Abstract
Background
Delusions, a core symptom of schizophrenia, are thought to arise from an alteration in predictive coding mechanisms that underlie perceptual inference. Here, we aimed to empirically test the hypothesized link between delusions and perceptual inference.
Method
28 patients with schizophrenia and 32 healthy controls matched for age and gender took part in a behavioral experiment that assessed the influence of stabilizing predictions on perception of an ambiguous visual stimulus.
Results
Participants with schizophrenia exhibited a weaker tendency towards percept stabilization during intermittent viewing of the ambiguous stimulus compared to healthy controls. The tendency towards percept stabilization in participants with schizophrenia correlated negatively with delusional ideation as measured with a validated questionnaire.
Conclusion
Our results indicate an association between a weakened effect of sensory predictions in perceptual inference and delusions in schizophrenia. We suggest that attenuated predictive signaling during perceptual inference in schizophrenia may yield the experience of aberrant salience, thereby providing the starting point for the formation of delusions.
Keywords: Schizophrenia, Delusions, Bistable perception, Structure-from-motion, Perceptual memory, Predictive coding
1. Introduction
Our perception not only is determined by the sensory input we receive, but is strongly shaped by previous experience. In line with this, perception can be described in the framework of predictive coding as an inferential process by which incoming information is combined with endogenous predictions (Friston, 2005, Kersten et al., 2004, Mumford, 1992). Such predictions are generated from an internal model that represents beliefs about the world built up by past experience, and these predictions enable stable and unequivocal percepts despite the noisiness and ambiguity of sensory signals. An important characteristic of such predictive coding is that it is adaptive: whenever a prediction is violated by incoming sensory information, a prediction error signal initiates learning by an update of the internal model resulting in adjusted beliefs about the world. An alteration in the brain's predictive machinery has been suggested to lie at the core of delusion formation in schizophrenia. In this context, delusions are explained by an altered integration of predictions with sensory signals, whereby the concomitant aberrant prediction error signal is assumed to drive erroneous updates of the internal world model resulting in maladaptive predictions and unfounded beliefs, i.e. delusions (Corlett et al., 2010, Fletcher and Frith, 2009, Adams et al., 2013). At the experiential level, the weakened influence of predictions in perceptual inference is thought to have the effect that expected events are perceived as if they were unpredicted and surprising (Hemsley, 2005), and the search for a cognitive explanation for this perceived aberrant salience is then assumed to result in the formation of delusions (Heinz, 2002, Kapur, 2003).
A powerful tool for probing the brain's predictive mechanisms and their alterations in delusions is ambiguous stimuli, which are compatible with two different, mutually exclusive perceptual interpretations. Prolonged viewing of such stimuli results in bistable perception fluctuating spontaneously between the two possible interpretations (Leopold and Logothetis, 1999, Sterzer et al., 2009). Such bistable perception has been conceptualized as an ongoing competition between two predictions that can never fully account for all the visual information in the ambiguous stimulus, with the effect that increasing prediction error signals trigger switches between the two interpretations (Clark, 2013, Hohwy et al., 2008). The ambiguity inherent in ambiguous stimuli maximizes the need for perceptual inference, and, hence, the potential influence of endogenous predictions. A simple and efficient way to experimentally induce such predictions is the intermittent presentation of an ambiguous stimulus: when an ambiguous stimulus is temporarily removed from view, the percept after re-onset of the stimulus strongly tends to be the same as the last percept before stimulus removal, resulting in a substantial reduction of perceptual fluctuations (Leopold et al., 2002, Orbach et al., 1963). This stabilization of perception during the intermittent presentation of an ambiguous stimulus can be conceptualized as a special case of perceptual priming, which is thought to be mediated by the incorporation of predictions based on previous perceptual outcomes (Friston, 2005). Such stabilizing predictions are thought to be implemented at low levels of the cortical hierarchy, within sensory cortices (Pearson and Brascamp, 2008, Sterzer and Rees, 2008). Accordingly, attenuated sensory predictions, as suggested to underlie the formation of delusions, would translate into a decreased tendency to percept stabilization. In line with this, we previously found a negative correlation between the propensity towards delusional ideation and the tendency towards percept stabilization in a cohort of healthy individuals (Schmack et al., 2013). Here we asked whether the observed alteration of perceptual inference might also account for delusions of clinical relevance. To this end we empirically tested whether a weakened influence of sensory predictions in perceptual inference is related to delusions in schizophrenia. Patients with schizophrenia and healthy controls participated in a visual perception experiment with intermittent presentation of an ambiguous stimulus. In addition, they underwent a specific quantitative assessment of delusional ideation. We hypothesized that the tendency towards percept stabilization (1) is lower in patients with schizophrenia compared to healthy controls, and, (2) is negatively correlated with the degree of delusional ideation.
