Abstract
Parkinson's disease (PD), characterized with bradykinesia, static tremor, rigidity and disturbances in balance, is the second most common neurodegenerative disorder. Along with the largely aging population in the world, the incidence is increasing year by year, which imposes the negative impacts on patients, their families and the whole society. Traditional Chinese medicine (TCM) has a positive prospect for the prevention and cure of PD due to its advantages of less side effects and multi-target effects. At present, the pathogenesis of PD is not yet fully discovered. This paper elaborates the mechanisms of TCM underlying the prevention and treatment of PD with regards to the inhibition of oxidative stress, the regulation of mitochondrial dysfunction, the reduction of toxic excitatory amino acids (EAA), the inhibition of neuroinflammation, the inhibition of neuronal apoptosis, and the inhibition of abnormal protein aggregation.
Keywords: Parkinson's disease, traditional Chinese medicine, oxidative stress, neuronal apoptosis, mitochondrial dysfunction
Introducion
Parkinson's disease (PD), the second most common neurodegenerative disorder among the aging population after Alzheimer disease, is characterized by a combination of typical motor symptoms that include akinesia, rigidity, bradykinesia, and often resting tremor (Michel et al., 2016; Rizek et al., 2016). The pathological changes in several areas of the brain are mainly marked by the degeneration of dopaminergic neurons (Damier et al., 1999). The disease is recognized as one of the most common, difficult and complicated neurological disorders identified by WHO. With the global trends in aging, the incidence of PD has increased year by year and the prevalence rate is high up to 1–2% among the elderly over the age of 65 years (Alves et al., 2008; Wang et al., 2012).
Clinically, the PD patients are usually treated with levodopa, dopamine receptor agonist, monoamine oxidase B inhibitors and other types of drugs. The clinical symptoms of the disease are mitigated by supplementing dopamine or reducing the degradation of it. However, the pathogenesis of PD is still not very clear, so that the efficacy of these drugs is not ideal and the unpleasant side effects are apparent after long-term administration such as the motor complications (Stocchi and Marconi, 2010), nausea, constipation, headache, and sleep disorder, etc. (Borovac, 2016) which would negatively influence the quality of life of the patients (Kum et al., 2011; Qin and Wu, 2016).
Considering the long-term side effects of western medicines, many patients are searching for a more safe and effective alternative treatment for PD. TCM has been used for centuries to treat diseases such as the tremor of head and hands, which is similar to the modern PD. The therapy of TCM for tremor, either single herb or herbal formula, could be traced back to the Huangdi Neijing (Huangdi's Internal Classic), the earliest existing classics in Chinese medicine (Zhang Y. et al., 2014). Up to the present, TCM is still very popular in the treatment of PD in some Asian countries such as China, Korea, and Japan (Kum et al., 2011). And much more attentions have been drawn to the TCM active ingredients showing a definite effect and a clear structure.
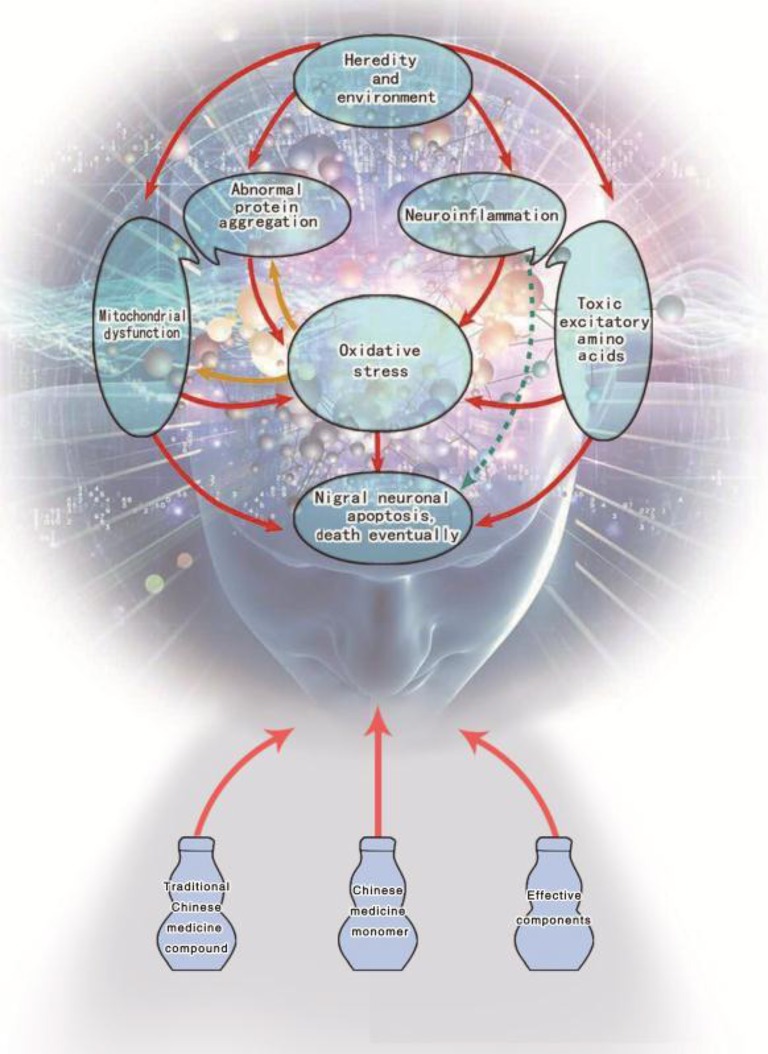
So far, there is still no exact cure for PD due to its diversity of etiology and complexity of symptoms. So, it is still a hot spot in the research of PD to figure out effective treatment methods and drugs. In Figure 1, it is clearly stated that under the effects of genetic or environmental factors the activation of any links (mechanism) can lead to injury or even death of dopaminergic neurons in the substantia nigra. Therefore, taking pathological mechanism of PD as the main stream, this paper attempts to summarize and analyze the mechanism of TCM which includes Chinese herbal compounds, herbal medicines, herbal formulations and TCM active ingredients in terms of prevention and treatment of PD.
Figure 1.
Potential mechanisms of traditional Chinese medicine for Parkinson's disease therapy.
Central nervous system activity
The inhibition of oxidative stress
The etiology and pathogenesis of PD so far have not been completely elucidated, but it has been generally acknowledged that the improvement of oxidative stress is one of the most important pathophysiological mechanisms (Avila et al., 2009; Chen et al., 2009; Sanyal et al., 2009).
Oxidative stress is incurred by the increase of free radicals in the organism, while the eliminative ability of free radicals decreased at the same time. There will be excessive free radicals in the body, which will be damaging cell components eventually (Sompol et al., 2008). PD patients are in a state of oxidative stress. In the substantia nigra of PD patients, the elevated concentration of ferric ion, weaken mitochondrial function and anomaly protection system of antioxidant (Such as low molecular free radical scavenger glutathione (GSH) and free radical scavenging enzyme SOD, GSH-Px) have contributed to the acceleration of oxidative stress and excessive generation of oxygen free radicals. Thereby, large amount of lipid peroxide, such as Malondialdehyde (MDA), hydroxyl, carbonyl, etc., will cause cell death, which leads to neuronal apoptosis ultimately (Wu et al., 2009; Wang et al., 2012).
Mainly distributed in grape, ginkgo, rhubarb, hawthorn, hypericum erectum, and other plants, procyanidin is a kind of bioflavonoid with special molecular structure, and it is currently recognized as the most effective natural antioxidant in the human body. After entering the body, procyanidin can be absorbed rapidly and directly involved in the physiological functions of the body, which shows stronger ability to scavenge hydroxyl radical, superoxide anion radical and other active oxygen. The ability of 100 mg/L procyanidin to scavenge superoxide anion and hydroxyl radical was 6.51 and 4.26 times higher than vitamin C, respectively (Bagchi et al., 1997). Procyanidin at dose of 400 mg/kg could significantly improve the grip function of PD mice which was established by intraperitoneal injection of 1-methyl-4-henyl-1,2,3, 6-tetrahydropyridine (MPTP). Meanwhile, the surging of MDA content, the decrease of SOD and GSH-Px activity in the substantia nigra of PD mice was effectively inhibited (Liang and Zhang, 2016). In the study of Clerodendranthus spicatus, the researchers found that the total flavonoids were the major active constituent which elicited affirmative effects on antioxidant activity and cleaning free radical. The total flavonoids of Clerodendranthus spicatus produced a markedly protective effect on the PD rat model and cell model induced by 6-Hydroxydopamine (6-OHDA). The protective effects may be related to reducing cell damages through reducing the level of oxidative stress (You et al., 2015).
In summary, oxidative stress plays a crucial role in the occurrence and deterioration of PD, and we can achieve the purpose of prevention and treatment against it by resisting oxidative stress. Many TCM or its effective components may act as a potential antioxidant. Therefore, the idea of screening TCM to delay the progression of PD has attracted the attention from many researchers. An overview of the improvement of oxidative stress is described in detail below, and the additional information is shown in Table 1.
Table 1.
