Abstract
During the menopause transition, women are at increased risk of subjective symptoms of executive dysfunction. Evidence from animal and human participant studies suggests adverse childhood experiences (ACE) may be a risk factor for executive complaints during this hormonal transition. Preclinical literature indicates early life adversity effects on serotonin function may play a role in this increased susceptibility. However, the mechanisms underlying this increase in vulnerability in human participants remain relatively unknown. Here we examined the impact of ACE and tryptophan depletion (TD), a paradigm used to lower central serotonin levels, on functional network connectivity in discovery and replication datasets. We hypothesized that ACE would be associated with decreased within-network connectivity. We predicted that TD would further lower connectivity in women with high levels of early adversity, but have no effect in women with low levels of early adversity. Forty women underwent two functional imaging sequences at two time points (141 total scans) in a double-blind, placebo controlled, crossover study. The effects of ACE and TD were evaluated using generalized estimating equations (GEE). As predicted, ACE was associated with lower within-network connectivity. While TD had no effect on connectivity in the low ACE group, TD increased connectivity in the high ACE group. The robust effect of ACE remained significant in the replication dataset, though the ACE x TD interaction did not. Together, these results suggest that early life adversity has lasting impacts on large-scale functional networks underlying executive function. Alterations in functional network connectivity may be one mechanism by which early life adversity increases the risk of cognitive disorders during menopause.
Keywords: Adverse childhood experiences, working memory, connectivity, menopause, tryptophan depletion
1. Introduction
The menopause transition is associated with increased susceptibility to subjective symptoms of cognitive dysfunction (Shanmugan and Epperson, 2014). Several reports have suggested that healthy women with no history of cognitive dysfunction experience increased difficulty with everyday tasks requiring executive processes such as working memory, focus, attention, organization, and planning (Epperson et al., 2015; Shanmugan and Epperson, 2014; Shanmugan et al., 2017b). There is preliminary evidence for adverse childhood experiences (ACE) such as abuse, neglect, and household dysfunction as a risk factor for executive dysfunction during this hormonal transition (Shanmugan and Epperson, 2014).
Significant adversity during childhood alters normal trajectories of brain maturation (Bruce et al., 2013; Hanson et al., 2015; Shanmugan and Satterthwaite, 2016) resulting in heightened vulnerability to executive dysfunction (Philip et al., 2016) in adult life. Neuroimaging studies have demonstrated that early adversity alters activation in brain regions subserving executive processes. In healthy adults, early trauma has been associated with executive system hyperactivation during working memory (Philip et al., 2016). In healthy menopausal women, estradiol treatment attenuates this ACE-associated executive hyperactivation (Shanmugan et al., 2017a), suggesting the effects of ACE may be magnified when women are hypogonadal.
While these studies demonstrate that ACE has effects on activation in particular brain regions, the impact of ACE on functional network architecture is less clear. There is growing evidence that the functional connectivity between brain regions as part of larger cognitive systems is critical to maintaining intact executive function (reviewed by Vaidya and Gordon, 2013). A recent large neuroimaging study in healthy adults ages 18–88 demonstrated that the degree of connectivity between and within large-scale brain networks becomes increasingly important to maintaining cognitive performance as we age (Tsvetanov et al., 2016). Additionally, altered neural connectivity has been suggested as a potential marker of menopausal women who may be at risk of late-life cognitive dysfunction (Vega et al., 2016). As such, understanding whether early adversity alters connectivity among these functional networks is particularly relevant in this middle-aged population.
During development, networks supporting cognitive processes segregate: brain regions become more connected with regions of the same network (increased within-network connectivity) and less connected with regions of other networks (decreased between-network connectivity) (Dosenbach et al., 2010; Fair et al., 2007a; Power et al., 2010; Satterthwaite and Baker, 2015; Shanmugan and Satterthwaite, 2016). Adversity-induced disruptions in this trajectory of network segregation have been observed as early as preschool (Demir-Lira et al., 2016) and last into adulthood (Philip et al., 2013). In healthy adults, early life trauma is associated with decreased connectivity between regions within the same network as well as increased connectivity between regions in different networks (Philip et al., 2013). Such patterns of dysconnectivity are associated with poorer working memory across the lifespan (Tsvetanov et al., 2016).
Together these studies suggest that early life adversity may confer a risk for executive dysfunction during menopause by altering connectivity between and within neural networks. However, the effects of ACE on functional network connectivity during this hormonal transition have not been studied. Converging evidence from animal and human studies suggests one mechanism by which ACE may alter brain function during periods of low estradiol may be via effects on serotonin. In non-human primates, both ovariectomy and social subordination stress are associated with reductions in tryptophan hydroxylase type 2 (TPH2) gene expression (Shively et al., 2003) and 5-HT1A binding potential (Michopoulos et al., 2014). Similarly, lowering central serotonin levels differentially impacts prefrontal cortex activation in menopausal women with high and low levels of early life adversity (Shanmugan et al., 2017a). However, whether ACE and sertononin exhibit a similar interactive effect on functional networks is not known.
Accordingly, we tested the hypothesis that ACE adversely impacts functional network connectivity during menopause via alterations in serotonin function. Forty healthy menopausal women with high and low levels of early life adversity underwent tryptophan depletion (TD) and performed two cognitive tasks during functional magnetic resonance imaging. Task effects were regressed to obtain discovery and replication timeseries approximate to the resting state (Fair et al., 2007b). The impact of ACE, TD, and the interaction between ACE and TD on within-network connectivity were examined in both datasets. We predicted that within-network connectivity would be lower in the high ACE group compared to the low ACE group during both active TD and sham TD. We also predicted that TD would further lower this measure in the high ACE group, but have no effect on connectivity in the low ACE group.