2. Methods
2.1. Participants
29 patients diagnosed with schizophrenia and 32 healthy controls matched for age and gender completed the study (see Table 1 for demographic and clinical characteristics). All patients fulfilled the ICD-10 criteria for paranoid schizophrenia and had no other psychiatric axis I disorder (SCID I). In addition to the specific assessment of delusional ideation (see 2.3 below), psychopathological symptoms were quantitatively assessed using the Positive and Negative Symptoms Scale (PANNS) (Kay et al., 1987). Patients were clinically stable and recruited as outpatients. Except for two unmedicated patients, all had been on stable doses of second generation antipsychotic medication for at least four weeks. Healthy volunteers had no axis I psychiatric disorder (SCID I) and no family history of psychiatric disorders. Exclusion criteria in both groups were neurological disorders and drug abuse up to seven days before testing. All participants had normal or corrected-to-normal vision and gave written informed consent before participation. The study was approved by the local ethics commitee of the Charité - Universitätsmedizin Berlin.
Table 1.
Demographic and clinical characteristics of schizophrenia patients and healthy controls.
| patients |
controls |
two-sided |
|
|---|---|---|---|
| (n = 29) | (n = 32) | p-value | |
| age | 34.0 ± 7.1a | 31.9 ± 6.9a | 0.25b |
| gender | 7 F–22 M | 8 F–24 M | 0.94c |
| PDI | |||
| yes/no | 17.6 ± 9.1a | 6.1 ± 5.9a | < 0.001b |
| conviction | 53.8 ± 36.3a | 15.6 ± 15.8a | < 0.001b |
| distress | 52.7 ± 32.7a | 11.4 ± 12.8a | < 0.001b |
| preoccupation | 49.7 ± 30.7a | 12.0 ± 13.1a | < 0.001b |
| sum | 156.2 ± 95.9a | 39.1 ± 40.7a | < 0.001b |
| PANNS | |||
| positive | 13.4 ± 4.6a | – | – |
| negative | 12.7 ± 5.2a | – | – |
| general | 25.3 ± 8.9a | – | – |
Mean ± SD.
Two-sample t-test.
Chi-squared test.
2.2. Experimental task
All participants took part in a visual perception experiment that was aimed at measuring the influence of sensory predictions in perceptual inference by the intermittent presentation of an ambiguous stimulus. Stimuli were presented on a on a CRT monitor (1024 × 768 pixels resolution, 60 Hz frame rate) using Matlab (MathWorks Inc.) and the Cogent2000 toolbox (http://www.vislab.ucl.ac.uk/cogent.php).
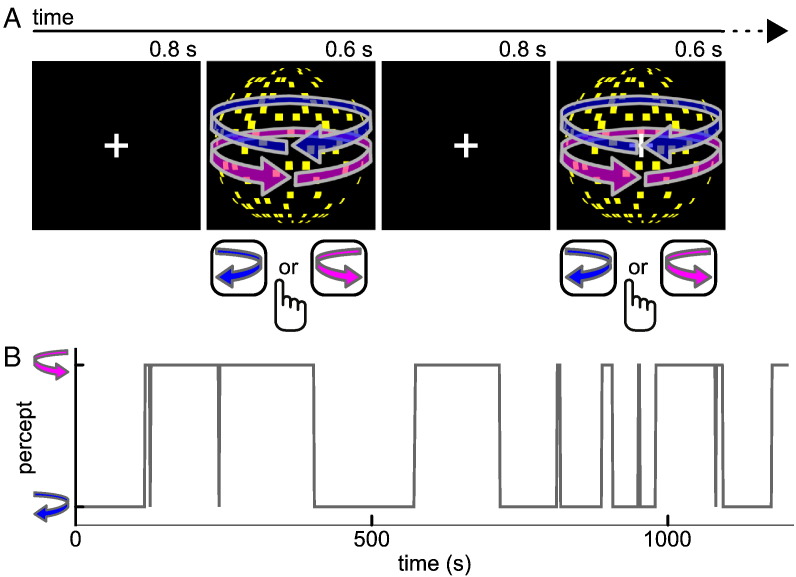
We used an ambiguous stimulus that consisted of 450 yellow square dots moving coherently on a black background with central white fixation cross and framed by a white square (Fig. 1). Due to the structure-from-motion phenomenon this stimulus is perceived as a sphere rotating in depth around a vertical axis (diameter 4.1° of visual angle, rotation speed 1/6 revolutions/s). The rotation direction of the sphere is ambiguous so that during prolonged viewing perception keeps fluctuating every few seconds between the percept of a sphere rotating towards the left and a sphere rotating towards the right. To familiarize participants with the two possible percepts evoked by the sphere, the experiment started with an initial training session during which the sphere was continuously presented for four minutes in which participants' perception fluctuated between the two percepts.