Effect of TCM on Oxidative Stress Responses in the Model of PD.
| TCM | Oxidative stress indexes | Model | References | ||||
|---|---|---|---|---|---|---|---|
| SOD | MDA | GSH-PX | GSH | CAT | |||
| Lycopene | ↑ | ↓ | ↑ | ↑ | Mice | Liu et al., 2013 | |
| Protocatechuic acid | ↑ | ↓ | Rat | Zhang Q. et al., 2015 | |||
| Proantho cyanidins | ↑ | ↓ | ↑ | Mice | Liang and Zhang, 2016 | ||
| Protocatechuic acid and chrysin | ↑ | ↓ | ↑ | PC12 cells | Zhang Z. J. et al., 2015 | ||
| Green tea polyphenols | ↑ | ↓ | Mice | Chen et al., 2013 | |||
| Total flavonoids of Clerodendranthus spicatus | ↑ | ↓ | ↑ | ↑ | Rat | You et al., 2015 | |
| Pine bark extract | ↑ | ↓ | ↑ | Mice | Lu et al., 2014 | ||
| Petroleum ether extract of Ficus religiosa (L.) leaves | ↑ | ↓ | ↑ | ↑ | Mice | Bhangale and Acharya, 2016 | |
| Powder of Gastrodia elata | ↑ | ↓ | ↑ | ↑ | Rat | Chen et al., 2014 | |
| Ganoderma lucidium spore | ↑ | ↓ | ↑ | ↑ | Rat | Bao, 2014 | |
| Zhenganxifeng decoction | ↓ | Rat | Li X. M. et al., 2016 | ||||
| Baichanting compound | ↑ | ↓ | ↑ | Mice | Ren et al., 2015 | ||
| Gui ling Pa An Wan | ↑ | ↓ | ↑ | Rat | Meng et al., 2014a | ||
The regulation of mitochondrial dysfunction
Abnormal morphology and dysfunction of mitochondrial are one of the important pathological mechanisms of PD.
Mitochondria, as the “power plant” and “energy conversion station” of cells, regulates the process of gene expression and apoptosis. Recent reports have suggested that mitochondrial dysfunction is closely related to a variety of neurodegenerative diseases including PD (Exner et al., 2012; Liu et al., 2012; Feng and Wu, 2014).
The decrease of mitochondrial complex I activity of substantia nigra neurons in autopsy of PD was firstly found by Shoffner et al. (1991). Shortly afterwards, Parker et al. found that the platelet mitochondrial complex I activity in patients with PD was also reduced (Parker et al., 1989). After the inhibition of mitochondrial complex I, there are obvious obstacles to the production of energy, which lead to a series of secondary reactions, even cell death occurs. Insufficient synthesis of ATP can also cause protein and lipid degradation. The degraded products may trigger or produce oxidative metabolic reactions, which aggravate the damage of substantia nigra. The production of large amount of reactive oxygen species elicited by the inhibition of complex I can drive its activity to continue to decline, thus form a vicious circle (Tada-Oikawa et al., 2003; Xiong et al., 2012).
MPTP which is an inhibitor of mitochondrial respiratory chain complex I can selectively damage dopaminergic neurons in the substantia nigra pars compacta. The mice are injected with MPTP to produce mitochondrial dysfunction and oxidative stress, which create similar clinical symptoms and pathological changes in PD (Blesa and Przedborski, 2014). In a study, locomotor activity, pole and rotarod test were used to evaluate the effects of Qianzheng San extract to the dyskinesia induced by MPTP. Compared with the model group, the MPTP-treated mice laid out a significant reduction in locomotor activity and ultrastructure of substantia nigra neuron was obviously harmed. However, Qianzheng San extract treatment largely increased autonomic activities, prolonged incubation period and shortened the pole-climbing time (P < 0.05), and reduced the impairment of ultrastructure of substantia nigra neurons. On the other hand, electron microscopy showed that the ultrastructure of substantia nigra neurons was ameliorated effectively and the high degree of mitochondrial damage was alleviated remarkedly by treatment of Qianzheng San extract. All these experimental results reveal that Qianzheng San extract may play a neuroprotective role through improving mitochondrial functions (Li et al., 2015). Catalpol, which is relatively abundant in the TCM such as Radix Rehmanniae and Radix Scrophulariae, is a small molecule compound of iridoids. It also showed that it has protective effects on mice brain mitochondrial damage induced by rotenone, partly through enhancing the activities of complex I, increasing the content of GSH, lowering the loss of mitochondrial membrane potential and restraining the release of LDH (Shi et al., 2012).
Baicalein, a well-known flavonoid compound isolated from dried roots of Scutellaria baicalensis, has been applied extensively as an antioxidant and anti-inflammatory agent (Ge et al., 2017). In recent years, with continuous studies on its mechanisms, it has been found that baicalein has some effect on the improvement of clinical symptoms and neuroprotection in neurodegenerative diseases such as PD. The protective effect of baicalein on mitochondria may be one of the pharmacological targets of neuroprotection against PD. The study confirmed that exposure of PC12 cells to 0.15 mM H2O2 for 20 min resulted in mitochondrial damage and cell apoptosis. And pre-treatment of PC12 cells with different concentrations of baicalein greatly cut down the cell viability loss. The protective effect of baicalein on mitochondrial function was related to inhibition of ROS production and the regulation of Bcl-2 family members first, and these regulations might adjust the mitochondrial membrane permeability, attenuate cytochrome c release to cytosol (Zhang et al., 2010).
The reduction of toxic EAA
In pathological conditions, glutamate (Glu) can produce the effects of excitotoxicity on nerve cells. The relationship between neurotoxicity of Glu and pathogenesis of PD has received elevating attention. At present, the treatment of PD with Glu release inhibitor has become one of the hottest spots in the research.
In recent years, a growing number of evidences suggests that in addition to dopamine (DA) and acetylcholine (Ach), there are also many other neurotransmitters such as Glu, gamma-aminobutyric acid (GABA) and enkephalin etc., and they can interact with each other in the substantia nigra and striatum (Papa and Chase, 1996). Under normal conditions, Glu creates excitatory effect on nerve cells, but it demonstrates toxic effects when DA neurons are fully or partially degenerated (Vital et al., 2003). The concentration of Glu in normal brain cells is close to 10 μmol/L, while the extracellular concentration is about 0.6 μmol/L. When the extracellular Glu concentration reaches 25 μmol/L, it can damage the cerebral cortex and hippocampus (Caragine et al., 1998; Zhou et al., 2003). The toxicity of EAA (Glu, aspartate) is mainly reflected in the activation of the corresponding receptor (NMDA-R, AMPA-R, KA-R) which mediate acute osmotic swelling or delayed injury of nerve cells. It was found that local and systemic application of EAA receptor antagonists could lower or prevent motor symptoms of the PD model rats induced by 6-OHDA, and postpone its the process of neurodegenerative (Hallett and Standaert, 2004). It was separately reported that low dose of NMDA receptor antagonist MK-801 combined with levodopa could enhance the efficacy and prolong its duration of action. Clinically, motor fluctuations and dyskinesia, caused by long term use of levodopa, can be effectively treated by MK-801. Anticholinergics are one of NMDA receptor non-competitive antagonists. Amantadine, a drug that has been used for years to moderately intervene symptoms of PD, has also been shown to be an NMDA receptor antagonist (Strugstad and Sager, 1998; Blesa and Przedborski, 2014).
Tetrandrine (Tet), a class of bisbenzylisoquinoline extracted from the roots of Radix stephania tetrandrae, (Wong et al., 2017) is a new type of reversible inhibitor of P-glycoprotein. The level of L-dopa in the brain can be increased by reversible P-glycoprotein inhibitors, which is conductive to clinical efficacy of neurodegenerative diseases, including PD (Schinkel, 1999). The researchers used Tet combined with GSH and L-dopa to explore the therapeutic mechanism of PD rats induced by 6-OHDA. By detecting the aspartate (Asp) and Glu in the affected side of striatum, it was evident that compared with the model group, the concentration of Asp was dramatically downgraded in the GSH treatment group; The level of Glu in the GSH + Tet treatment group was much lower than that in the GSH treatment group; The concentration of Glu and Asp in the L-dopa treatment group was notably higher than that in the model group; The concentration of Glu and Asp in the GSH + L-dopa + Tet treatment group was considerably lower than the model group. The above results show that Tet, by the means of increasing the concentration of anti-PD drugs in the brain, can protect the brain neuron from the toxic effect of EAA (Jin and Bao, 2010). In a separate study, Glu treatment largely increased LDH release and produced a great deal of NO in primary cultured rat brain neurons. While Baicalein, at 3.5 μmol·L−1, could exert neuroprotective effects against Glu stimulation by reducing the generation of LDH and NO (Yu et al., 2012). Puerarin, a kind of flavonoid compound, was extracted from Puerariae Radix. It stated neuroprotective effects on a variety of brain damage by sharply reducing the content of EAA. The results demonstrated that puerarin can promote the expression of Glu decarboxylase mRNA in rats with cerebral ischemia and increase the contents of cerebral GABA to antagonize the toxic effect of EAA (Huang and Wang, 2015).
The inhibition of neuroinflammation
Neuroinflammation is a common and important pathological mechanism in nervous system diseases and different neurological diseases are involved in neuroinflammation at some stage. At present, it is believed that neuroinflammation was involved in an important cascade reaction in neuronal degeneration of PD (Niranjan, 2014).
Along with aging, dysregulation of immune and inflammatory will gradually appear in the body, and the activation of microglia is considered to be related to the pathogenesis of PD nerve degeneration. When the central nervous system suffers from exogenous antigens stimulus, such as pathogenic microorganisms or foreign bodies, microglia will be rapidly activated. Then, the activated microglia cells can secrete various cytokines such as IL-1β, IL-2, IL-4, IL-6, TNF-α, and IFN-γ, etc. (Hunot and Hirsch, 2003).