2. Methods
2.1. Participants
Participants were healthy menopausal women ages 48–60 with no current psychiatric diagnoses and within 10 years of their last menstrual period. Participants were right-handed and had a normal mammogram and pap smear within the last year and a clear urine toxicology. Follicle stimulating hormone (FSH) levels increase during menopause as estradiol levels decrease; FSH > 30 IU/ml was used to confirm menopausal status (Epperson et al., 2012) and was required for inclusion. Exclusion criteria included use of estrogen or hormone therapy within the last year, contraindications to hormone therapy, IQ less than 95, Mini Mental Status Exam Score less than 25, lifetime history of DSM-V psychotic or bipolar disorder, other psychiatric or substance use disorder in the last year, Hamilton depression score > 14, psychotropic medication use within the last month, metallic implants, and claustrophobia. All participants provided written informed consent. The sample considered here constitutes a superset of participants previously included in a report focusing on the impact of early adversity on prefrontal cortex activation during working memory (Shanmugan et al., 2017a).
2.2 Study design
This study used a double-blind, placebo-controlled, cross-over design. Each participant underwent 2 imaging sessions: active TD or sham TD. The order of condition (active TD vs sham TD) was counterbalanced and double-blind. On each test day, participants presented after an overnight fast and ingested 70 capsules containing either 31.5g of amino acids without tryptophan (active TD) or 31.5g of microcellulose (sham TD). The active capsules consisted of L-isoleucine 4.2g, L-leucine 6.6g, L-lysine 4.8g, L-methionine 1.5g, L-phenylalanine 6.6g, L-threonine 3.0g and L-valine 4.8g. Blood was taken for free tryptophan analysis and mood ratings were completed prior to consumption of the capsules and approximately 6 hours later, after which participants underwent cognitive testing and neuroimaging.
2.3 Assessment of early life adversity
The Adverse Childhood Experiences (ACE) Questionnaire (Felitti et al., 1998) was used to assess history of emotional, physical, or sexual abuse, childhood neglect, and household dysfunction. The ACE questionnaire assesses the number of exposure categories and has been used extensively to assess the association between early life adversity and later life health-related outcomes. The number of exposures was summed to create the ACE score (range: 0–10). Participants with an ACE score greater than or equal to 2 were considered “high ACE” while participants with ACE score of less than 2 were considered “low ACE”. A threshold of 2 was used to define the high ACE group based on studies of depression prevalence in later life that demonstrate increased susceptibility at this level of exposure (Chapman et al., 2004; Epperson et al., 2017). The continuous effects of ACE were explored in secondary analyses utilizing total ACE score.
2.4 Assessment of mood
Mood was measured as a possible confound. Participants completed the Profile of Mood States prior to TD procedure and again before fMRI. Total score and the “Depression-Dejection” sub-score were used to assess changes in overall mood and depressive symptoms, respectively. Difference in mood score (morning - afternoon) served as the outcome variable in regression models used to assess the effects of TD and ACE on mood.
2.5 Task paradigms
Prior to each fMRI scan, subjects completed the Penn Continuous Performance Task (CPT) number and letter version (Kurtz et al., 2001) to assess sustained attention. During the task, subjects were instructed to respond when the image seen was a complete letter or number. Behavioral outcome measures of interest were CPT true positive count, false positive count, and median true positive reaction time.
A letter version of the n-back task was used to probe working memory and executive system function during fMRI scans. The task consisted of four conditions: 0-back, 1-back, 2-back and 3-back. Stimuli were 4-letter non-words without vowels presented for 500 ms, followed by an interstimulus interval of 2500 ms. During the 0-back, participants responded to a target 4-letter stimulus with a button press. During other conditions, participants responded to a stimulus “n” number of stimuli before it. Each condition consisted of a 20-trial block (60s) and each class of blocks was repeated three times. A target-foil ratio of 1:2 was maintained in all blocks. Visual instructions (9s) preceded each block. The task began with a 48s baseline rest period. Additional 24s baseline rest periods occurred at the beginning, middle, and end of the acquisition. Total task duration was 924s. Equivalent n-back tasks with unique stimuli were used for the four sessions and version order was counter-balanced.
An emotional identification task was used to probe emotional bias and affective processing. The task consisted of 60 faces displaying neutral, happy, sad, angry or fearful expressions presented in a fast event-related design. Each face was displayed for 5.5 seconds followed by a variable inter-stimulus interval (0.5–18.5 seconds) during which a complex crosshair (matched to the faces’ perceptual qualities) was displayed. Participants were asked to select one of five labels (happy, sad, anger, fear, neutral) for each face presented using a scroll wheel. Equivalent versions of the task with unique stimuli were used for the four sessions and version order was counter-balanced. Total task duration was 630s.
2.6 Image acquisition
Imaging data were acquired on 3T Siemens Trio scanner. A magnetization-prepared, rapid acquisition gradient echo T1-weighted image (TR = 1810 ms, TE = 3.51 ms, FOV = 180 × 241 mm, matrix = 192 × 256, 160 slices, effective voxel resolution of 0.94 × 0.94 × 1 mm) was acquired to aid spatial normalization to standard space. Functional images were acquired using a whole-brain, single-shot gradient-echo (GE) echoplanar sequence with the following parameters: TR/TE = 3000/32 ms, FOV= 192 × 192 mm, matrix = 64 × 64, slice thickness/gap = 3/0 mm, 46 slices, effective voxel resolution of 3 × 3 × 3 mm.