Fig. 1.
Schematic illustration of experimental procedure. A, Sensory predictions were induced by repeated presentation of an ambiguous structure-from-motion stimulus that can be perceived as a sphere rotating either leftward or rightward. The stimulus was presented repeatedly for 0.6 s interleaved by blank screens of 0.8 s duration. Upon each appearance of the stimulus, participants reported the perceived rotation direction by button press. B, Perceptual time course from one exemplary individual. Due to the stabilizing effect of sensory predictions that are built up during intermittent presentation of the ambiguous stimulus, participants tended to have the same percept across many successive presentation cycles.
In the main experimental session, we then assessed sensory predictions by presenting the ambiguous sphere stimulus intermittently. Such a repeated exposure to an ambiguous stimulus results in the generation of sensory predictions that facilitate perceptual inference at each recurrence of the stimulus such that the appearance of the stimulus is stabilized. In other words, when removing an ambiguous stimulus temporarily from view, perception after re-onset of the stimulus strongly tends to be the same as the last percept before stimulus removal. During 20 min, participants viewed the sphere stimulus repeatedly for short intervals of 0.6 s interleaved by blank screens of 0.8 s duration (Fig. 1A). At each stimulus reappearance, participants indicated whether they perceived the sphere rotating to the left or to the right by key presses on the computer keyboard.
To quantify the influence of sensory predictions, we calculated the survival probability of percepts across temporary stimulus removals in each participant. A lower survival probability indicates a weaker tendency towards percept stabilization and thus a weaker influence of sensory predictions in perceptual inference. Due to the relatively fast but long sequence of stimulus presentations, in some trials participants missed the required response, and we found the number of missed trials to be higher in schizophrenia patients than in healthy controls (4.2% vs 1.1% of trials [medians], z = 2.7, p < 0.01, Wilcoxon rank-sum test). This raises the possibility that between-group differences in the number of missed responses would systematically bias the results in favor of our hypothesis of lower survival probabilities in schizophrenia patients compared to healthy controls. To preclude such a type-I-error, we considered trials in which participants did not make a response as trials in which the percept had survived the preceding stimulus removal. This procedure not only seems reasonable given that in the vast majority of trials the stimulus was indeed perceived in the same configuration as in the preceding trial (see Results), but also implies that in participants with high numbers of missed responses (i.e. schizophrenia patients) survival probabilities would be rather over- than underestimated, thereby rather decreasing than inflating the hypothesized group difference between schizophrenia patients and healthy controls.
2.3. Measurement of delusional ideation
As we hypothesized that the influence of sensory predictions on perception would relate particularly to delusions, we obtained a specific measure of delusional ideation in all participants using the Peters et al. Delusions Inventory (PDI, Peters et al., 1999). This self-rating questionnaire was originally designed to measure delusional ideation in the general population, but is also an established tool for the quantitative assessment of delusions in patients with schizophrenia (Preti et al., 2007). It consists of 40 items covering the most common delusional themes including paranoia, grandiosity or experiences of reference. In addition to dichotomous yes–no statements measuring the occurrence of delusional ideas, dimensional ratings assess distress, preoccupation and conviction associated with delusional ideas.
2.4. Data analysis
Statistical analyses were performed in Matlab the Statistics Toolbox (MathWorks Inc.) using non-parametric tests as the distribution of the variable of interest, i.e. survival probability of percepts across temporary stimulus removals, deviated significantly from normality (p < 0.001, Kolmogorov–Smirnov–Lilliefors Test). To test whether the influence of sensory predictions in perceptual inference differed between schizophrenia patients and healthy controls, survival probabilities of percepts were compared in a two-sample Wilcoxon rank-sum test. To relate the influence of sensory predictions in perceptual inference to delusional ideation, we conducted correlation analyses between survival probability and PDI scores. As we expected PDI scores to differ between schizophrenia patients and healthy controls, correlation analyses were performed separately for patients and controls. To reduce the number of statistical tests, in each group we adopted a two-step approach as described previously (Schmack et al., 2013). First, an overall delusion score representing the sum of the four sub-scores of the PDI was correlated in a product–moment correlation with the survival probability of percepts. Upon significance, the most predictive sub-scales were identified in a stepwise regression analysis using forward selection and a criterion of p < 0.05. In all steps, significance was assessed by non-parametric permutation testing with 10,000 permutations.