The increased levels of cytokines can cause inflammatory response and neuronal damage, induce the cell to undergo programmed death by increasing the level of nitric oxide (NO) in the brain and lead to the onset of neurodegenerative diseases eventually (Mosley et al., 2006; Béraud et al., 2013). Among them, IL-1β and TNF-α appear especially important because they can promote macrophages and other cells to secrete IL-6, IL-8 and other cytokines. For the animal model of PD induced by the neurotoxin such as MPTP and 6-OHDA, there is obvious activation of microglia in the early stage of degeneration of dopaminergic neurons (Barnum and Tansey, 2010; Miller et al., 2011). The enhancement of the expression of IL-1β, TNF-α and other inflammatory cytokines are observed in the nigrostriatal system of PD patients at autopsy, mainly in activated microglia, and the expression of inflammatory factors is positively correlated with the loss of DA neurons (Miklossy et al., 2006; Tansey and Goldberg, 2010). In addition, up-regulation of cyclooxygenase-2 (COX-2) expression is also vital to immunity and inflammatory responses immunity and inflammatory responses. As an inflammatory response gene, it is involved in the inflammatory response of the body, and generated neuronal apoptosis in the pathological process of PD (Wei et al., 2009; Yu et al., 2012).
In recent years, some progress has been made in the research of anti-inflammatory TCM in the treatment of PD. The protective effect of triptolide on dopaminergic neurons in MPP+-induced hemiparkinsonian rats may be concerned to the inhibition of microglial cell activation (Hirsch et al., 2005; Gao et al., 2008). Curcumin can effectively antagonize the loss of dopaminergic neurons in the parkinsonian mouse model caused by MPTP. Its mechanism is associated with the decrease of the active oxygen content of dopaminergic neuron and inhibition of inflammation (Pan et al., 2007). Through the antioxidant and anti-inflammatory effects, celastrol also can efficiently prevent or delay the progression of PD (Faust et al., 2009; Zhang et al., 2012). In the study of Polygona-Polysaccharose on PD, it was found that the expression of Peroxisome proliferator-activated receptor-γ (PPAR-γ) was up-regulated in treatment group as compared with model group. PPAR-γ is a class of ligand-activated type 2 nuclear transcription factor belonging to the nuclear receptor superfamily. It has neuroprotective effects and attenuate the neuronal damages from neurodegenerative diseases such as Alzheimer's disease, PD, cerebral ischemia and multiple sclerosis. Meanwhile, the study results about Polygona-Polysaccharose also revealed that the mechanism might be related to the up-regulation of PPAR-γ expression, thereby inhibiting the inflammatory reaction and promoting the regeneration of dopaminergic neurons (Chen et al., 2010). Polyphenols from toona sinensis seeds (PTSS) can exert the protective effect to DA neurons of substantia nigra of PD rats by reducing the number of microglia and astrocytes in the substantia nigra and down-regulating expression levels of protein and mRNA of inflammatory factors COX-2 and TNF-α (Li X. J. et al., 2016). Parthenolide, as an active ingredient obtained from Chinese herbs tansy, possesses extensive biological functions, such as anti-inflammation, antioxidation et al., and it also has apparent protective effects against the damage of DA neurons induced by MPTP in substantia nigra. The research showed that compared with the mice in control group, the model mice represented the typical symptoms of PD. The numbers of COX-2, PGE2 and iNOS positive cells were reduced noticeably (P < 0.01), the number of TH-positive neurons in substantia nigra was decreased from 58 to 27% after the intervention with parthenolide. Taken together, the protective effect of parthenolide for dopaminergic neurons may be related to its activity as an anti-inflammatory in the expression of COX-2, PGE2, and iNOS in substantia nigra of PD mice model (Zhang H. et al., 2015).
The inhibition of neuronal apoptosis
With the in-depth study, the researchers find that another way of loss of dopaminergic neurons is abnormal apoptosis (Golpich et al., 2015). Apoptosis may be one of the most important factors in the death of dopamine neurons, and accelerate the occurrence and progression of PD (Valdeolivas et al., 2013). Energy consumption of normal activities of brain cells are derived directly from aerobic energy, and there is little energy storage. Once the brain damage occurs, it will cause nerve cell apoptosis or death (Kermer et al., 1999; Li R. et al., 2013). Apoptosis, which is an active programmed cell death, is the terminal phenomenon of gene-induced biological cascade reaction under the stimulation of both in vitro and in vivo. The Bcl-2 family is a kind of important apoptosis-regulatory genes. It is divided into two categories: anti-apoptosis gene (such as Bcl-2, Bcl-xL, Bcl-w, Bcl-1, etc.) and pro-apoptosis gene (such as Bax, Bak, Bad, Bid, etc.). Bcl-2, located in the outer mitochondrial membrane primarily, can counter pro-apoptotic factor. The early phase of the apoptotic cascade depends mainly on the balance between the pro- and anti-apoptotic proteins of the Bcl-2 family, while the Bcl-2/Bax ratio is regarded as a better predictor of apoptosis than the expression of either Bcl-2 or Bax alone (Sa et al., 2015; Wu et al., 2015). Studies have shown that the overexpression of bcl-2 can break off the apoptosis of various nerve cell, can also restrain the toxic effects of MPTP and 6-OHDA on dopaminergic neurons, thus reducing the apoptosis of dopaminergic neurons in substantia nigra (Burke, 2011; Chen et al., 2015b).
Studies indicated that Guiling pa'an Wan (Chinese patent medicine) could improve pathology and behavior of the PD model rats induced by 6-OHDA. Based on this background, the mechanism of action was further studied. After treatment with Guiling pa'an Wan, the expression of Bcl-2 and Bcl-2/Bax ratio was increased while Bax and Caspase-3 expression were dropped in substantia nigra neurons, which showed that Guiling pa'an could provide protection for dopaminergic neurons by reducing the apoptosis of nerve cells in PD rats (Meng et al., 2014b; Zhang H. Z. et al., 2016). Some flavonoids have good effect on the prevention and treatment of PD. Puerarin can inhibit the expression of p53, Bax, PUMA and caspase 3 via the activation of pi3k/akt signaling pathway in SH-SY5Y cells stimulated by MPP+ (Zhu et al., 2012). In 6-OHDA-induced neurotoxicity in a dopaminergic cell line, SN4741 cells, isoliquiritigenin can significantly ameliorate the expression of Bcl-2 and lower expression of Bax and the release of cytochrome C, which can be reversed by the inhibitor of PI3K/Akt/PKB (Hwang and Chun, 2012; Qin and Wu, 2016). Ginkgo biloba Pingchan Recipe has protective impacts on dopaminergic neurons in PD model mice induced by MPTP. The researchers, through SH-SY5Y cell model induced by MPP+, made a further study of its mechanism. After the drug treatment of Ginkgo biloba Pingchan Recipe, the proliferation speed of the cells was accelerated while apoptosis was substantially plunged, and the expression levels of apoptosis related gene PARP and PTEN were vastly declined (Wu et al., 2016). As a tumor suppressor gene, PTEN regulates the cell division cycle by preventing fast cell growth and division or uncontrolled cell division (Li and Yang, 2012; Li D. et al., 2013; Zhang G. et al., 2014). Geniposide, an iridoid glycoside compound extracted from the TCM Gardenia jasminoides Ellis fruit, exerts neuroprotective effects by alleviating inflammation responses and oxidative damages (Zhao et al., 2016). The behavioral experiment including rotarod and swimming trials indicated that the Geniposide could substantially improve the abnormal behavior caused by MPTP. Meanwhile, the number of TH positive neuron sharply increased (P < 0.001) and the apoptotic neurons fell (P < 0.001) after treatment with Geniposide. Thereby suggesting that Geniposide has protective effect on dopaminergic neurons in substantia nigra of PD mice model induced by MPTP, and its mechanism may be related to the inhibition of neuronal apoptosis (Chen et al., 2015a).
The inhibition of abnormal protein aggregation
Currently, impaired degradation of misfolded and aggregated proteins has been proposed to play a key role in the pathogenesis of PD (Le and Chen, 2009). The abnormal deposition of protein in brain tissue is characteristic of several age-related neurodegenerative diseases, such as PD. Although, the composition and position (i.e., intra- or extracellular) of protein aggregates are different from disease to disease, this common characteristic shows that protein deposition per se, or some related event, is toxic to neurons (Dauer and Przedborski, 2003).
The pathological changes of PD are characterized by degeneration of DA neurons in the substantia nigra and formation of Lewy body (LB) in neurons (Scherfler et al., 2007). Many proteins, including α-synuclein (α-syn), ubiquitin and its related enzymes, aregathered in LB. The study showed that the death of nerve cells in the brain was caused by the α-synprotein conformational change, the formation of amyloid filaments and abnormal accumulation (Dekundy et al., 2015). α-syn is the main component of LB, which is the firstly identified as a protein with gene mutation associated with PD. The abnormal aggregation of the protein is closely related to the pathogenesis of PD (Lubbe and Morris, 2014; Zhang X. et al., 2016). The ubiquitin proteasome system (UPS), a new protein degradation pathway, is regarded as the major pathway of non-lysosomal protein degradation in eukaryotic cells. The study confirmed that the activity of the proteasome dropped substantially in substantia nigra of patients with PD, which weakened the effect of the substantia nigra on the degradation of α-syn and other proteins (Masliah et al., 2005). The overexpression and mutation of α-syn can accelerate mitochondrial disorder, enhance the sensitivity to oxidative stress and promote cell death due to its cytotoxicity mediated by DAT (Alberio et al., 2012).