2.7 Network construction and visualization
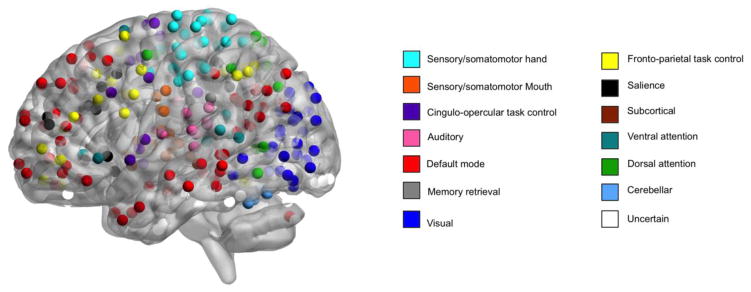
We examined the effects of ACE and TD on functional connectivity using a system of functional networks described by Power et al. (Power et al., 2011). In this system, networks are composed of spheres with a 5-mm radius (264 nodes) (Figure 1) and the connections between these nodes (34,716 unique edges). These nodes compose 14 functional network modules that correspond to brain networks that are present during both task performance and at rest (Power et al., 2011). This node system provides good coverage of the whole brain (Power et al., 2011) and has been used to examine functional connectivity between and within brain networks during cognitive tasks and at rest (Dosenbach et al., 2010; Satterthwaite et al., 2013b).
Figure 1. Power nodes.
Nodes in the system of functional networks described by Power et al. (Power et al., 2011). In this system, networks are composed of spheres with a 5-mm radius (264 nodes) and the connections between these nodes (34,716 unique edges). These nodes compose 14 functional network modules that correspond to brain networks that are present during both task performance and at rest. This node system provides good coverage of the whole brain and has been used to examine functional connectivity between and within brain networks during cognitive tasks and at rest. Time series were extracted from each of the 264 nodes in subject space using the residuals of this subject-level analysis that modeled task and motion. Image is displayed using BrainNet Viewer.
Image registration
Subject-level functional and anatomical T1 images were co-registered using boundary-based registration (Greve and Fischl, 2009). The anatomical image was normalized to the MNI 152 T1 1mm template using the top-performing diffeomorphic SyN registration in ANTs (Avants et al., 2011; Klein et al., 2009). Co-registration, normalization, and down-sampling of network nodes to subject space were concatenated so only one interpolation was performed.
2.8 Image Processing
BOLD time series data were skull stripped with BET (Smith, 2002), despiked with AFNI’s 3dDespike (Cox, 1996), motion-corrected with MCFLIRT (Jenkinson et al., 2002), high pass filtered (120 s), spatially smoothed (6 mm FWHM), and mean-based intensity normalized. The event related emotion identification task time series were also slice-time corrected. Subject-level time series analyses were carried out using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction (Woolrich et al., 2009). Task condition (0-back, 1-back, 2-back, and 3-back blocks for the n-back or happy, sad, anger, fear, and neutral events for emotion identification) and their temporal derivatives were modeled using a canonical (double-gamma) hemodynamic response function with 24 motion parameters (Friston et al., 1996; Yan et al., 2013a) included as nuisance covariates. The rest condition (fixation point) served as the unmodeled baseline. Images were assessed for excessive motion (mean relative displacement > 0.3 mm) (Satterthwaite et al., 2013b). Time series were extracted from each of the 264 nodes in subject space using the residuals of this subject-level analysis that modeled task and motion. Task regression has been shown to aide test-retest reliability of connectivity measurements obtained during tasks (Cao et al., 2014).
2.9 Graph construction and analyses of network topology
For each participant, pair-wise Pearson correlations were calculated using the time series extracted from the 264 nodes. These values were then transformed using a Fisher-Z transformation to construct a 264 × 264 symmetric connectivity matrix for each participant. Our primary outcome of interest was within-network connectivity. Within-network connectivity is the mean strength of edges between nodes within a network, averaged across all networks. We also calculated total network strength to account for potential motion artifact (Saad et al., 2013; Yan et al., 2013b). Total network strength is the average strength of the connectivity matrix (Saad et al., 2013; Yan et al., 2013b).
2.9 Discovery and replication data sets
Prior studies have demonstrated that removing the effects of task from BOLD data acquired during cognitive tasks produces “resting-state” timeseries that are qualitatively similar to “true” resting-state data (Fair et al., 2007b). Several groups have used these residual timeseries to examine the effect of pathology on “resting-state” functional connectivity (Fleisher et al., 2009; Weisberg et al., 2014). However, given that differences between task-regressed residuals and “true” resting-state timeseries have been noted (Fair et al., 2007b), we tested our hypotheses in both a discovery and replication dataset. We obtained these two approximate resting-state timeseries by regressing task effects during working memory and emotion identification tasks. Given the differences in both task design (block vs event-related) and task demands, consistency of results across timeseries would suggest differences due to intrinsic functional network architecture rather than task performance, while discrepancies in results between timeseries may be due to residual, non-linear task effects.
We defined the timeseries obtained during the n-back as the discovery timeseries for several reasons. First, the n-back task was longer and provided an additional 98 volumes for calculation of correlations between network nodes. Second, Cao et al. (2014) demonstrated that the test-retest reliability of graph-based connectivity measures calculated from residual timeseries was higher for the n-back than for an emotional face-processing task. Third, as described below in the results (section 3.1 Participants), a greater number of timeseries were available for the n-back than the emotional identification task. Given these differences, we chose to first examine the impact of ACE and TD in the residuals of the n-back timeseries, which may be more consistent and a closer approximate to resting-state. The main effect of task on within-network connectivity was evaluated using generalized estimating equations to assess the validity of using residual timeseries from a separate task as the replication dataset.
2.9 Statistical analysis
Two-sample t-tests were used to assess whether the sample available for analyses differed significantly from the remaining subjects enrolled. Generalized estimating equations implemented using the geepack package (Højsgaard et al., 2006) in R (R Core Team, 2015) were employed to evaluate the effect of ACE, TD, and their interaction on out-of-scanner task performance and within-network connectivity. This method accommodates multiple assessments per participant and adjusts for non-independence among these repeated measures. All models controlled for age, estradiol level, and time since last menstrual period. As previous studies have demonstrated that the impact of TD on brain function varies with estradiol level in menopausal women (Epperson et al., 2012), we also controlled for this interaction in our analyses. Both mean relative displacement (Power et al., 2015; Satterthwaite et al., 2013a) and mean network strength (Saad et al., 2013; Yan et al., 2013a; Yan et al., 2013b) were included as covariates to minimize the impact of motion. The effect of the number of ACEs (continuous) on functional connectivity was evaluated in supplementary analyses that examined interactions between TD and total ACE score. Generalized estimating equations controlling for the biological and imaging covariates mentioned above as well as ACE score, TD status, and their interaction were used to examine the association between within-network connectivity and out-of-scanner task performance. Statistical tests were two-sided with p<=0.05 considered significant.