2.5. Motion perception test
Previous work has suggested that patients with schizophrenia might be impaired at detecting coherent motion (Chen et al., 2003, Chen et al., 2005, Slaghuis et al., 2007, Stuve et al., 1997). This raises the possibility that the hypothesized lower survival probabilities in patients with schizophrenia compared to healthy controls might reflect difficulties with the perception of coherent motion rather than attenuated effects of sensory predictions. To probe this possibility, we conducted a psychophysical test to quantify each participant's threshold to perceive coherent motion, and relate it to performance in the main experiment.
To this end, we used dynamic random dot pattern that consisted of 100 grey dots moving on a black background with a velocity of 4° of visual angle per second. The stimulus was composed of a signal component – a portion of dots moving coherently towards the left or the right – and a noise component — a portion of dots moving in random directions. Both components were spatially overlaid within a circular window (diameter 4.1° of visual angle). On each trial, the stimulus was presented for 1 s, before participants had to indicate the motion direction of the signal component by key presses indicating leftward or rightward motion. If unsure, participants were instructed to make their best guess. Trials were separated by blank screens that were shown for an average period of 1 s jittered by 0.35 s.
A staircase procedure was used to determine each individual's threshold for detecting coherent motion. The procedure began with a maximal signal component of 100% coherently moving dots. Applying a three-down-one-up-rule, the percentage of coherently moving dots was decreased after three correct responses and increased after one incorrect response. Importantly, steps down were chosen smaller than steps up in order to circumvent potentially biased threshold estimates (García-Pérez, 1998). The ratio between steps down and steps up was set at 0.7393, ensuring that the staircase converged at a performance level of 83.15% (García-Pérez, 1998). Decrements and increments in motion coherence followed a logarithmic scale. The staircase was continued until eight reversals had occurred. To calculate each individual's motion perception threshold, the motion coherence proportions at the last six reversals were averaged in logarithmic space and converted to linear space. Each participant completed two subsequent staircase runs over which motion perception thresholds were averaged.
3. Results
3.1. Instable perceptual inference in patients with schizophrenia
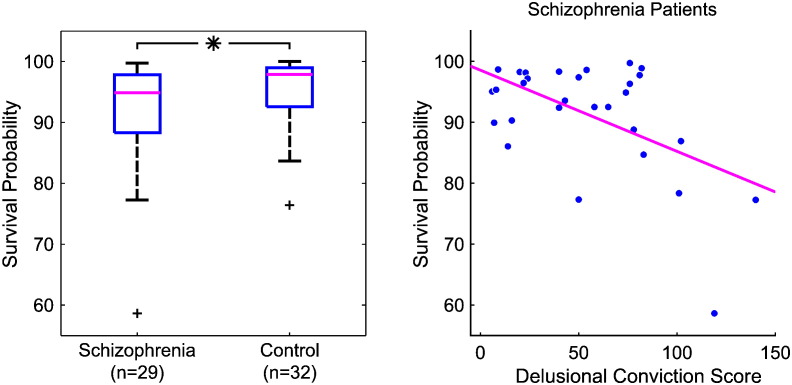
As expected, we found high percept survival probabilities across temporary stimulus removals (median 96.3%, interquartile distance 8.4%), indicating the stabilizing effect of sensory predictions on perception of the ambiguous stimulus. Remarkably, survival probabilities were significantly lower in patients with schizophrenia than in healthy controls (Fig. 2A, Z = − 2.0, p = 0.02, one-sided Wilcoxon rank-sum test), in line with our first hypothesis of a weaker influence of stabilizing sensory predictions in perceptual inference in schizophrenia.
Fig. 2.
Instable perceptual inference in schizophrenia and delusions during intermittent presentation of an ambiguous stimulus. A, The tendency towards percept stabilization as measured by survival probabilities of percepts across stimulus removals was lower in schizophrenia patients than in healthy controls (Z = − 2.0, p = 0.02, one-sided Wilcoxon rank-sum test), indicating a weaker effect of stabilizing sensory predictions in perceptual inference in schizophrenia. B, In schizophrenia patients, the tendency towards percept stabilization as measured by the perceptual survival probabilities was negatively correlated to the delusional conviction scores quantified by the PDI (r = − 0.53, p = 0.002), indicating a link between the weaker effect of stabilizing sensory predictions in perceptual inference in schizophrenia and delusions.