In a study, rotenone was used to stimulate PC12 cells to develop a cell model of PD with over expression of α-syn. After treatment with bilobalide, the oligomer of α-syn were effectively restrained, cell activity was intensified and apoptosis was decreased accordingly. Before that, some scholars found that the bilobalide could regulate the metabolism of the amyloid precursor protein (APP), increase the proportion of soluble APP alpha, reduce the formation of β-amyloid protein (Shi et al., 2011). The similar experimental results were presented that bilobalide could inhibit the formation of abnormal aggregation of different protein by some common mechanism and alleviate the toxic effects of abnormal proteins on cells. Therefore, it acts as a neuroprotective role in this kind of “protein folding diseases” (Zeng et al., 2013).
In another open study, to produce the symptoms of PD, researchers injected the trace amounts of the proteasome inhibitor lactacystin into the substantia nigra pars compacta (SNC) and ventral tegmental area (VTA) in the brain of SD rats. Compared with the model control, the aggregation of α-syn and the apoptosis of substantia nigra were obviously inhibited in rats with PD after treatment with the Anchanling (Chinese patent medicine), which presented that the mechanism of Anchanling might be related to the improvement of UPS function. When the damaged UPS function was improved, the UPS raised up the degradation of α-syn, thereby reducing the accumulation of intracellular proteins and the formation of inclusion bodies. So, the improving the function of UPS may be of great significance for the prevention and treatment of PD (Gao, 2007; Wu et al., 2009). Some studies have proved that baicalein can protect nerve cells by inhibiting fibrosis procedure of α-syn protein. 12.5 mol·L−1 baicalein can significantly inhibit the oligomerization of α-syn and its cytotoxic effect on SH-SY5Y cells (Lu et al., 2011). Clinically, Bushen Huoxue Granule was proved to be effective in treating PD for many years, a Chinese herbal compound granule (Li et al., 2012). The therapeutic mechanisms of Bushen Huoxue Granule against PD might be related with up-regulation of the TrkB expression that could strengthen the effect of repairing nerve injury factors and down-regulation of the Tau expression that could contribute to reduce the condensed expression of proteins in the cells (Yu et al., 2016).
Conclusion
In summary, PD is regarded as a complex disease caused by interaction among multiple factors (environmental factors, genetic factors) and various mechanisms. Considering curative effect and symptom control, in short term, western medicine is superior to TCM. However, the long-term effect of treatment is debilitated and a series of side effects will be produced. In contrast, TCM has become a research hotspot in recent years due to its the advantages of multiple components and holistic regulation. In particular, some progress has been made in the study of inhibition of oxidative stress, improvement of mitochondrial energy metabolism, resistance to EAA toxicity and suppression of cell apoptosis. A range of TCM is summarized in Table 2, which exhibits neuroprotective effects on dopamine neurons in substantia nigra or shows beneficial improvements on PD symptoms through one or more biological interventions. Although TCM has the glorious history in the treatment of PD, the experimental studies have only been carried out in recent years, especially for the adoption of the PD model. The PD models are mainly divided into two categories: in vitro, which includes PC12 cell, SH-SY5Y cell, SN4741, cell and in vivo, which includes mouse, rat, zebrafish, Drosophila DJ-1A and so on. Due to the complexity of TCM and its active ingredients, it is difficult to choose the right model to explore its mechanism comprehensively. And, the existing models of PD can only screen some TCM. Therefore, in order to better reveal the pharmacological effects and mechanisms of TCM against PD, several models are firstly applied simultaneously to compensate for the shortage of a single model, and secondly, it is essential to develop more models that conform to the human disease characteristics.
Table 2.
Summary of TCM on Mechanisms of Anti-PD.
| TCM or extract of TCM | Anti-PD mechanisms | Model | Inducer | References |
|---|---|---|---|---|
| Anchanling | The inhibition of abnormal protein aggregation; The inhibition of neuronal apoptosis | SD rats | Lactacystin | Gao, 2007; Wu et al., 2009 |
| Baicalein | The inhibition of oxidative stress; The regulation of mitochondrial dysfunction; The reduction of toxic EAA; The inhibition of abnormal protein aggregation | PC12 cells/C57BL/6 mice/SD rats | H2O2/Rotenone/Rotenone | Zhang et al., 2010, 2017; Lu et al., 2011; Hu et al., 2016 |
| Baichanting Compound | The inhibition of oxidative stress | C57BL/6 mice | MPTP | Ren et al., 2015 |
| Bilobalide | The inhibition of abnormal protein aggregation | PC12 cells | Rotenone | Shi et al., 2011; Zeng et al., 2013 |
| Bushen Huoxue Granule | The inhibition of abnormal protein aggregation | SD rats | 6-OHDA | Li et al., 2012; Yu et al., 2016 |
| Carnosic acid | The inhibition of oxidative stress; The inhibition of neuronal apoptosis | SH-SY5Y cells/Wistar rats | 6-OHDA/6-OHDA | Wu et al., 2015 |
| Catalpol | The regulation of mitochondrial dysfunction | Kunming mice | Rotenone | Shi et al., 2012 |
| Celastrol | The inhibition of neuroinflammation | Drosophila DJ-1A | – | Faust et al., 2009; Zhang et al., 2012 |
| Curcumin | The inhibition of oxidative stress; The inhibition of neuroinflammation | C57BL/6 mice/Lewis rats | MPTP/Rotenone | Pan et al., 2007; Cui et al., 2016 |
| Forsythia suspense extract | The inhibition of oxidative stress; The inhibition of neuroinflammation | PC12 cell/SD rats | Rotenone/Rotenone | Zhang S. et al., 2016 |
| Ganoderma lucidium spore | The inhibition of oxidative stress; The inhibition of neuroinflammation | Wistar rats | 6-OHDA | Bao, 2014 |
| Geniposide | The inhibition of neuronal apoptosis | C57BL/6 mice | MPTP | Chen et al., 2015a,c; Zhao et al., 2016 |
| Ginkgo biloba Pingchan Recipe | The inhibition of neuronal apoptosis | SH-SY5Y cells/C57BL mice | MPP+/MPTP | Wu et al., 2016 |
| Ginsenoside Rg1 | The inhibition of neuroinflammation, The inhibition of abnormal protein aggregation | C57BL/6 mice | MPTP&probenecid | Heng et al., 2016 |
| Green tea polyphenols | The inhibition of oxidative stress | C57BL/6J mice | MPTP | Chen et al., 2013 |
| Gui Ling Pa An Wan | The inhibition of oxidative stress; The inhibition of neuronal apoptosis | SD rats | 6-OHDA | Meng et al., 2014a,b; Zhang H. Z. et al., 2016 |
| Icariin | The inhibition of neuronal apoptosis | C57BL/6 mice | MPTP | Chen et al., 2017 |
| Isoliquiritigenin | The inhibition of neuronal apoptosis | SN4741 cells | 6-OHDA | Hwang and Chun, 2012; Qin and Wu, 2016 |
| Kukoamine A | The inhibition of oxidative stress; The inhibition of neuroinflammation; The reduction of toxic EAA; The inhibition of neuronal apoptosis | SH-SY5Y cells/C57BL/6 mice | MPP+/MPTP | Hu et al., 2017 |
| Lycopene | The inhibition of oxidative stress | C57BL/6 mice | Rotenone | Liu et al., 2013 |
| Matrine | The inhibition of oxidative stress | C57BL mice | MPTP | Meng et al., 2017 |
| Paeoniflorin | The inhibition of neuronal apoptosis | C57BL/6 mice | MPTP | Zheng et al., 2017 |
| Paeonolum | The inhibition of oxidative stress; The inhibition of neuronal apoptosis | PC12 cells/zebrafish | MPP+ | Lu et al., 2015 |
| Parthenolide | The inhibition of neuroinflammation | C57BL/6 mice | MPTP | Zhang H. et al., 2015 |
| Petroleum Ether Extract of Ficus religiosa (L.) Leaves | The inhibition of oxidative stress | Wistar rats | 6-OHDA | Bhangale and Acharya, 2016 |
| pine bark extract | The inhibition of oxidative stress | C57BL/6 mice | Rotenone | Lu et al., 2014 |
| Piperine | The inhibition of oxidative stress; The inhibition of neuroinflammation; The inhibition of neuronal apoptosis | C57BL/6 mice | MPTP | Yang et al., 2015 |
| Polygona-Polysaccharose | The inhibition of neuroinflammation | SD rats | 6-OHDA | Chen et al., 2010 |
| Polyphenols from toona sinensis seeds | The inhibition of neuroinflammation | SD rats | 6-OHDA | Li X. J. et al., 2016 |
| Polysaccharide from Spirulina platensis | The inhibition of oxidative stress | C57BL/6J mice | MPTP | Zhang F. et al., 2015 |
| Powder of Gastrodia elata | The inhibition of oxidative stress; The inhibition of neuroinflammation | Wistar rats | 6-OHDA | Chen et al., 2014; Wang et al., 2014 |
| Proantho cyanidins | The inhibition of oxidative stress | C57BL/6 mice | MPTP | Liang and Zhang, 2016 |
| Protocatechuic acid | The inhibition of oxidative stress | SD rats | 6-OHDA | Liu et al., 2013 |
| Protocatechuic acid and chrysin | The inhibition of oxidative stress; The inhibition of neuroinflammation | PC12 cells/zebrafish/mice | 6-OHDA/6-OHDA/MPTP | Zhang Z. J. et al., 2015 |
| Puerarin | The reduction of toxic EAA; The inhibition of neuronal apoptosis | C57BL/6 mice | MPTP | Zhu et al., 2012; Huang and Wang, 2015; Jiang et al., 2016 |
| Qianzheng San Extract | The regulation of mitochondrial dysfunction | Kunming mice/C57BL/6 mice | Arecoline Hydrobromide/Oxotremorine/MPTP | Li et al., 2015 |
| Salidroside | The inhibition of oxidative stress; The inhibition of neuronal apoptosis; The inhibition of abnormal protein aggregation | PC12 cells/C57BL/6 mice | MPP+/MPTP | Wang et al., 2015 |
| Salvianolic Acid B | The inhibition of oxidative stress; The inhibition of neuroinflammation | Mesencephalic cells/C57BL/6 mice | MPP+/LPS/MPTP | Zhou et al., 2014 |
| Schisantherin A | The inhibition of neuronal apoptosis | SH-SY5Y cells/C57BL/6 mice | MPP+/MPTP | Sa et al., 2015 |
| Tetrandrine(Tet) | The reduction of toxic EAA | SD rats | 6-OHDA | Jin and Bao, 2010 |
| Total flavonoids from Scutellaria baicalensis | The inhibition of oxidative stress | C57BL/6J mice | MPTP | Li X. L. et al., 2016 |
| Total Flavonoids of Clerodendranthus spicatus | The inhibition of oxidative stress | SH-SY5Y cells/Wistar rats | 6-OHDA/6-OHDA | You et al., 2015 |
| Trehalose | The inhibition of abnormal protein aggregation | SD Rats | AAV1/2 A53T α-synuclein | He et al., 2015 |
| Triptolide | The inhibition of neuroinflammation | SD rats | MPP+ | Hirsch et al., 2005; Gao et al., 2008 |
| Zhenganxifeng decoction | The inhibition of oxidative stress | Wistar rats | 6-OHDA | Li X. M. et al., 2016 |
PD is a result of the interaction of many neuroendocrine factors in the aging state. The use of TCM alone can effectively control the early signs of PD, avoid toxic side effects of western medicine and enhance the compliance of patients with medication greatly. Although TCM have showed the magic effect for the disease, it is difficult to ignore the problem that the composition of the TCM is complex and the mechanism of action is not completely clear. The following suggestions should be particularly considered: (1) more active components should be isolated and screened from TCM, just like artemisinin for malaria; (2) as TCM compound, therapeutic material basis will continue to be searched for the fight against PD, just like compound Danshen dripping pills for coronary heart disease. The compound of active ingredients of TCM, whose material base is relatively clear, adheres to the concept and advantages of formula compatibility of TCM. Thus, it is one of the most important approaches to modern TCM research. With the rapid development of molecular biology, neurobiology, behavioral science and other disciplines of knowledge, single herbs, effective component and TCM compound, through a variety of mechanisms, will create comprehensive and effective prevention and treatment of PD with the anticipation of different time, links, and levels.