3. Results
3.1 Participants
Sixty participants completed screening and were enrolled in the present study. Of these 60 participants, 2 withdrew from the study before the first test day, and 3 were lost to follow up. Six participants did not complete the first test day due to inability to complete the depletion procedure (n=1), claustrophobia (n=4), or lack of space (n=1) inside the MRI scanner. Three participants withdrew prior to the second test day, and one participant was excluded due to artifacts produced by dental fillings. Participants were excluded from analyses for excessive motion (n-back n=1; emotion identification n=2), having a percent change in tryptophan level approximately greater than 3 SD from the average for the assigned depletion status (n=3), or missing data regarding ACE (n=3), imaging (n-back n=1; emotion identification n=5), or tryptophan levels (n=4). The final sample included in the discovery dataset consisted of 24 low ACE participants (43 sessions) and 16 high ACE participants (30 sessions; see Table 1 and Table S1). Four of these participants did not complete the emotional identification task; the final sample included in the replication dataset consisted of 24 low ACE participants (40 sessions) and 15 high ACE participants (28 sessions). The sample included in the discovery dataset (n=40) did not significantly differ from the sample remaining subjects that were enrolled (n=20) in terms of age, race, estradiol level, FSH level, or time since last menstrual period.
Table 1.
Participant characteristics
| Low ACE | High ACE | |
|---|---|---|
|
| ||
| Mean (SD) or number (%) | ||
|
| ||
| n | 24 | 16 |
| Age (years) | 55.5 (3.3) | 55.1 (3.1) |
| Months since last menstrual period | 53.7 (33.0) | 62.3 (30.9) |
| Follicle-stimulating hormone (IU/L) | 93.5 (32.5) | 74.0 (40.8) |
| Estradiol (pg/mL) | 27.1 (17.7) | 22.7 (11.3) |
| Education | ||
| Graduate | 14 (58) | 2 (13) |
| Some Graduate | 1 (4) | 3 (29) |
| College | 4 (17) | 6 (38) |
| Some College | 2 (8) | 5 (31) |
| Associates | 1 (4) | 0 (0) |
| High School | 2 (8) | 0 (0) |
| Race | ||
| Caucasian | 19 (79) | 11 (69) |
| African American | 3 (13) | 4 (25) |
| Asian | 1 (4) | 1 (6) |
| Other | 1 (4) | |
| Employment | ||
| Full time | 16 (67) | 10 (63) |
| Part time | 2 (8) | 5 (31) |
| Unemployed | 1 (4) | 0 (0) |
| Retired | 2 (8) | 0 (0) |
| Unknown or preferred not to disclose | 0 (0) | 1 (6) |
3.2 Tryptophan level, mood, and behavioral results
Blood was taken for free tryptophan analysis before consumption of the study capsules (morning) and approximately 6 hours later (afternoon). The effect of active depletion vs sham depletion was assessed using a generalized estimating equation with percent change in free tryptophan concentration as the outcome. Active TD resulted in a significant decrease in tryptophan level in comparison to sham TD (p<0.0001). There was no significant difference in percent change in tryptophan levels between ACE groups. There was no significant effect of ACE, TD, or ACE x TD on mood. CPT true positive reaction time was significantly slower in the high ACE group compared to the low ACE group (p=0.004). Active TD was associated with greater true positive responses (p=0.03) but slower reaction time (p=0.09). There was no other significant effect of ACE, TD, or ACE x TD on out-of-scanner task performance.
3.3 Validation of discovery and replication timeseries
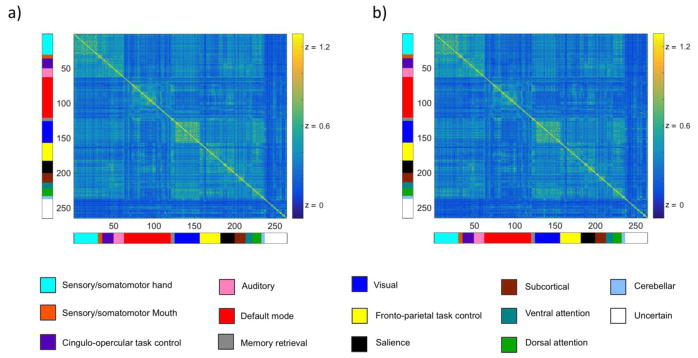
As expected, nodes demonstrated greater connectivity with nodes of the same network than with nodes of different networks in both the discovery (Figure 2a) and replication (Figure 2b) data sets. The mean symmetric connectivity matricies from these two timeseries qualitatively appeared very similar. However, prior to testing our hypotheses regarding the impact of ACE and TD on functional connectivity, we evaluated the main effect of cognitive task performed on within-network connectivity to assess the validity of using residual timeseries from a separate task as the replication dataset. There was no significant effect of task (n-back vs emotional identification) on within-network connectivity. This effect remained non-significant when accounting for total network strength as well as motion and was not evaluated further. The lack of significant task effect supported our use of these two sets of timeseries as discovery and replication datasets.
Figure 2. Mean symmetric connectivity matrices.
a. The mean symmetric connectivity matrix of discovery (a) and replication (b) timeseries confirmed nodes demonstrated greater connectivity with nodes of the same network than with nodes of different networks. The mean symmetric connectivity matrices from these two timeseries qualitatively appeared very similar. We evaluated the main effect of cognitive task performed on within-network connectivity to assess the validity of using residual timeseries from a separate task as the replication dataset. There was no significant effect of task (n-back vs emotional identification) on within-network connectivity. This effect remained non-significant when accounting for total network strength as well as motion. Values displayed are Fisher Z transformed pair-wise Pearson’s correlations. Color bars to the left and bottom of connectivity matrices indicate community membership.