3.2. Delusional ideation and instable perceptual inference
We then tested whether the decreased tendency of percept stabilization in patients with schizophrenia compared to healthy controls was related to delusions. Notably, in the patient group we found a negative correlation between percept survival probabilities and the overall PDI score (r = − 0.42, p = 0.01, product–moment correlation, p-value based on 10,000 permutations). A stepwise forward linear regression showed that the dimension “delusional conviction” alone accounted best for this correlation (Fig. 2B; r = − 0.53, p = 0.002, stepwise linear forward regression, one-sided p- value based on 10,000 permutations), showing that patients with higher degrees of delusional convictions exhibited less perceptual stabilization and suggesting a link between delusions and weakened sensory predictions in accordance with our second hypothesis. No significant correlation was found between perceptual stability and the severity of psychopathological symptoms as assessed by the PANNS. In the control group, correlations between survival probabilities and PDI scores failed to show a significant relationship between perceptual stability and delusional ideation (r = 0.18, p = 0.17). Thus, we did not confirm our previous findings in a larger group of healthy individuals (Schmack et al., 2013).
We therefore further examined the PDI scores in our current cohort of healthy controls. We found that a relatively large number of participants scored zero on all four sub-scales (9 out of 32). Furthermore, all four PDI sub-scores in healthy control participants were lower than the respective PDI sub-scores in a large sample of healthy individuals from a previous study (Schmack et al., 2013) (all p < 0.05, two-sided two-sample t-tests). The differences in PDI scores could not be entirely attributed to between-sample differences in age and gender (all p < 0.08, ANCOVAs with factor sample and covariates age and gender). These observations raise the possibility that in our current study, healthy participants unconsciously or consciously biased their responses in order to appear less delusional, possibly because they were aware of the fact that they served as controls for schizophrenia patients after they had been informed beforehand that the current study was aimed at investigating the mechanisms related to schizophrenia.
3.3. Coherent motion perception
Given previous reports of impaired motion perception in schizophrenia (Chen et al., 2003, Chen et al., 2005, Slaghuis et al., 2007, Stuve et al., 1997), we then probed whether the ability to form a coherent motion percept might mediate the found relationship between decreased tendency towards percept stabilization and delusions in schizophrenia. In contrast to previous reports, we did not find a difference in motion perception thresholds between schizophrenia patients and healthy controls (Z = 0.40, p = 0.69, two-sided Wilcoxon rank-sum test). Moreover, there was no correlation between motion perception thresholds and percept survival probabilities, either in schizophrenia patients (r = 0.07, p = 0.38) or in healthy controls (r = 0.15, p = 0.22) or in all individuals together (r = 0.12, p = 0.16, product–moment correlations, p-value based on 10.000 permutations). Thus, there was no evidence for a relationship between coherent motion perception and perceptual stability.
4. Discussion
The current study aimed at investigating the effect of sensory predictions in perceptual inference in schizophrenia patients, and relating it to delusions. Using intermittent presentation of an ambiguous stimulus, we assessed patients' tendency towards percept stabilization as a marker of the influence of sensory predictions in perceptual inference. Our results show that patients with schizophrenia exhibit a reduced tendency towards percept stabilization, indicating a weakened influence of sensory predictions in perceptual inference. Furthermore, in schizophrenia patients the tendency towards percept stabilization was inversely related to the degree of delusional convictions, in accordance with the notion that attenuated sensory predictions might provide the starting point for the formation of delusions in schizophrenia (Fletcher and Frith, 2009, Hemsley, 2005, Corlett et al., 2010, Adams et al., 2013). Moreover, perceptual thresholds for coherent motion detection were not related to the tendency towards percept stabilization and did not differ between schizophrenia patients and healthy controls, suggesting the that interindividual differences in the ability to perceive coherent motion did not account for the found relationship between perceptual inference and delusions.