Author contributions
XL summarized the literature and wrote the manuscript. YZ helped with the manuscript writing. YW revised the manuscript. JX wrote part of the manuscript. PX designed the diagram. YM and QW revised the manuscript and provided critical comments. HK supervised all the works. All the authors approved its final version, and agreed to be accountable for all aspects of the work.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Major State Basic Research Development Program (973 Program) of China (2013CB531800), and the National Natural Science Foundation of China (81473351 and 81773904).
Glossary
Abbreviations
- TCM
Traditional Chinese medicine
- PD
Parkinson's disease
- EAA
excitatory amino acids
- MPTP
1-methyl-4-henyl-1,2,3,6-tetrahydropyridine
- GSH
Glutathione
- MDA
Malondialdehyde
- 6-OHDA
6-Hydroxydopamine
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- Glu
glutamate
- Tet
Tetrandrine
- Asp
aspartate
- NO
nitric oxide
- DA
dopamine
- Ach
acetylcholine
- GABA
gamma-aminobutyric acid
- COX-2
cyclooxygenase-2
- LB
Lewy body
- α-syn
α-synuclein
- UPS
ubiquitin proteasome system.
References
- Alberio T., Bossi A. M., Milli A., Parma E., Gariboldi M. B., Tosi G., et al. (2012). Proteomic analysis of dopamine and α-synuclein interplay in a cellular model of Parkinson's disease pathogenesis. FEBS J. 277, 4909–4919. 10.1111/j.1742-4658.2010.07896.x [DOI] [PubMed] [Google Scholar]
- Alves G., Forsaa E. B., Pedersen K. F., Dreetz Gjerstad M., Larsen J. P. (2008). Epidemiology of Parkinson's disease. J. Neurol. 255, 18–32. 10.1007/s00415-008-5004-3 [DOI] [PubMed] [Google Scholar]
- Avila I., Reilly M. P., Reilly F., Posadas-Sanchez D., Chavez C. L., Banerjee N., et al. (2009). Modeling operant behavior in the Parkinsonian rat. Behav. Brain Res. 198, 298–305. 10.1016/j.bbr.2008.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D., Garg A., Krohn R. L., Bagchi M., Tran M. X., Stohs S. J. (1997). Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 95, 179–189. [PubMed] [Google Scholar]
- Bao C. (2014). Effect of Ganoderma lucidium spore on oxidative stress and inflammatory responses in rats with parkinson's disease. Pract. Pharm. Clin. Rem. 17, 402–404. 10.14053/j.cnki.ppcr.2014.04.028 [DOI] [Google Scholar]
- Barnum C. J., Tansey M. G. (2010). Modeling neuroinflammatory pathogenesis of Parkinson's disease. Prog. Brain Res. 184, 113–132. 10.1016/S0079-6123(10)84006-3 [DOI] [PubMed] [Google Scholar]
- Béraud D., Hathaway H. A., Trecki J., Chasovskikh S., Johnson D. A., Johnson J. A., et al. (2013). Microglial activation and antioxidant responses induced by the Parkinson's disease protein alpha-synuclein. J. Neuroimmune Pharmacol. 8, 94–117. 10.1007/s11481-012-9401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangale J. O., Acharya S. R. (2016). Anti-parkinson activity of petroleum ether extract of Ficus religiosa (L.) leaves. Adv. Pharmacol. Sci. 12, 1–9. 10.1155/2016/9436106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J., Przedborski S. (2014). Parkinson's disease: animal models and dopaminergic cell vulnerability. Front. Neuroanat. 8:155. 10.3389/fnana.2014.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovac J. A. (2016). Side effects of a dopamine agonist therapy for Parkinson's disease: a mini-review of clinical pharmacology. Yale J. Biol. Med. 89, 37–47. [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. (2011). Programmed cell death in Parkinson's disease. Handb. Clin. Neurol. 83, 591–605. 10.1016/S0072-9752(07)83029-6 [DOI] [PubMed] [Google Scholar]
- Caragine L. P., Park H. K., Diaz F. G., Phillis J. W. (1998). Real time measurement of ischemia-evoked glutamate release in the cerebral cortex vessel rat occlusion models. Brain Res. 793, 255–264. 10.1016/S0006-8993(98)00182-6 [DOI] [PubMed] [Google Scholar]
- Chen H., Ai G., Huang M. (2014). Comparative study on neuroethology, oxidative stress and inflammatory responses in rats with parkinson's disease of superfine and common powder of Gastrodia elata. Chin. J. Exp. Tradit. Med. Formulae. 3, 144–148. 10.11653/syfj2014030144 [DOI] [Google Scholar]
- Chen J., Li Y. Y., Tian W., Cheng W. (2010). Effect of Polygona-Polysaccharose (PP) on expression of PPAR-γ in brain tissue of rats with Parkinson disease. Prog. Mod. Biomed. 10, 814–817. 10.13241/j.cnki.pmb.2010.05.016 [DOI] [Google Scholar]
- Chen M., Huang X. F., Ye J., Yu L., Liu C. W. (2013). Protective effect of green tea polyphenols on substantia nigra dopaminergic neurons of MPTP induced Parkinson's disease mice. Chongqing Med. 7, 721–723. 10.3969/j.issn.1671-8348.2013.07.001 [DOI] [Google Scholar]
- Chen W. F., Wu L., Du Z. R., Chen L., Xu A. L., Chen X. H., et al. (2017). Neuroprotective properties of icariin in MPTP-induced mouse model of parkinson's disease: involvement of PI3k/AKt and MEK/ERK signaling pathways. Phytomedicine 25, 93–99. 10.1016/j.phymed.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Chen W. Q., Hou X. L., Li S. T., Hong Y., Wang D. L., Cheng Y. Y. (2009). Protective effects of green tea polyphenols on cognitive impairments induced by psychological stress in rats. Behav. Brain Res. 202, 71–76. 10.1016/j.bbr.2009.03.017 [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Li L., Zhang Y. F., Li Y. W., Liu W. Z. (2015a). Neuroprotective effects of geniposide to MPTP induced Parkinson's disease mice model. Chin. J. Neuroanat. 31, 629–634. 10.16557/j.cnki.1000-7547.2015050017 [DOI] [Google Scholar]
- Chen Y. M., Zhang Y. F., Li L. (2015b). Neuroprotective effect of geniposide on Parkinson's disease model mice. Chin. J. Contemp. Neurol. Neurosurg. 15, 481–487. 10.3969/j.issn.1672-6731.2015.06.012 [DOI] [Google Scholar]
- Chen Y. M., Zhang Y. F., Li L., Hölscher C. (2015c). Neuroprotective effects of geniposide in the MPTP mouse model of parkinson's disease. Eur J. Pharmacol. 768, 21–27. 10.1016/j.ejphar.2015.09.029 [DOI] [PubMed] [Google Scholar]
- Cui Q. L., Li X., Zhu H. C. (2016). Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the AKt/Nrf2 pathway. Mol. Med. Rep. 13, 1381–1388. 10.3892/mmr.2015.4657 [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E. C., Agid Y., Graybiel A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122, 1437–1448. 10.1093/brain/122.8.1437 [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. (2003). Parkinson's disease: mechanisms and models. Neuron 39, 889–909. 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- Dekundy A., Mela F., Hofmann M., Danysz W. (2015). Effects of dopamine uptake inhibitor MRZ-9547 in animal models of Parkinson's disease. J. Neural Transm. (Vienna) 122, 809–818. 10.1007/s00702-014-1326-8 [DOI] [PubMed] [Google Scholar]
- Exner N., Lutz A. K., Haass C., Winklhofer K. F. (2012). Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences. EMBO J. 31, 3038–3062. 10.1038/emboj.2012.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K., Gehrke S., Yang Y., Yang L., Beal M. F., Lu B. (2009). Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson's disease. BMC Neurosci. 10:109. 10.1186/1471-2202-10-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Wu Y. C. (2014). Research progress of Mitochondrial Dysfunction in pathogenesis of Parkinson's disease. J. Int. Neurol. Neurosurg. 41, 349–352. 10.16636/j.cnki.jinn.2014.04.015 [DOI] [Google Scholar]
- Gao J. P., Sun S., Li W. W., Chen Y. P., Cai D. F. (2008). Triptolide protects against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats: implication for immunosuppressive therapy in Parkinson's disease. Neurosci Bull. 24, 133–142. 10.1007/s12264-008-1225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. W. (2007). Anchanling's Effect on Ubiquitin Proseasome System of Parkinson's Disease Rat Models. Hubei: University of Chinese Medicine. [Google Scholar]