3.4 Discovery timeseries results
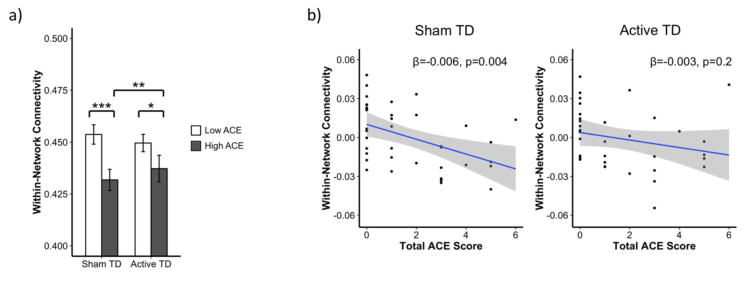
To test our hypotheses that ACE would be associated with lower within-network connectivity and that tryptophan depletion would selectively further lower connectivity only in the high ACE group, we used a model that evaluated the impact of ACE group, TD status, and ACE group x TD status on within-network connectivity. The effect of ACE in this model was robust; as predicted, connectivity was significantly lower in the high ACE group than in the low ACE group during sham depletion (p=0.002). In line with our hypothesis that TD would selectively impair the high ACE group, TD had no effect on connectivity in the low ACE group. Also as predicted, an interaction between ACE group and depletion status on within-network connectivity was observed at a trend level (p=0.1). However, contrary to our hypothesis that active TD would exacerbate the effect of ACE observed during sham TD, active depletion actually increased connectivity in the high ACE group (p=0.02; Figure 3a). This attenuated the effect of ACE during active TD. As in the sham condition, the high ACE group also displayed lower within-network connectivity during active TD, though this trend did not reach statistical significance (p=0.1).
Figure 3. ACE and TD effects on within-network connectivity in discovery timeseries.
a. To test our hypotheses that ACE would be associated with lower within-network connectivity and that tryptophan depletion would selectively further lower connectivity only in the high ACE group, we used a model that evaluated the impact of ACE group, TD status, and ACE group x TD status on within-network connectivity. The effect of ACE in this model was robust; as predicted, connectivity was significantly lower in the high ACE group than in the low ACE group during sham depletion (p=0.002). In line with out hypothesis that TD would selectively impair the high ACE group, TD had no effect on connectivity in the low ACE group. Also as predicted, an interaction between ACE group and depletion status on within-network connectivity was observed at a trend level (p=0.1). However, contrary to our hypothesis that TD would exacerbate the effect of ACE observed during sham TD, active depletion actually increased connectivity in the high ACE group (p=0.02). This attenuated the effect of ACE during active TD. As in the sham condition, the high ACE group also displayed lower within-network connectivity during active TD, though this trend did not reach statistical significance (p=0.1). Bars represent least-square means adjusted for age, time since last menstrual period, estradiol level, total network strength, and motion. Error bars represent standard error. b. We also examined the effect of the number of ACEs (continuous) using a model that evaluated the impact of total ACE score, TD status, and ACE score x TD status. As hypothesized and in agreement with our results using ACE group, there was a robust effect of ACE score as ACE score was significantly negatively associated with within-network connectivity during sham depletion (p=0.004). Additionally, there was a significant ACE score x TD status interaction on within-network connectivity (p=0.04). Consistent with our TD x ACE group analysis, active TD diminished the impact of ACE score; there was no association between total ACE score and within-network connectivity during active TD (p=0.2). The effect of all covariates included in analyses have been regressed from the connectivity values depicted. ACE= adverse childhood experiences; TD = tryptophan depletion; *p ≤ 0.1, **p ≤ 0.5, ***p ≤ 0.01
Previous studies using the ACE questionnaire to evaluate the impact of early life adversity on mental health outcomes in adult life have used a threshold of ≥ 2 to define the highACE group (Epperson et al., 2017). As such, our primary analysis utilized the same binary definitions of ACE. However, it is not clear whether such a threshold applies to ACE effects on functional network connectivity. Therefore, we also examined the effect of the number of ACEs (continuous) using a model that evaluated the impact of total ACE score, TD status, and ACE score x TD status. As hypothesized and in agreement with our results using ACE group, there was a robust effect of ACE score, as ACE score was significantly negatively associated with within-network connectivity during sham depletion (p=0.004; Figure 3b). Additionally, there was a significant ACE score x TD status interaction on within-network connectivity (p=0.04). Consistent with our TD x ACE group analysis, active TD diminished the impact of ACE score; there was no association between total ACE score and within-network connectivity during active TD (p=0.2).
3.5 Replication timeseries results
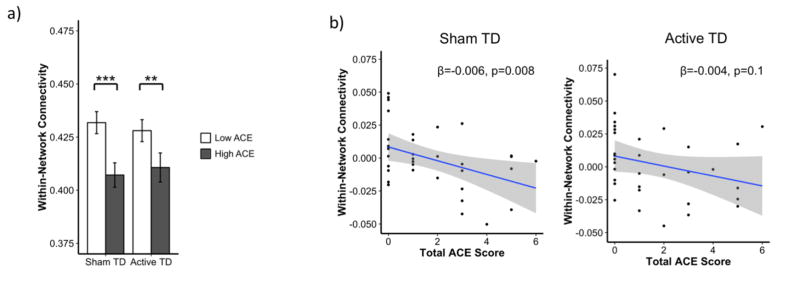
To probe the validity of our results, we tested our hypotheses regarding the impact of ACE and TD on functional connectivity using a separate set of task-regressed timeseries data acquired in a subsample of our participants. As in the discovery data set, we used a model that evaluated the impact of ACE group, TD status, and ACE group x TD status on within-network connectivity to test our hypotheses that ACE would be associated with lower connectivity and that tryptophan depletion would selectively lower connectivity only in the high ACE group. The effect of ACE group was robust and consistent with discovery results. Within-network connectivity was significantly lower in the high ACE group during both sham TD (p=0.002) and active TD (p=0.05; Figure 4a). The effect of TD in the low ACE group was also replicated and remained non-significant. However in contrast to discovery results, there was no significant ACE group x TD status interaction and no effect of TD in the high ACE group.
