysis characterized subsequent therapy and treatment patterns among patients with mCRPC who progressed on AA. Patients were commonly treated with subsequent therapy, although older patients were almost three times more likely not to receive any subsequent therapy in comparison younger patients. Docetaxel was the FST for a large majority of patients, irrespective of age group.
The observed post-treatment PSA declines ≥50%support an antitumor effect of docetaxel as FST in some patients who progressed with AA. Although 27% of patients had a confirmed PSA response, the data overall on PSA decline suggest that docetaxel may still impact clinical benefit in the post-AA setting. The median TTPP was 7.6 months which would be, similar to that from contemporaneous reports of AA-naïve patients treated with docetaxel in large phase 3 trials [19–21]. However, this observation needs to be interpreted with caution owing to the high censoring rate (71%), which is likely to have led to overestimation of this value. Moreover, the median duration of docetaxel therapy was based on patients with known docetaxel start and end dates, whereas the median number of docetaxel courses administered may not have been captured. With this consideration, the median treatment duration in the 100-patient cohort described here was 4.2 mo, compared to 7.7 mo in the TAX-327 trial. The confirmed PSA response rate among patients from the AA arm who received docetaxel as FST was 27%, which is lower than the 45% rate reported for docetaxel therapy for mCRPC in the phase 3 TAX-327 study [4]. However, the rate of confirmed and unconfirmed post-treatment PSA decline ≥50% was 40%, which is closer to the TAX-327 findings. In addition, patients in TAX-327 may have been vigorously selected and prescreened in terms of performance status and prognosis as part of the eligibility criteria for the trial, which specifically investigated docetaxel use.
There is conflicting evidence that mCRPC patients who experience disease progression after androgen signaling–directed therapy may be less responsive to taxane-based chemotherapy. Such cross-resistance could possibly be mediated in part by taxane-induced disruption of androgen receptor (AR) trafficking along microtubules [22]. Results from two retrospective studies suggest partial cross-resistance between AA and docetaxel. In a study by Mezynski et al. [23], subsequent therapy with docetaxel resulted in PSA declines ≥50% in 26% of cases and a median TTPP of 4.6 mo (95% CI, 4.2–5.9) among mCRPC patients previously treated with AA (n = 35). In a second study [24], mCRPC patients who received AA before docetaxel (n = 24) had median PFS of 4.1 mo compared to 6.7 mo in the docetaxel-only group (p = 0.002). In the same study, PSA declines ≥50% were less frequent among patients who received AA before docetaxel (38% vs 63%; p = 0.02); however, PSA responses to docetaxel were observed in 30% (7/23) of men previously treated with AA [24]. In addition, other reports have suggested no or minimal cross-resistance between AA and docetaxel [25] and between ketoconazole and docetaxel [26]. Additional results supporting a clinical benefit for taxane-based chemotherapy following AA were reported by Al Nakouzi et al. [27]. In this retrospective study of 79 patients with progressive mCRPC after docetaxel and AA, PSA declines ≥50% were achieved in 35% (28/79) of patients who received subsequent therapy with cabazitaxel [27].
The potential role of AR splice variants as a resistance mechanism is further evidence that all subsequent therapy for mCRPC may not be effective [28]. In a prospective study of 62 men with mCRPC, detection of AR-V7 mRNA in circulating tumor cells was associated with resistance to AA and enzalutamide [29]. Results from two retrospective studies suggest that the effects of AA following enzalutamide treatment for mCRPC are associated with limited response rates for chemotherapy-pretreated and chemotherapy-naïve men [30,31]. Similar observations were reported for enzalutamide following AA treatment [32]. However, a recent report suggests that AR-V7 is not associated with primary resistance to taxane chemotherapy [33]. Thus, it is plausible that some patients in the current analysis progressed on AA treatment because of AR-V7, but retained sensitivity to docetaxel. Overall, our results suggest that a proportion of AA-unresponsive patients may still derive a benefit from subsequent therapy with docetaxel.
The treatment of mCRPC is evolving rapidly and there may be geographic differences in terms of regional practice patterns and available agents. While COU-AA-302 was an international study, the availability of other drugs approved for mCRPC (including enzalutamide, radium-223, and cabazitaxel) varied by country, and this may have influenced post-AA treatment patterns. In addition, subsequent to the conclusion of COU-AA-302, information from two data sets emerged to support the use of upfront docetaxel in the metastatic hormone-sensitive setting. The impact of docetaxel in this earlier application on post-AA treatment patterns and treatment efficacy will need to be evaluated in future studies.