We have previously reported an inverse relationship between the tendency towards percept stabilization during intermittent viewing of an ambiguous stimulus and the degree of delusional convictions in healthy individuals, suggesting that varying propensities towards delusional ideation in the general population might be linked to inter-individual differences in the influence of predictions in perceptual inference (Schmack et al., 2013). Our current results failed to show a significant correlation between delusional ideation and perceptual inference in healthy individuals. We suggest that this negative finding might be attributed to the smaller sample size as well as to the healthy participants' response behavior in the delusional ideation questionnaire in our study. A post-hoc analysis of the delusional ideation scores in healthy control group suggested a bias towards reporting lower degrees of delusional ideation, presumably because participants were aware of the fact that they served as controls for patients suffering from delusions. Nevertheless, the present results showing a relationship between weakened sensory predictions in perceptual inference and delusional ideation in schizophrenia extend our previous finding of such a relationship in healthy individuals (Schmack et al., 2013). Taken together, our findings therefore provide further support for the continuity view of psychosis, which describes psychotic symptoms, such as delusions, as an extreme expression of a continuously distributed phenotype (Freeman, 2006, Meehl, 1962, Van Os et al., 2009). We thus propose that both non-clinical delusional ideation in the general population and clinical delusions in individuals suffering from schizophrenia can be explained in terms of attenuated sensory predictions during perceptual inference, such that predicted sensory events are experienced as overly surprising and salient, and the attempt to cope with these perceptual experiences results in the formation of delusional interpretations (Corlett et al., 2010, Fletcher and Frith, 2009, Hemsley, 2005). However, the exact causal mechanisms involved in attenuated predictive signaling require further investigation. On the one hand, it is conceivable that deficits in feedforward processing of sensory information lead to an inadequate build-up of predictions. On the other hand, sensory processing might be preserved but the generation of appropriate feedback predictions in higher levels of the cortical hierarchy is disturbed. Future work is needed to disentangle these two possibilities.
Our current results are in line with findings from previous studies examining perceptual inference in schizophrenia by the use of visual illusions that critically depend on endogenous predictions (Notredame et al., 2014). For instance, schizophrenia patients compared to healthy individuals have been consistently found to be less susceptible to the hollow mask illusion (Dima et al., 2009, Emrich et al., 1997, Keane et al., 2013, Koethe et al., 2006, Koethe et al., 2009, Schneider et al., 1996, Schneider et al., 2002). In this visual illusion, a face or another familiar object is presented depth-inverted (e.g. concave) but nevertheless clearly perceived in normal 3D-shape (e.g. convex), reflecting a strong effect of learned predictions overriding sensory evidence. Thus, the resistance of schizophrenia patients to this depth inversion illusion is in support of the theoretical claim of a weakened effect of predictions in perceptual inference in delusions. Further examples suggesting a link between weakened predictions and delusions include the decreased susceptibility of schizophrenia patients compared to healthy individuals to apparent and illusory motion (Crawford et al., 2010, Sanders et al., 2013), as well as the weakened effects of temporal and spatial context on visual processing in schizophrenia (Dakin et al., 2005, Fogelson et al., 2011, Joseph et al., 2013, Must et al., 2004, Seymour et al., 2013, Tadin et al., 2006, Uhlhaas et al., 2006; but see Barch et al., 2012 for an alternative explanation for weakened context effects). However, previous results regarding a direct link between the reported alterations of perceptual inference in schizophrenia and delusions have remained mixed and inconclusive. Only one study has found an inverse correlation between the effect of predictions in perceptual inference and a specific score of delusional ideation (Sanders et al., 2013), whereas other work using more general psychopathological scales indicated relationships between the observed perceptual alterations and the severity of positive (Keane et al., 2013, Uhlhaas et al., 2006), cognitive (Uhlhaas et al., 2006), and negative symptoms (Koethe et al., 2009, Tadin et al., 2006), or none of the assessed symptoms (Seymour et al., 2013). Our current results failed to show an association between the effect of predictions in perceptual inference and severity of positive, negative or general symptoms assessed by the PANSS, a general interview-based psychopathological scale. However, we were able to establish a direct relationship between altered perceptual inference and delusions by using the PDI, a specific self-rating measure for delusional ideation, thereby confirming a conceptual model that explains the formation of delusions in terms of altered predictive coding with attenuated predictions at sensory processing levels. We suggest that the fine-scaled and subjective assessment of single symptoms might be superior to coarse and objective measures of symptom dimensions when empirically probing theories of psychopathology.
In conclusion, our results show that patients with schizophrenia exhibit less perceptual stabilization during intermittent viewing of an ambiguous stimulus, and that the tendency towards perceptual stabilization decreases with increasing delusional ideation. These results support the notion that an alteration in predictive coding with a weakening of sensory predictions may be the starting point for the formation of delusions.
Role of Funding Source
This work was supported by the German Research Foundation (STE-1430/2-1 and STE-1430/7-1). The sponsor had no role in study design, collection of data, data analysis, interpretation of results, or writing of the manuscript.