Figure 4. ACE and TD effects on within-network connectivity in replication timeseries.
a. To probe the validity of our results, we tested our hypotheses regarding the impact of ACE and TD on functional connectivity using a separate set of task-regressed timeseries data acquired in a subsample of our participants. As in the discovery dataset, we used a model that evaluated the impact of ACE group, TD status, and ACE group x TD status on within-network connectivity to test our hypotheses that ACE would be associated with lower connectivity and that tryptophan depletion would selectively lower connectivity only in the high ACE group. The effect of ACE group was robust and consistent with discovery results. Within-network connectivity was significantly lower in the high ACE group during both sham TD (p=0.002) and active TD (p=0.05). The effect of TD in the low ACE group was also replicated and remained non-significant. However in contrast to discovery results, there was no significant ACE group x TD status interaction and no effect of TD in the high ACE group. Bars represent least-square means adjusted for age, time since last menstrual period, estradiol level, global connectivity, and motion. Error bars represent standard error. b. We also attempted to replicate our findings regarding the continuous effects of ACE using a model that evaluated the impact of total ACE score, TD status, and ACE score x TD status. Consistent with our hypothesis and analyses using ACE group, total ACE score was significantly negatively associated with within-network connectivity during sham TD (p=0.008). As in the discovery dataset, this association did not reach significance during active TD. However, it was present at a trend level (p=0.1), and the interaction between ACE score and TD status was not significant. The effect of all covariates included in analyses have been regressed from the connectivity values depicted. ACE= adverse childhood experiences; TD = tryptophan depletion; *p ≤ 0.1, **p ≤ 0.5, ***p ≤ 0.01
We also attempted to replicate our findings regarding the continuous effects of ACE using a model that evaluated the impact of total ACE score, TD status, and ACE score x TD status. Consistent with our hypothesis and analyses using ACE group, total ACE score was significantly negatively associated with within-network connectivity during sham TD (p=0.008; Figure 4b). As in the discovery dataset, this association did not reach significance during active TD. However, it was present at a trend level (p=0.1), and the interaction between ACE score and TD status was not significant.
3.6 Connectivity associations with behavior
Results described in sections 3.4 and 3.5 demonstrate that both ACE group and ACE score are associated with lower within-network connectivity, a finding reflective of executive dysfunction (Vaidya and Gordon, 2013; Satterthwaite and Baker, 2015; Shanmugan and Satterthwaite, 2016). To verify that this relationship between decreased within-network connectivity and executive dysfunction was present in the current study sample, we examined associations between connectivity and performance on a task of sustained attention. As expected, there was a positive relationship between within-network connectivity and CPT true positive count (discovery dataset p=0.03; replication dataset p=0.03). Similarly, there was also a negative association between within-network connectivity and false positive count in the replication dataset (discovery dataset p=NS; replication dataset p=0.05). These increases in true positive count and decreases in false positive count with increasing connectivity were accompanied by an increase in reaction time (discovery dataset p=0.1; replication dataset p= 0.0001).
4. Discussion
In this double-blind, placebo-controlled, cross-over study, we examined the effects of ACE and TD on functional network connectivity in healthy menopausal women. In our discovery dataset, high ACE women displayed lower within-network connectivity than low ACE women regardless of depletion status. Similarly, total ACE score was negatively associated with within-network connectivity. Additionally, active TD increased within-network connectivity in women with high levels of early adversity but had no effect in women with low levels of early adversity. While the robust effects of both ACE group and ACE score were replicated, the more marginal ACE x TD interaction was not. These results indicate early life adversity has lasting impacts on intrinsic large-scale functional networks and provide preliminary evidence that serotonin’s effect on these networks may be task-dependent.
As expected, we found a main effect of ACE on functional connectivity. High ACE participants displayed lower within-network connectivity in both the discovery and replication datasets. Connectivity within functional networks has been shown to underlie multiple cognitive domains including working memory (Dosenbach et al., 2007; Fair et al., 2007a; Power et al., 2010; Tsvetanov et al., 2016). Decrements in working memory are associated with lower connectivity within default and cingulo-opercular networks (Vaidya and Gordon, 2013). Decreases in within-network connectivity have been observed in psychiatric disorders exhibiting dysfunction in executive domains (Satterthwaite and Baker, 2015; Shanmugan and Satterthwaite, 2016). While the impact of ACE on similar measures of within-network connectivity has not been examined, our findings are globally convergent with seed-based resting-state connectivity studies examining the impact of early life stress on functional connectivity in healthy adults (Philip et al., 2013) and adult women with post traumatic stress disorder (Bluhm et al., 2009). Although seed regions selected and networks examined vary, these studies found that early adversity is associated with lower connectivity between regions within the same network (Bluhm et al., 2009; Philip et al., 2013).