- Ge G. F., Shi W. W., Yu C. H., Jin X. Y., Zhang H. H., Zhang W. Y., et al. (2017). Baicalein attenuates vinorelbine-induced vascular endothelial cell injury and chemotherapeutic phlebitis in rabbits. Toxicol. Appl. Pharmacol. 318, 23–32. 10.1016/j.taap.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Golpich M., Amini E., Hemmati F., Ibrahim N. M., Rahmani B., Mohamed Z., et al. (2015). Glycogen synthase kinase-3 beta (GSK-3β) signaling: implications for Parkinson's disease. Pharmacol. Res. 97, 16–26. 10.1016/j.phrs.2015.03.010 [DOI] [PubMed] [Google Scholar]
- Hallett P. J., Standaert D. G. (2004). Rationale for and use of NMDA receptor antagonists in Parkinson' s disease. Pharmacol. Ther. 102, 155–174. 10.1016/j.pharmthera.2004.04.001 [DOI] [PubMed] [Google Scholar]
- He Q., Koprich J. B., Wang Y., Yu W. B., Xiao B. G., Brotchie J. M., et al. (2015). Treatment with trehalose prevents behavioral and neurochemical deficits produced in an AAV α-synuclein rat model of parkinson's disease. Mol. Neurobiol. 53, 2258–2268. 10.1007/s12035-015-9173-7 [DOI] [PubMed] [Google Scholar]
- Heng Y., Zhang Q. S., Mu Z., Hu J. F., Yuan Y. H., Chen N. H. (2016). Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting α-synuclein abnormalities in the substantia nigra. Toxicol. Lett. 243, 7–21. 10.1016/j.toxlet.2015.12.005 [DOI] [PubMed] [Google Scholar]
- Hirsch E. C., Hunot S., Hartmann A. (2005). Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat. Disord. 11, S9–S15. 10.1016/j.parkreldis.2004.10.013 [DOI] [PubMed] [Google Scholar]
- Hu Q., Uversky V. N., Huang M. Y., Kang H. C., Xu F., Liu X. Y., et al. (2016). Baicalein inhibits α-synuclein oligomer formation and prevents progression of α-synuclein accumulation in a rotenone mouse model of parkinson's disease. Biochim. Biophys. Acta 1862, 1883–1890. 10.1016/j.bbadis.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Hu X. L., Song Q., Li X., Li D. D., Zhang Q., Meng W. H., et al. (2017). Neuroprotective effects of kukoamine A on neurotoxin-induced parkinson's model through apoptosis inhibition and autophagy enhancement. Neuropharmacology 117:352. 10.1016/j.neuropharm.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Huang X. F., Wang J. M. (2015). Research progress of neuroprotective mechanisms of puerarin. Chin. J. Exp. Tradit. Med. Formulae 21, 224–230. 10.13422/j.cnki.syfjx.2015040224 [DOI] [Google Scholar]
- Hunot S., Hirsch E. C. (2003). Neuroinflammatory processes in Parkinson's disease. Ann. Neuro. 53, 49–60. 10.1002/ana.10481 [DOI] [PubMed] [Google Scholar]
- Hwang C. K., Chun H. S. (2012). Isoliquiritigenin isolated from licorice Glycyrrhiza uralensis prevents 6-hydroxydopamine-induced apoptosis in dopaminergic neurons. Biosci. Biotechnol. Biochem. 76, 536–543. 10.1271/bbb.110842 [DOI] [PubMed] [Google Scholar]
- Jiang M., Yun Q., Niu G., Gao Y., Shi F., Yu S. (2016). Puerarin prevents inflammation and apoptosis in the neurocytes of a murine parkinson's disease model. Genet. Mol. Res. 15:gmr.15047501. 10.4238/gmr.15047501 [DOI] [PubMed] [Google Scholar]
- Jin X. H., Bao S. Y. (2010). Effects of glutathione combined with tetrandrine on excitatory amino acids of striatum corpora in Parkinson's disease. Chin. J. Pract. Nerv. Dis. 13, 1–4. [Google Scholar]
- Kermer P., Klöcker N., Bähr M. (1999). Neuronal death after brain injury. Models, mechanisms, and therapeutic strategies in vivo. Cell Tissue Res. 298, 383–395. 10.1007/s004410050061 [DOI] [PubMed] [Google Scholar]
- Kum W. F., Durairajan S. S., Bian Z. X., Man S. C., Lam Y. C., Xie L. X., et al. (2011). Treatment of idiopathic Parkinson's disease with traditional chinese herbal medicine: a randomized placebo-controlled pilot clinical study. Evid. Based Complement. Alternat. Med. 2011:724353. 10.1093/ecam/nep116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le W. D., Chen S. (2009). Etiopathogenesis of Parkinson disease: a new beginning. Neuroscientist 15, 28–35. 10.1177/1073858408319974 [DOI] [PubMed] [Google Scholar]
- Li D., Zhang Y., Xie Y., Xiang J., Zhu Y., Yang J. (2013). Enhanced tumor suppression by adenoviral PTEN gene therapy combined with cisplatin chemotherapy in small-cell lung cancer. Cancer Gene Ther. 20, 251–259. 10.1038/cgt.2013.14 [DOI] [PubMed] [Google Scholar]
- Li H., Yang B. B. (2012). Stress response of glioblastoma cells mediated by miR-17-5p targeting PTEN and the passenger strand miR-17-3p targeting MDM2. Oncotarget 3, 1653–1668. 10.18632/oncotarget.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Yang M. H., Liu Y., Gao L. P. (2012). Effects of Bushen Huoxue Granules on tension of extremity muscles of Parkinson's patients. CJTCMP 27, 599–602. [Google Scholar]
- Li R., Xu L. Y., Liang T., Zhang S. J., Li Y. W., Duan X. J. (2013). Effect of puerarin on the expressions of BDNF, TrkB, caspase- 3in substantia nigra tissue of Parkinson's rats. Chin. J. Exp. Tradit. Med. Formulae 19, 208–211. 10.13422/j.cnki.syfjx.2013.03.069 [DOI] [Google Scholar]
- Li X. J., Zhuang W. X., Lv E., Fei X. C., Liu F., Liu J. C., et al. (2016). The anti-inflammatory and neuroprotective effects of polyphenols from toona sinensis seeds in the rat model of 6-OHDA- induced Parkinson's disease. Chin. J. Neuroanat. 32, 653–659. 10.16557/j.cnki.1000-7547.2016.05.0018 [DOI] [Google Scholar]
- Li X. L., Xu X. F., Bu Q. X., Jin W. R., Sun Q. R., Feng D. P., et al. (2016). Effect of total flavonoids from scutellaria baicalensis on dopaminergic neurons in the substantia nigra. Biomed. Rep. 5, 213–216. 10.3892/br.2016.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. M., Zhang H. Y., Qi Y. Q., Sun Y., Zhu L. H., Dong M. X. (2016). Effect of Zhenganxifeng decoction on oxidative stress in the midbrain of Parkinson's disease rats with syndrome of hyperactivity of liver-Yang. Lishizhen Med. Mater. Med. Res. 11, 2588–2590. 10.3969/j.issn.1008-0805.2016.11.011 [DOI] [Google Scholar]
- Li X. X., Wang J. P., Jin K., Wang C. S., Dong X., Huang M. J., et al. (2015). Effect of Qianzheng San extract on parkinsonian tremor, motor dysfunction and ultrastructure of DA nergic neurons in mice. Chin. J. Exp. Tradit. Med. Formulae 21, 130–133. 10.13422/j.cnki.syfjx.2015210130 [DOI] [Google Scholar]
- Liang C. B., Zhang F. (2016). The effect of proantho cyanidins on oxidize stress in the substantia nigra of mouse model with Parkinson's disease. Clin. J. Med. Off. 44, 1172–1174. 10.16680/j.1671-3826.2016.11.20 [DOI] [Google Scholar]
- Liu C. B., Wang R., Pan H. B., Ding Q. F., Liu F. B. (2013). Effect of lycopene on oxidative stress and behavioral deficits in rotenone induced model of Parkinson's disease. Chin. J. Appl. Physiol. 29, 380–384. 10.13459/j.cnki.cjap.2013.04.027 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang J. J., Wu Y. C. (2012). Research progress in neuroprotection of Silent mating-type information regulator 2 homolog 1 on neurodegenerative disease. J. Int. Neurol. Neurosurg. 39, 562–566. 10.16636/j.cnki.jinn.2012.06.018 [DOI] [Google Scholar]
- Lu F. B., Wang R., Chen L., Chen L. N., Zhang L. P., Liu C. B. (2014). Protective effect of pine bark extract on impaired neuron in rotenone induced Parkinson's disease mouse model. Mod. Prev. Med. 8, 1468–1471. [Google Scholar]
- Lu J. H., Ardah M. T., Durairajan S. S., Xie L. F., Fong W. F., Hasan M. Y., et al. (2011). Baicalein inhibits formation of α-synuclein oligomers within living cells and prevents Aβ peptide fibrillation and oligomerisation. Chembiochem. 12, 615–624. 10.1002/cbic.201000604 [DOI] [PubMed] [Google Scholar]