Contributors
Katharina Schmack and Philipp Sterzer conceived and designed the study. Katharina Schmack, Josef Priller and Alexandra Schnack collected the data. Katharina Schmack and Alexandra Schnack analyzed the data. Katharina Schmack and Philipp Sterzer discussed the results and wrote the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
Katharina Schmack is participant in the Charité Clinical Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
References
- Adams R.A., Stephan K.E., Brown H.R., Frith C.D., Friston K.J. The computational anatomy of psychosis. Front. Psychiatry. 2013;4:47. doi: 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Carter C.S., Dakin S.C., Gold J., Luck S.J., Macdonald A. The clinical translation of a measure of gain control: the contrast–contrast effect task. Schizophr. Bull. 2012;38:135–143. doi: 10.1093/schbul/sbr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Nakayama K., Levy D., Matthysse S., Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr. Res. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Chen Y., Bidwell L.C., Holzman P.S. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr. Res. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013;36:181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Corlett P.R., Taylor J.R., Wang X.-J., Fletcher P.C., Krystal J.H. Toward a neurobiology of delusions. Prog. Neurobiol. 2010;92:345–369. doi: 10.1016/j.pneurobio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford T.J., Hamm J.P., Kean M., Schmechtig A., Kumari V., Anilkumar A.P. The perception of real and illusory motion in schizophrenia. Neuropsychologia. 2010;48:3121–3127. doi: 10.1016/j.neuropsychologia.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Dakin S., Carlin P., Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr. Biol. 2005;15:R822–R824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Dima D., Roiser J.P., Dietrich D.E., Bonnemann C., Lanfermann H., Emrich H.M. Understanding why patients with schizophrenia do not perceive the hollow-mask illusion using dynamic causal modelling. Neuroimage. 2009;46:1180–1186. doi: 10.1016/j.neuroimage.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Emrich H.M., Leweke F.M., Schneider U. Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacol. Biochem. Behav. 1997;56:803–807. doi: 10.1016/s0091-3057(96)00426-1. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C., Frith C.D. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Fogelson N., Ribolsi M., Fernandez-Del-Olmo M., Rubino I.A., Romeo D., Koch G. Neural correlates of local contextual processing deficits in schizophrenic patients. Psychophysiology. 2011;48:1217–1226. doi: 10.1111/j.1469-8986.2011.01195.x. [DOI] [PubMed] [Google Scholar]
- Freeman D. Delusions in the nonclinical population. Curr. Psychiatry Rep. 2006;8:191–204. doi: 10.1007/s11920-006-0023-1. [DOI] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez M.A. Forced-choice staircases with fixed step sizes: asymptotic and small-sample properties. Vis. Res. 1998;38:1861–1881. doi: 10.1016/s0042-6989(97)00340-4. [DOI] [PubMed] [Google Scholar]
- Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia–psychopathological and behavioral correlates. Eur. Psychiatry. 2002;17:9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- Hemsley D.R. The schizophrenic experience: taken out of context? Schizophr. Bull. 2005;31:43–53. doi: 10.1093/schbul/sbi003. [DOI] [PubMed] [Google Scholar]
- Hohwy J., Roepstorff A., Friston K. Predictive coding explains binocular rivalry: an epistemological review. Cognition. 2008;108:687–701. doi: 10.1016/j.cognition.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Joseph J., Bae G., Silverstein S.M. Sex, symptom, and premorbid social functioning associated with perceptual organization dysfunction in schizophrenia. Front. Psychol. 2013;4:547. doi: 10.3389/fpsyg.2013.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keane B.P., Silverstein S.M., Wang Y., Papathomas T.V. Reduced depth inversion illusions in schizophrenia are state-specific and occur for multiple object types and viewing conditions. J. Abnorm. Psychol. 2013;122:506–512. doi: 10.1037/a0032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten D., Mamassian P., Yuille A. Object perception as Bayesian inference. Annu. Rev. Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- Koethe D., Gerth C.W., Neatby M.A., Haensel A., Thies M., Schneider U. Disturbances of visual information processing in early states of psychosis and experimental delta-9-tetrahydrocannabinol altered states of consciousness. Schizophr. Res. 2006;88:142–150. doi: 10.1016/j.schres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Koethe D., Kranaster L., Hoyer C., Gross S., Neatby M.A., Schultze-Lutter F. Binocular depth inversion as a paradigm of reduced visual information processing in prodromal state, antipsychotic-naïve and treated schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259:195–202. doi: 10.1007/s00406-008-0851-6. [DOI] [PubMed] [Google Scholar]