A substantial proportion (43%) of patients aged ≥75 yr who progressed with AA received no subsequent therapy with mCRPC drugs, suggesting that treatment nihilism may exist, in part potentially because of the toxicity profile of docetaxel in this population, although patient acceptance and other disease characteristics may also be factors [34,35]. Although the proportion of older patients receiving no subsequent therapy is high, this finding is consistent with other observations of treatment patterns among elderly men with mCRPC [36,37]. Interestingly, a high proportion of patients in the AA treatment arm received subsequent therapy, suggesting that patients remained fit enough for subsequent therapy after progression on AA. Overall, these observations suggest that the favorable toxicity profile of AA may allow a greater proportion of mCRPC patients, especially older men, to receive effective mCRPC medical therapy. Treatment patterns are important for older patients with mCRPC for several reasons. In comparison to younger patients, elderly men are more likely to present with advanced disease [38]. Although age-related changes may affect the risk of toxicities, age alone should not prevent patients from deriving benefit from cancer treatment [38,39]. Indeed, the clinical benefit of AA and enzalutamide among elderly patients with mCRPC has been demonstrated in post hoc analyses for randomized, double-blind phase 3 trials [16,17,40].
There are several important limitations to the analysis. Subsequent therapy and treatment patterns were evaluated retrospectively, and no specific end points were defined; investigators were instructed to follow PCWG2 criteria. Since patients were under routine clinical care and no longer on trial, PSA data were not available for most patients to confirm PSA response or progression data, and thus there was a high censoring rate. Among the 261 AA patients who received docetaxel as FST, post-trial baseline and post-baseline PSA values were not available for 161 men. Thus, the confirmed PSA response was limited to the 100 patients with baseline and post-baseline PSA values available, which may have introduced selection bias. For example, patients who progressed rapidly on docetaxel may be under-represented in the analysis compared to patients who had a more favorable clinical course and possibly more folllow-up PSA data available.
5. Conclusions
This post hoc analysis for chemotherapy-naïve patients with mCRPC who experienced disease progression on AA suggests that docetaxel has meaningful antitumor activity as FST. While acknowledging the limitations of a retrospective analysis, our observations suggest that docetaxel may be considered for patients with mCRPC who progress on AA treatment. A substantial proportion of older patients with mCRPC who progressed on AA received no subsequent therapy with drugs approved for mCRPC. This may be explained by a broader group of mCRPC patients considered eligible for first-line AA therapy but not considered candidates for other subsequent mCRPC treatments such as docetaxel after progression on AA. Taken together, these data indicate that further assessment of subsequent therapy and treatment patterns following AA treatment for mCRPC is warranted, particularly among older patients.
Presented in part at the 2016 Genitourinary Cancers Symposium (ASCO GU), January 6–9, 2016, San Francisco, CA, USA (abstract 168); the 2015 Genitourinary Cancers Symposium (ASCO GU), February 26–28, 2015, Orlando, FL, USA (abstract 184); and the 2015 European Association of Urology (EAU) Congress, March 20–24, 2015, Madrid, Spain (abstract 668).
Supplementary Material
Fig. 3.
First subsequent therapy by age subgroup. (A) Docetaxel as first subsequent therapy and no subsequent therapy. (B) All first subsequent therapy. AA = abiraterone acetate plus prednisone; P = placebo plus prednisone.
Acknowledgments
The authors thank Thomas W. Griffin for his critical review of the analyses. Writing assistance was provided by Ann Tighe of PAREXEL and was funded by Janssen Global Services LLC.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.06.033.
Footnotes
Author contributions: Johann S. de Bono had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: de Bono, Flaig, De Porre, Kheoh, Li, Todd, Griffin.
Acquisition of data: de Bono, Flaig, Saad, Shore, Rathkopf, Smith, Fizazi, Mulders, North, Small, Ryan.
Analysis and interpretation of data: de Bono, Flaig, De Porre, Kheoh, Li, Todd, Griffin.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Kheoh, Li.
Obtaining funding: De Porre.
Administrative, technical, or material support: De Porre.
Supervision: All authors.
Other: None.
Financial disclosures: Johann S. de Bono certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Johann S. de Bono is a consultant/advisor to and has received honoraria from Johnson & Johnson. Matthew R. Smith is a consultant/advisor to and has received research funding from Janssen. Fred Saad is a consultant/advisor to and has received honoraria and research funding from Astellas and Janssen. Dana E. Rathkopf provides uncompensated research funding for Celgene, Janssen/Johnson & Johnson, Medication/Astellas, Millenium, and Novartis. She is also a consultant/advisor to Johnson & Johnson. Peter F.A. Mulders and Eric J. Small have nothing to disclose. Neal D. Shore is a consultant/advisor to Amgen, Astellas, Bayer, BNI, Dendreon, Ferring, Janssen, Medivation, Sanofi, Takeda, and Tolmar. Karim Fizazi is a consultant/advisor to and has received honoraria from Janssen. Peter De Porre, Thian Kheoh, Jinhui Li, and Mary B. Todd are employees of Janssen Research and Development and hold stock in Johnson & Johnson. Charles J. Ryan has received honoraria from Janssen. Thomas W. Flaig is a consultant/advisor to GTx; has received honoraria from BN ImmunoTherapeutics and GTx; receives research funding from Amgen, Aragon, Astellas, AstraZeneca, Bavarian Nordic, Bristol-Myers Squibb, Cougar Biotechnology, Dendreon, Exelixis, GlaxoSmithKline, GTx, Janssen Oncology, Lilly, Medivation, Novartis, Pfizer, Roche/Genentech, Sanofi, Sotio, and Tokai; and holds stock in Aurora Oncology.