- Lu X. L., Lin Y. H., Wu Q., Su F. J., Ye C. H., Shi L., et al. (2015). Paeonolum protects against mpp+-induced neurotoxicity in zebrafish and pc12 cells. BMC Complement. Altern. Med. 15:137. 10.1186/s12906-015-0661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbe S., Morris H. R. (2014). Recent advances in Parkinson's disease genetics. J. Neurol. 261, 259–266. 10.1007/s00415-013-7003-2 [DOI] [PubMed] [Google Scholar]
- Masliah E., Rockenstein E., Adame A., Alford M., Crews L., Hashimoto M., et al. (2005). Effects of α-synuclein immunization in a mouse model of Parkinson's disease. Neuron 46, 857–868. 10.1016/j.neuron.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Meng F. H., Wang J. H., Ding F. X., Xie Y. L., Zhang Y. J., Zhu J. (2017). Neuroprotective effect of matrine on MPTP-induced parkinson's disease and on Nrf2 expression. Oncol. Lett. 13, 296–300. 10.3892/ol.2016.5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Qiao M. L., Chang X. H. (2014a). Effect of Gui Ling Pa An Wan on oxidation stress response of Parkinson disease rats. China J. Chin. Med. 29, 230–232. 10.16368/j.issn.1674-8999.2014.02.042 [DOI] [Google Scholar]
- Meng Y., Qiao M. L., Ning Y. H., Chang X. H. (2014b). Effect of Guiling Pa'an Pill on apoptosis of the dopaminergic neurons in rats with Parkinson disease. Tradit. Chin. Med. Res. 27, 65–67. [Google Scholar]
- Michel P. P., Hirsch E. C., Hunot S. (2016). Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90, 675–691. 10.1016/j.neuron.2016.03.038 [DOI] [PubMed] [Google Scholar]
- Miklossy J., Doudet D. D., Schwab C., Yu S., McGeer E. G., McGeer P. L. (2006). Role of ICAM-1 in persisting inflammation in Parkinson's disease and MPTP monkeys. Exp. Neurol. 197, 275–283. 10.1016/j.expneurol.2005.10.034 [DOI] [PubMed] [Google Scholar]
- Miller J. A., Trout B. R., Sullivan K. A., Bialecki R. A., Roberts R. A., Tjalkens R. B. (2011). Low-dose 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine causes inflammatory activation of astrocytes in nuclear factor-κB reporter mice prior to loss of dopaminergic neurons. J. Neurosci. Res. 89, 406–417. 10.1002/jnr.22549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Benner E. J., Kadiu I., Thomas M., Boska M. D., Hasan K., et al. (2006). Neuroinflammation, oxidative stress and the pathogenesis of Parkinson's disease. Clin. Neurosci. Res. 6, 261–281. 10.1016/j.cnr.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan R. (2014). The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinsons disease: focus on astrocytes. Mol. Neurobiol. 49, 28–38. 10.1007/s12035-013-8483-x [DOI] [PubMed] [Google Scholar]
- Pan J., Ding J. P., Chen S. D. (2007). The protection of curcumin in nigral dopaminergic neuronal injury of mice model of Parkinson disease. Chin. J. Contemp. Neurol. Neurosurg. 7, 421–426. [Google Scholar]
- Papa S. M., Chase T. N. (1996). Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann. Neurol. 39, 574–578. 10.1002/ana.410390505 [DOI] [PubMed] [Google Scholar]
- Parker W. D., Boyson S. T., Parks J. K. (1989). Abnormalities of electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 26, 719–723. 10.1002/ana.410260606 [DOI] [PubMed] [Google Scholar]
- Qin L. Y., Wu X. J. (2016). Flavonoids from medicinal plants in prevention and treatment of Parkinson disease: advances and prospection on pharmacology. Chin. J. Pharmacol. Toxicol. 30, 1125–1135. 10.3867/j.issn.1000-3002.2016.11.001 [DOI] [Google Scholar]
- Ren Y. D., Jing Y. E., Zhang S. X., Wang H. X., Lu F., Liu S. M. (2015). Effect of baichanting compound on oxidative stress responses in mice model of Parkinson's disease. Chin. J. Exp. Tradit. Med. Formulae 22, 154–157. 10.13422/j.cnki.syfjx.2015220154 [DOI] [Google Scholar]
- Rizek P., Kumar N., Jog M. S. (2016). An update on the diagnosis and treatment of Parkinson disease. CMAJ 188, 1157–1165. 10.1503/cmaj.151179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa F., Zhang L. Q., Chong C. M., Guo B. J., Li S., Zhang Z. J., et al. (2015). Discovery of novel anti-parkinsonian effect of schisantherin A in in vitro and in vivo. Neurosci. Lett. 593, 7–12. 10.1016/j.neulet.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Sanyal J., Bandyopadhyay S. K., Banerjee T. K., Mukherjee S. C., Chakraborty D. P., Ray B. C., et al. (2009). Plasma levels of lipid peroxides in patients with Parkinson's disease. Eur. Rev. Med. Pharmacol. Sci. 13, 129–132. [PubMed] [Google Scholar]
- Scherfler C., Schwarz J., Antonini A., Grosset D., Valldeoriola F., Marek K., et al. (2007). Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov. Disord. 22, 1229–1238. 10.1002/mds.21505 [DOI] [PubMed] [Google Scholar]
- Schinkel A. H. (1999). P-Glycoprotein, a gatekeeper in the blood-brain barrier, Adv. Drug Deliv. Rev. 36, 179–194. 10.1016/S0169-409X(98)00085-4 [DOI] [PubMed] [Google Scholar]
- Shi C., Wu F., Xu J., Zou J. (2011). Bilobalide regulates soluble amyloid precursor protein release via phosphatidylinositol 3 kinase-dependent pathway. Neurochem. Int. 59, 59–64. 10.1016/j.neuint.2011.03.028 [DOI] [PubMed] [Google Scholar]
- Shi G. F., Wang H. S., Mao Y. R., Liu X. Y., Jiang B. (2012). Protective effect of catalpol against mice brain mitochondrial injuries induced by rotenone. Prog. Mod. Biomed. 12, 5661–5664. 10.13241/j.cnki.pmb.2012.29.043 [DOI] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. (1991). Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann. Neurol. 30, 332–339. 10.1002/ana.410300304 [DOI] [PubMed] [Google Scholar]
- Sompol P., Ittarat W., Tangpong J., Chen Y., Doubinskaia I., Batinic-Haberle I., et al. (2008). Aneuronal model of Alzheimer's disease: an insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience 153, 120–130. 10.1016/j.neuroscience.2008.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchi F., Marconi S. (2010). Factors associated with motor fluctuations and dyskinesia in Parkinson disease: potential role of a new melevodopa plus carbidopa formulation (Sirio). Clin. Neuropharmacol. 33, 198–203. 10.1097/WNF.0b013e3181de8924 [DOI] [PubMed] [Google Scholar]
- Strugstad E., Sager G. (1998). Mechanisms behind drug dependence. Tidsskr. Nor. Lageforen. 118, 1866–1869. [PubMed] [Google Scholar]
- Tada-Oikawa S., Hiraku Y., Kawanishi M., Kawanishi S. (2003). Mechanism for generation of hydrogen peroxide and change of mitochondrialmembrane potential during rotenone-induced apoptosis. Life Sci. 73, 3277–3288. 10.1016/j.lfs.2003.06.013 [DOI] [PubMed] [Google Scholar]
- Tansey M. G., Goldberg M. S. (2010). Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 37, 510–518. 10.1016/j.nbd.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdeolivas S., Pazos M. R., Bisogno T., Piscitelli F., Iannotti F. A., Allarà M., et al. (2013). The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: a potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis. 4:e862. 10.1038/cddis.2013.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital A., Vital C., Lagueney A., Ferrer X., Ribière-Bachelier C., Latour P., et al. (2003). Inflammatory dymyelinnation in a patient with CMT1A. J. Muscle Nerve 28, 373–376. 10.1002/mus.10404 [DOI] [PubMed] [Google Scholar]
- Wang D. M., Hai J. R., Wei F., Mo Y. S., Feng J. (2012). Effects of paning decoction on behavior and oxidative stress reaction in rats with Parkinson's disease. Chin. J. Exp. Tradit. Med. Formulae 18, 199–202. 10.13422/j.cnki.syfjx.2012.10.062 [DOI] [Google Scholar]