The mechanisms by which ACE imparts this negative impact on connectivity are likely multifold. While we hypothesized that TD would selectively impair connectivity in the high ACE group, we found that TD instead selectively improved connectivity in the high ACE group. Though contrary to our hypothesis, this result is consistent with previous findings demonstrating that tryptophan depletion attenuates ACE effects on dorsolateral prefrontal cortex activation during the n-back in menopausal women (Shanmugan et al., 2017a). That TD attenuated the negative effects of ACE on connectivity suggests that serotonergic differences may be contributing to baseline differences in functional connectivity between ACE groups. Non-human primate (Shively et al., 2003) and human participant (Shanmugan et al., 2017a) studies have shown that estradiol attenuates the effects of early adversity on serotonin (Shively et al., 2003; Shanmugan et al., 2017a) and DLPFC function (Shanmugan et al., 2017a), suggesting estradiol may similarly attenuate the impact of ACE on connectivity observed in this study. Importantly, many women have contraindications to hormone therapy and results of this study suggest future research aimed at alleviating executive difficulties during menopause should examine serotonergic targets, particularly in the context of significant childhood adversity. However, we were not able to replicate this ACE x TD interaction in our replication timeseries. This discordance may be due to differences in task duration, task design, or variations in non-linear effects of task remaining in the residual timeseries (Fair et al., 2007b). Alternatively, the lack of ACE x TD effect in the replication time series may suggest that childhood adversity exerts enduring effects on functional connectivity by mechanisms not involving serotonin.
Given that functional connections may be reflective of underlying structural connections (Damoiseaux and Greicius, 2009), the effects of early adversity on neuronal development during childhood and adolescence likely also play a role. During development, within-network connectivity increases while between-network connectivity decreases (Satterthwaite and Baker, 2015; Shanmugan and Satterthwaite, 2016). Significant adversity during this developmental period may preclude this network segregation (Vaidya and Gordon, 2013), possibly via structural changes. Early life stress in mice has been shown to induce alterations in PFC dendritic architecture (Yang et al., 2015). Female mice that experience peripubertal stress also display increases in PFC myelin basic protein gene expression and proteolipid protein gene expression, both of which are indicators of myelination (Morrison et al., 2016). Importantly, estradiol supports the normal development and maintenance of serotonergic function (Epperson et al., 2012), dendritic morphology (Shanmugan and Epperson, 2014), and neuronal myelination (Luo et al., 2016).
4.1 Limitations
Certain limitations of the present study should be noted. First, we focused on middle-aged females who were within 10 years of their final menstrual period, as women are at risk of executive dysfunction during the menopause transition (Epperson et al., 2015; Shanmugan and Epperson, 2014; Shanmugan et al., 2017b). Given the impact of estradiol on prefrontal cortex structure and function (Shanmugan and Epperson, 2014) as well as evidence for sex differences in the effects of early adversity (Shanmugan and Satterthwaite, 2016) on neural response, these data cannot be extrapolated to similar-aged males or pre-menopausal females. Similarly, while results demonstrate that ACE is associated with maladaptive connectivity patterns during menopause, it is not clear whether ACE has comparable effects prior to menopause. Longitudinal studies testing women pre- and post-menopause would be necessary to determine whether the impacts of ACE and menopause on functional connectivity are synergistic or additive. Second, given the association between ACE and many adverse health-related outcomes (Felitti et al., 1998), the resilient sample of highly educated, healthy hypogonadal women without psychiatric disorders studied here reduces generalizability to the typical menopausal population, particularly those with substantial early life adversity. However, this sample allowed us to examine the impact of early adversity on functional network connectivity while limiting the confounding effects of psychiatric symptomatology and psychotropic medication use. Third, residuals from task-regressed timeseries, while similar, are not identical to true resting state data and non-linear effects associated with performing the task may not have been removed (Fair et al., 2007b). However, that ACE results were replicated in a separate set of residual timeseries lends confidence to our results. Fourth, the sample size of 40 is relatively small and the unequal sample sizes of the two ACE groups impacts the statistical power for comparisons between the two groups. We report a statistically significant interaction between continuous ACE score and TD in the discovery dataset. While patterns of association were similar in the replication timeseries, the variance of corrected within-subject connectivity estimates was ~ 20% higher than in the discovery dataset, which resulted in decreased power to detect an ACE score x TD interaction in the replication dataset. However, the double-blind, placebo-controlled, cross-over study design using repeated-measures allowed for decreased variance in estimates of condition effects and increased power to detect between group differences.
4.2 Conclusions
In summary, these data suggest that early life adversity has lasting impacts on functional network connectivity in healthy menopausal women. These results provide preliminary evidence regarding the mechanisms by which early life adversity confers a vulnerability for executive dysfunction during menopause. Further research in a larger sample would be helpful in elucidating the impact of timing of early life adversity on brain networks and whether sex-differences exist with regard to TD and ACE effects on connectivity.
Supplementary Material
Highlights.
Early adversity is associated with dysconnectivity of large-scale brain networks
Level of adversity is negatively associated with within-network connectivity
Tryptophan depletion may disrupt ACE effects on functional connectivity
Acknowledgments
Funding and Disclosure
This research was supported by P50 MH099910 (Epperson), Penn PROMOTES Research on Sex and Gender in Health (Epperson), R01MH107703 (Satterthwaite), and F30AG055256 (Shanmugan). Dr. Epperson reports that she has received funding from Shire Pharmaceuticals for investigator initiated research and is site investigator for a multi-site, randomized clinical trial funded by Sage Therapeutics. Dr. Epperson also discloses personal investments in the following companies; Pfizer, Johnson and Johnson, Merck, Abbott, and Abbvie.