- Leopold, Logothetis Multistable phenomena: changing views in perception. Trends Cogn. Sci. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- Leopold D.A., Wilke M., Maier A., Logothetis N.K. Stable perception of visually ambiguous patterns. Nat. Neurosci. 2002;5:605–609. doi: 10.1038/nn0602-851. [DOI] [PubMed] [Google Scholar]
- Meehl Paul. Schizotaxia, schizotypy, schizophrenia. Am. Psychol. 1962;17:827–838. [Google Scholar]
- Mumford D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol. Cybern. 1992;66:241–251. doi: 10.1007/BF00198477. [DOI] [PubMed] [Google Scholar]
- Must A., Janka Z., Benedek G., Kéri S. Reduced facilitation effect of collinear flankers on contrast detection reveals impaired lateral connectivity in the visual cortex of schizophrenia patients. Neurosci. Lett. 2004;357:131–134. doi: 10.1016/j.neulet.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Notredame C.-E., Pins D., Deneve S., Jardri R. What visual illusions teach us about schizophrenia. Front. Integr. Neurosci. 2014;8:63. doi: 10.3389/fnint.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach J., Ehrlich D., Heath H.A. Reversibility of the Necker Cube. I. An examination of the concept of “satiation of orientation”. Percept. Mot. Skills. 1963;17:439–458. doi: 10.2466/pms.1963.17.2.439. [DOI] [PubMed] [Google Scholar]
- Pearson J., Brascamp J. Sensory memory for ambiguous vision. Trends Cogn. Sci. (Regul. Ed.) 2008;12:334–341. doi: 10.1016/j.tics.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Peters E.R., Joseph S.A., Garety P.A. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory) Schizophr. Bull. 1999;25:553–576. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- Preti A., Rocchi M.B.L., Sisti D., Mura T., Manca S., Siddi S. The psychometric discriminative properties of the Peters et al Delusions Inventory: a receiver operating characteristic curve analysis. Compr. Psychiatry. 2007;48:62–69. doi: 10.1016/j.comppsych.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Sanders L.L.O., De Millas W., Heinz A., Kathmann N., Sterzer P. Apparent motion perception in patients with paranoid schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:233–239. doi: 10.1007/s00406-012-0344-5. [DOI] [PubMed] [Google Scholar]
- Schmack K., Gòmez-Carrillo de Castro A., Rothkirch M., Sekutowicz M., Rössler H., Haynes J.-D. Delusions and the role of beliefs in perceptual inference. J. Neurosci. 2013;33:13701–13712. doi: 10.1523/JNEUROSCI.1778-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U., Leweke F.M., Sternemann U., Weber M.M., Emrich H.M. Visual 3D illusion: a systems-theoretical approach to psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 1996;246:256–260. doi: 10.1007/BF02190277. [DOI] [PubMed] [Google Scholar]
- Schneider U., Borsutzky M., Seifert J., Leweke F.M., Huber T.J., Rollnik J.D. Reduced binocular depth inversion in schizophrenic patients. Schizophr. Res. 2002;53:101–108. doi: 10.1016/s0920-9964(00)00172-9. [DOI] [PubMed] [Google Scholar]
- Seymour K., Stein T., Sanders L.L.O., Guggenmos M., Theophil I., Sterzer P. Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology. 2013;38:2607–2612. doi: 10.1038/npp.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaghuis W.L., Holthouse T., Hawkes A., Bruno R. Eye movement and visual motion perception in schizophrenia II: global coherent motion as a function of target velocity and stimulus density. Exp. Brain Res. 2007;182:415–426. doi: 10.1007/s00221-007-1003-3. [DOI] [PubMed] [Google Scholar]
- Sterzer P., Rees G. A neural basis for percept stabilization in binocular rivalry. J. Cogn. Neurosci. 2008;20:389–399. doi: 10.1162/jocn.2008.20039. [DOI] [PubMed] [Google Scholar]
- Sterzer P., Kleinschmidt A., Rees G. The neural bases of multistable perception. Trends Cogn. Sci. (Regul. Ed.) 2009;13:310–318. doi: 10.1016/j.tics.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Stuve T.A., Friedman L., Jesberger J.A., Gilmore G.C., Strauss M.E., Meltzer H.Y. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol. Med. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- Tadin D., Kim J., Doop M.L., Gibson C., Lappin J.S., Blake R. Weakened center-surround interactions in visual motion processing in schizophrenia. J. Neurosci. 2006;26:11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Phillips W.A., Mitchell G., Silverstein S.M. Perceptual grouping in disorganized schizophrenia. Psychiatry Res. 2006;145:105–117. doi: 10.1016/j.psychres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Van Os J., Linscott R.J., Myin-Germeys I., Delespaul P., Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]