- Wang S. H., He H., Chen L., Zhang W., Zhang X. J., Chen J. Z. (2015). Protective effects of salidroside in the MPTP/MPP+-induced model of parkinson's disease through ROS-NO-related mitochondrion pathway. Mol. Neurobiol. 51:718. 10.1007/s12035-014-8755-0 [DOI] [PubMed] [Google Scholar]
- Wang X. L., Xing G. H., Hong B., Li X. M., Zou Y., Zhang X. J., et al. (2014). Gastrodin prevents motor deficits and oxidative stress in the MPTP mouse model of parkinson's disease: involvement of ERK/2-Nrf2 signaling pathway. Life Sci. 114, 77–85. 10.1016/j.lfs.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Wei Z. F., Wang Y. S., Ma L. R., Wang Q., Zhang Z. F., Zhang Y. X. (2009). P38MAPK pathway regulates COX-2 and caspase-3 expression in a mouse model of Parkinson disease. J. South. Med. Univ. 29, 2010–2013. [PubMed] [Google Scholar]
- Wong V. K. W., Zeng W., Chen J., Yao X. J., Leung E. L. H., Wang Q. Q., et al. (2017). Tetrandrine, an activator of autophagy, induces autophagic cell death via pkc-α inhibition and mtor-dependent mechanisms. Front. Pharmacol. 8:351. 10.3389/fphar.2017.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. R., Tsai C. W., Chang S. W., Lin C. Y., Huang L. C., Tsai C. W. (2015). Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of parkinson's disease: involvement of antioxidative enzymes induction. Chem. Biol. Interact. 225, 40–46. 10.1016/j.cbi.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Wu Y. D., Liang P. P., Long D. Y., Gao B. M. (2016). Effects of Ginkgo biloba Pingchan Recipe on loss and apoptosis of dopamine neurons in mouse models of Parkinson's disease. Chin. J. Tissue Eng. Res. 20, 7327–7333. 10.3969/j.issn.2095-4344.2016.49.005 [DOI] [Google Scholar]
- Wu Z., Zhang J., Zhao B. (2009). Superoxide anion regulates the mitochondrial free Ca2+ through uncoupling proteins. Antioxid. Redox Signal. 11, 1805–1818. 10.1089/ars.2009.2427 [DOI] [PubMed] [Google Scholar]
- Wu Z. Z., Andrew C. J. H., Gao X. W., Zhu Q. W., Li Y. H. (2009). Study of impact of anchanling on rotation behavior and UPS function in Parkinson disease models. World J. Integr. Tradit. West. Med. 4, 162–165. 10.13935/j.cnki.sjzx.2009.03.027 [DOI] [Google Scholar]
- Xiong P., Chen X., Zhang N. (2012). Advance in studies on pathological mechanism of Parkinson's disease and traditional chinese medicine experiments in prevention and treatment of Parkinson's disease. China J. Chin. Mater. Med. 37, 686–691. 10.4268/cjcmm20120527 [DOI] [PubMed] [Google Scholar]
- Yang W., Chen Y. H., Liu H., Qu H. D. (2015). Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinson's disease mouse model. Int. J. Mol. Med. 36, 1369–1376. 10.3892/ijmm.2015.2356 [DOI] [PubMed] [Google Scholar]
- You J. J., Li G., Li Y. C., Wang W. L., Li Y. H. (2015). Neuroprotective effect of total flavonoids of Clerodendranthus spicatus on Parkinson disease. Chin. J. Exp. Tradit. Med. Formulae 4, 139–143. 10.13422/j.cnki.syfjx.2015040139 [DOI] [Google Scholar]
- Yu L., Li S. D., Liu Y., Xu R. X., Yang M. H. (2016). Effects of Bushen Huoxue Granule on the expression of TrkB and Tau in cerebral substantia nigra cells of rats with Parkinson's disease. CJTCMP 31, 1380–1382. [Google Scholar]
- Yu X., He G. R., Guan G. H. (2012). Neuroprotective effect of baicalein in patients with Parkinson's disease. China J. Chin. Mater. Med. 37, 421–425. 10.4268/cjcmm20120403 [DOI] [PubMed] [Google Scholar]
- Zeng Z. F., Jing X. N., Liang Y. R., Yang L. H., Liu J., Xiao S. H., et al. (2013). Bilobalide restrains the oligomer of α-synuclein in PC12 cells treated with rotenone. Chin. J. Brain Dis. Rabil. (Electronic Edition) 3, 25–29. 10.3877/cma.j.issn.2095-123X.2013.01.007 [DOI] [Google Scholar]
- Zhang F., Lu J., Zhang J. G., Xie J. X. (2015). Protective effects of a polysaccharide from spirulina platensis on dopaminergic neurons in an MPTP-induced parkinson's disease model in C57BL/6J mice. Neural Regener. Res. 10, 308–313. 10.4103/1673-5374.152387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Zhou H., Xiao H., Liu Z., Tian H., Zhou T. (2014). MicroRNA-92a functions as an oncogene in colorectal cancer by targeting PTEN. Dig. Dis. Sci. 59, 98–107. 10.1007/s10620-013-2858-8 [DOI] [PubMed] [Google Scholar]
- Zhang H., Tao J. F., Wang A. J., Sun N., Wang Q. (2015). Studies on the anti-inflammation effect of parthenolide in MPTP Parkinson's disease mice model. J. Apoplexy Nerv. Dis. 32, 158–160. [Google Scholar]
- Zhang H. Z., Chang X. H., Ning Y. H. (2016). Guiling Pa'an Pill on behavior, Caspase-3 and SYN of Parkinson's disease rats. Chin. Arch. Tradit. Chin. Med. 11, 2673–2675. 10.13193/j.issn.1673-7717.2016.11.032 [DOI] [Google Scholar]
- Zhang M. R., Sun F. L., Ai H. X., Zhang L., Jing Y., Wei S. R., et al. (2012). Inflammatory and anti-inflammatory for Parkinson's disease. Chin. J. Rehabil. Theory Pract. 18, 1040–1043. 10.3969/j.issn.1006-9771.2012.11.014 [DOI] [Google Scholar]
- Zhang Q., Liu Y., Zhang Z. H. (2015). Effect of protocatechuic acid on serum TNF-α, IL-1β and oxidative stress products levels in Parkinson rats. Chin. J. Biochem. Pharm. 11, 37–39. [Google Scholar]
- Zhang S., Shao S. Y., Song X. Y., Xia C. Y., Yang Y. N., Zhang P. C., et al. (2016). Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. Neurotoxicology 52, 72–83. 10.1016/j.neuro.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Zhang S., Ye J., Dong G. (2010). Neuroprotective effect of baicalein on hydrogen peroxide-mediated oxidative stress and mitochondrial dysfunction in PC12 cells. J. Mol. Neurosci. 40, 311–320. 10.1007/s12031-009-9285-5 [DOI] [PubMed] [Google Scholar]
- Zhang X., Yang Y. L., Du L. D., Zhang W., Du G. H. (2017). Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int. Immunopharmacol. 50, 38–47. 10.1016/j.intimp.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang W., Du L. D., Gao L., Du G. H. (2016). Advances in anti-Parkinson's disease drugs and their related pharmacological targets. J. Int. Pharm. Res. 43, 87–96. 10.13220/j.cnki.jipr.2016.01.013 [DOI] [Google Scholar]
- Zhang Y., Wang Z. Z., Sun H. M., Li P., Li Y. F., Chen N. H. (2014). Systematic review of traditional chinese medicine for depression in parkinson's disease. Am. J. Chin. Med. 42, 1035–1051. 10.1142/S0192415X14500657 [DOI] [PubMed] [Google Scholar]
- Zhang Z. J., Li G. H., Szeto S. S., Chong C. M., Quan Q., Huang C., et al. (2015). Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 84, 331–334. 10.1016/j.freeradbiomed.2015.02.030 [DOI] [PubMed] [Google Scholar]
- Zhao C. H., Zhang H. J., Li H., Lv C., Liu X. L., Li Z., et al. (2016). Geniposide ameliorates cognitive deficits by attenuating the cholinergic defect and amyloidosis in middle-aged Alzheimer model mice. Neuropharmacology 116, 18–29. 10.1016/j.neuropharm.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Zheng M. Z., Liu C. M., Fan Y. J., Yan P., Shi D. F., Zhang Y. C. (2017). Neuroprotection by paeoniflorin in the MPTP mouse model of parkinson's disease. Neuropharmacology 116, 412–420. 10.1016/j.neuropharm.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Zhou C., Gu Y. J., Jiang Z. L. (2003). Study on the protective effect of Acathopanax Senticosus to the neuron by toxicity of Glu. J. Clin. Neurol. 16, 6–8. [Google Scholar]
- Zhou J., Qu X. D., Li Z. Y., Ji W., Liu Q., Ma Y. H., et al. (2014). Salvianolic acid b attenuates toxin-induced neuronal damage via nrf2-dependent glial cells-mediated protective activity in parkinson's disease models. Plos ONE 9:e101668. 10.1371/journal.pone.0101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G. Q., Wang X. C., Wu S. B., Li Q. L. (2012). Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem. Int. 60, 400–408. 10.1016/j.neuint.2012.01.003 [DOI] [PubMed] [Google Scholar]