Funding/Support and role of the sponsor: This work was supported by Janssen Research & Development (formerly Ortho Biotech Oncology Research & Development, Cougar Biotechnology Unit). The sponsor played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24:1141–62. doi: 10.1093/annonc/mds624. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal N, Di LG, Sonpavde G, Bellmunt J. New agents for prostate cancer. Ann Oncol. 2014;25:1700–9. doi: 10.1093/annonc/mdu038. [DOI] [PubMed] [Google Scholar]
- 7.Oudard S. Progress in emerging therapies for advanced prostate cancer. Cancer Treat Rev. 2013;39:275–89. doi: 10.1016/j.ctrv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26:1589–604. doi: 10.1093/annonc/mdv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17α-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 11.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 12.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–25. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MR, Rathkopf DE, Mulders PF, et al. Efficacy and safety of abiraterone acetate in elderly (75 years or older) chemotherapy naive patients with metastatic castration resistant prostate cancer. J Urol. 2015;194:1277–84. doi: 10.1016/j.juro.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulders PF, Molina A, Marberger M, et al. Efficacy and safety of abiraterone acetate in an elderly patient subgroup (aged 75 and older) with metastatic castration-resistant prostate cancer after docetaxel-based chemotherapy. Eur Urol. 2014;65:875–83. doi: 10.1016/j.eururo.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Guidance for industry. E7 studies in support of special populations: geriatrics, questions and answers. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM189544.pdf. [PubMed]
- 19.Fizazi K, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–7. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 20.Petrylak DP, Vogelzang NJ, Budnik N, et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2015;16:417–25. doi: 10.1016/S1470-2045(15)70025-2. [DOI] [PubMed] [Google Scholar]
- 21.Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14:760–8. doi: 10.1016/S1470-2045(13)70184-0. [DOI] [PubMed] [Google Scholar]
- 22.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezynski J, Pezaro C, Bianchini D, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23:2943–7. doi: 10.1093/annonc/mds119. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer MT, Zhou XC, Wang H, et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;66:646–52. doi: 10.1016/j.eururo.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal R, Harris A, Formaker C, et al. Response to subsequent docetaxel in a patient cohort with metastatic castration-resistant prostate cancer after abiraterone acetate treatment. Clin Genitourin Cancer. 2014;12:e167–72. doi: 10.1016/j.clgc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal R, Halabi S, Kelly WK, et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance) Cancer. 2013;119:3636–43. doi: 10.1002/cncr.28285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Nakouzi N, Le Moulec S, Albiges L, et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol. 2015;68:228–35. doi: 10.1016/j.eururo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 31.Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–7. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 32.Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30–6. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 33.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–91. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horgan AM, Seruga B, Pond GR, et al. Tolerability and efficacy of docetaxel in older men with metastatic castrate-resistant prostate cancer (mCRPC) in the TAX 327 trial. J Geriatr Oncol. 2014;5:119–26. doi: 10.1016/j.jgo.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Italiano A, Ortholan C, Oudard S, et al. Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur Urol. 2009;55:1368–76. doi: 10.1016/j.eururo.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 36.Lissbrant IF, Garmo H, Widmark A, Stattin P. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52:1593–601. doi: 10.3109/0284186X.2013.770164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flaig TW, Potluri RC, Ng Y, Todd MB, Mehra M. Treatment evolution for metastatic castration-resistant prostate cancer with recent introduction of novel agents: retrospective analysis of real-world data. Cancer Med. 2016;5:182–91. doi: 10.1002/cam4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherji D, Pezaro CJ, Shamseddine A, de Bono JS. New treatment developments applied to elderly patients with advanced prostate cancer. Cancer Treat Rev. 2013;39:578–83. doi: 10.1016/j.ctrv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Fung C, Dale W, Mohile SG. Prostate cancer in the elderly patient. J Clin Oncol. 2014;32:2523–30. doi: 10.1200/JCO.2014.55.1531. [DOI] [PubMed] [Google Scholar]
- 40.Sternberg CN, de Bono JS, Chi KN, et al. Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol. 2014;25:429–34. doi: 10.1093/annonc/mdt571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.