Footnotes
Conflict of Interest
Dr. Epperson reports that she has received funding from Shire Pharmaceuticals for investigator initiated research and is site investigator for a multi-site, randomized clinical trial funded by Sage Therapeutics. Dr. Epperson also discloses personal investments in the following companies; Pfizer, Johnson and Johnson, Merck, Abbott, and Abbvie. Dr. Gur has received royalties from the Brain Resource Centre. No other authors report commercial relationships with financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuro Image. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RW, Theberge J, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of psychiatry & neuroscience: JPN. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Graham AM, Moore WE, Peake SJ, Mannering AM. Patterns of brain activation in foster children and nonmaltreated children during an inhibitory control task. Development and psychopathology. 2013;25:931–941. doi: 10.1017/S095457941300028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Plichta MM, Schafer A, Haddad L, Grimm O, Schneider M, Esslinger C, Kirsch P, Meyer-Lindenberg A, Tost H. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. Neuro Image. 2014;84:888–900. doi: 10.1016/j.neuroimage.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of affective disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain structure & function. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Demir-Lira OE, Voss JL, O’Neil JT, Briggs-Gowan MJ, Wakschlag LS, Booth JR. Early-life stress exposure associated with altered prefrontal resting-state fMRI connectivity in young children. Developmental cognitive neuroscience. 2016;19:107–114. doi: 10.1016/j.dcn.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr, Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science (New York, NY) 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology. 2012;37:372–382. doi: 10.1016/j.psyneuen.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Sammel MD, Bale TL, Kim DR, Conlin S, Scalice S, Freeman K, Freeman EW. Adverse Childhood Experiences and Risk for First-Episode Major Depression During the Menopause Transition. The Journal of clinical psychiatry. 2017;78:e298–e307. doi: 10.4088/JCP.16m10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Shanmugan S, Kim DR, Mathews S, Czarkowski KA, Bradley J, Appleby DH, Iannelli C, Sammel MD, Brown TE. New onset executive function difficulties at menopause: a possible role for lisdexamfetamine. Psychopharmacology. 2015;232:3091–3100. doi: 10.1007/s00213-015-3953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007a;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuro Image. 2007b;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JB, Chen K, Buxton RB. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuro Image. 2009;47:1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic resonance in medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuro Image. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højsgaard S, Halekoh UJY. The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software. 2006;15(2):1–11. [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biological psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuro Image. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuro Image. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophrenia research. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Luo Y, Xiao Q, Chao F, He Q, Lv F, Zhang L, Gao Y, Qiu X, Huang C, Li Y, Wang S, Jiang R, Gu H, Tang Y. 17beta-estradiol replacement therapy protects myelin sheaths in the white matter of middle-aged female ovariectomized rats: a stereological study. Neurobiology of aging. 2016;47:139–148. doi: 10.1016/j.neurobiolaging.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Perez Diaz M, Embree M, Reding K, Votaw JR, Mun J, Voll RJ, Goodman MM, Wilson M, Sanchez M, Toufexis D. Oestradiol alters central 5-HT1A receptor binding potential differences related to psychosocial stress but not differences related to 5-HTTLPR genotype in female rhesus monkeys. Journal of neuroendocrinology. 2014;26:80–88. doi: 10.1111/jne.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Narasimhan S, Fein E, Bale TL. Peripubertal Stress With Social Support Promotes Resilience in the Face of Aging. Endocrinology. 2016;157:2002–2014. doi: 10.1210/en.2015-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Carpenter SL, Albright SE, Price LH, Carpenter LL. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain imaging and behavior. 2016;10:124–135. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2013;23:24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuro Image. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, Cox RW. Correcting brain-wide correlation differences in resting-state FMRI. Brain connectivity. 2013;3:339–352. doi: 10.1089/brain.2013.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Current opinion in neurobiology. 2015;30:85–91. doi: 10.1016/j.conb.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, Hopson R, Jackson C, Prabhakaran K, Bilker WB, Calkins ME, Loughead J, Smith A, Roalf DR, Hakonarson H, Verma R, Davatzikos C, Gur RC, Gur RE. Functional maturation of the executive system during adolescence. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013a;33:16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, Gennatas ED, Jackson C, Prabhakaran K, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuro Image. 2013b;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Human brain mapping. 2014;35:847–865. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan S, Loughead J, Cao W, Sammel MD, Satterthwaite TD, Ruparel K, Gur RC, Epperson CN. Impact of Tryptophan Depletion on Executive System Function during Menopause is Moderated by Childhood Adversity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2017a doi: 10.1038/npp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan S, Loughead J, Nanga RP, Elliott M, Hariharan H, Appleby D, Kim D, Ruparel K, Reddy R, Brown TE, Epperson CN. Lisdexamfetamine Effects on Executive Activation and Neurochemistry in Menopausal Women with Executive Function Difficulties. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2017b;42:437–445. doi: 10.1038/npp.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan S, Satterthwaite TD. Neural Markers of the Development of Executive Function: Relevance for Education. Current opinion in behavioral sciences. 2016;10:7–13. doi: 10.1016/j.cobeha.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. The pharmacogenomics journal. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- Tsvetanov KA, Henson RN, Tyler LK, Razi A, Geerligs L, Ham TE, Rowe JB. Extrinsic and Intrinsic Brain Network Connectivity Maintains Cognition across the Lifespan Despite Accelerated Decay of Regional Brain Activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:3115–3126. doi: 10.1523/JNEUROSCI.2733-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Gordon EM. Phenotypic variability in resting-state functional connectivity: current status. Brain connectivity. 2013;3:99–120. doi: 10.1089/brain.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JN, Zurkovsky L, Albert K, Melo A, Boyd B, Dumas J, Woodward N, McDonald BC, Saykin AJ, Park JH, Naylor M, Newhouse PA. Altered Brain Connectivity in Early Postmenopausal Women with Subjective Cognitive Impairment. Frontiers in neuroscience. 2016;10:433. doi: 10.3389/fnins.2016.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg J, Milleville SC, Kenworthy L, Wallace GL, Gotts SJ, Beauchamp MS, Martin A. Social perception in autism spectrum disorders: impaired category selectivity for dynamic but not static images in ventral temporal cortex. Cerebral cortex (New York, NY : 1991) 2014;24:37–48. doi: 10.1093/cercor/bhs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuro Image. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuro Image. 2013a;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Craddock RC, Zuo XN, Zang YF, Milham MP. Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuro Image. 2013b;80:246–262. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Liao XM, Uribe-Marino A, Liu R, Xie XM, Jia J, Su YA, Li JT, Schmidt MV, Wang XD, Si TM. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:1203–1215. doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.