Abstract
The validity and significance of normal range neurocognition in schizophrenia remain unclear and controversial. We assessed whether normal range patients and controls demonstrate evidence of decline relative to premorbid ability and differ in performance profiles across measures, including those external to the normality criterion. In addition, we compared below normal range healthy control participants with patients at the same ability level. Performance normality was defined as a MATRICS Consensus Cognitive Battery (MCCB) composite T score between 40 and 60. Patients (n = 17) and controls (n = 24) meeting the criterion were compared on MCCB domain scores and on independent measures of reading ability, probabilistic and social reasoning. Patients (n = 19) and controls (n = 20) scoring below 40 on the MCCB composite were compared on the same set of measures. Cognitively normal range patients and controls did not differ on estimated premorbid ability or decline and differed only on the Processing Speed domain of the MCCB. Performance did not differ across other domains or on social and probabilistic reasoning tasks. Cognitively below normal range patients and controls showed marked discrepancies between premorbid and current ability, but there were no group differences. In addition, below normal range groups did not differ on any MCCB domain score or in terms of external cognitive measures. Cognitively normal range schizophrenia patients may be largely indistinguishable from normal range controls, with the exception of processing speed performance. More typical schizophrenia patients below the normal range may be indistinguishable from low-performing controls even in terms of processing speed.
Keywords: Neurocognitive normality, Schizophrenia, MATRICS consensus cognitive battery
Cognitive impairment is prevalent in schizophrenia, but substantial minorities of the patient population perform within normal limits on many standard measures (Heinrichs, 2005). Mean effect sizes (standardized group differences) for even the most sensitive tasks suggest considerable distribution overlap between patients and non-psychiatric control populations. For example, measures of processing speed are frequently impaired, but aggregated studies imply that approximately 28% of patients and controls overlap in terms of performance (Dickinson et al., 2007). In a seminal study Palmer et al. (1997) reported that 11% of their patients demonstrated no impairments across all components of a comprehensive neuropsychological test battery. Moreover, a small number of monozygotic twins with schizophrenia score as well or better than their unaffected co-twins on some cognitive measures (Goldberg et al., 1995, Torrey et al., 1994). Subsequent reports have supported the existence of variable proportions of cognitively normal and even above normal range schizophrenia patients across settings and samples (e.g. Holthausen et al., 2002, Kremen et al., 2000, Leung et al., 2008, MacCabe et al., 2012).
Nevertheless, the validity and meaning of cognitive normality findings are complicated by dubious classification criteria and the possibility of undetected or under-estimated deficits in patients meeting these criteria. For example, an average-range IQ may not represent normal cognition if performance prior to illness onset was above average. Proxy measures of premorbid ability like reading skill reveal declines when compared with current ability in many schizophrenia patients (Ammari et al., 2014, Weickert et al., 2000). It follows that differences in estimated premorbid ability may exist between patients and controls even when there are no differences in current ability. In addition, summary indices like IQ are problematic in themselves by combining subtests, thereby obscuring cognitive strengths and weaknesses (Wilk et al., 2005). This drawback can be addressed through subtest profile analysis, but recent evidence suggests that IQ measures fail to capture the true breadth of cognitive impairment expressed in schizophrenia (Gray et al., 2013). Therefore, defining cognitive normality primarily in terms of IQ values is questionable. However, even patients scoring within normal limits across multiple neuropsychological tasks may have deficiencies in executive, attention-related and motor abilities when compared directly with healthy control participants (e.g. Allen et al., 2003). Nonetheless, other studies report no deficits relative to healthy controls on any measure (Heinrichs et al., 2008) and critics of the cognitive normality concept acknowledge that “islands” of proficient function seem to exist. For example, aspects of attention, procedural memory and emotion processing may be spared in schizophrenia (Gold et al., 2009).
A further consideration involves testing the validity of cognitive normality with measures that are external to the normality criterion. It is not surprising to find performance similarity between groups on ability measures used to create the groups in the first place. Thus Muharib et al. (2014) found no differences between schizophrenia patients and controls on subtests of a cognitive battery when the composite summary score was used as a normality criterion. However, independent measures external to the criterion provide a stronger test of cognitive normality. Thus it is not known whether cognitively normal range schizophrenia patients differentiate from healthy controls on specialized tasks with sensitivity to psychotic psychopathology. Relevant tasks include measures of probabilistic thinking (Garety and Freeman, 2013) and aspects of social cognition including Theory of Mind (Mehl et al., 2010) and attribution bias (Bentall et al., 1994).
A novel and complementary approach to these issues involves the comparative study of cognitively low-performing, but psychiatrically unremarkable, control participants. For example, 25% of the general population scores below the average range on IQ measures (Wechsler, 2008), thereby approximating the performance level typically found in schizophrenia. Moreover, more than a third of neurologically normal adults perform at least a standard deviation below the mean on multiple neuropsychological test measures even with adjustment for age and education (Schretlen et al., 2008). Here again, this performance level is the most frequently reported degree of impairment observed in schizophrenia patients (Dickinson et al., 2007, Heinrichs, 2005). However, the low-performing portion of the general population is seldom accessed to establish control comparisons in schizophrenia research. Accordingly, little is known about whether differences in, say, information processing speed, persist when patients are compared with low-performing rather than high-performing healthy controls. This kind of comparison may reveal cognitive abnormalities intrinsic to schizophrenia and eliminate those that occur as a simple function of general ability level across populations. In light of these considerations we asked whether schizophrenia patients meeting or failing to meet a composite neurocognitive normality criterion differ from healthy control groups defined by the same criterion in terms of: 1) estimated premorbid ability; 2) component neurocognitive profiles; and 3) independent measures of probabilistic and social reasoning. In addition, we asked whether cognitively normal and below normal range patient groups differ in clinical status and symptom severity.
1. Methods
1.1. Participants
Patients (n = 96) were recruited from several programs in Hamilton, Ontario, Canada: the Cleghorn Early Intervention Clinic (St. Joseph’s Healthcare Hamilton), the Hamilton Program for Schizophrenia, the Schizophrenia Outpatient Clinic (St. Joseph’s Healthcare Hamilton), Schizophrenia Services of Ontario, Hamilton Chapter, Path Employment Services and the Wellington Psychiatric Outreach Program. Criteria for study entry included: 1) a diagnosis of schizophrenia or schizoaffective disorder confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996), with no concurrent diagnosis of substance use disorder; 2) a history free of developmental or learning disability; 3) history free of neurological or endocrine disorder; and 4) age 18–65. Healthy control participants (n = 144) were recruited through local newspaper and online classified advertisements for paid research participation. Interested individuals were screened for psychiatric history and substance use disorders. All participants provided written informed consent and the research was approved by institutional ethics review boards.
1.2. Measures
Standard cognitive tests forming the criterion for performance normality comprised the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008). The MCCB includes individual measures of working memory, attention, verbal memory, processing speed, reasoning and problem-solving, visual learning and social cognition and yields a composite index of overall performance.
The following adjunct cognitive tests were included. Early or premature decision-making in probabilistic reasoning (“jumping to conclusions”) was measured with the classic “beads” task described by Garety and Freeman (2013). The task requires participants to decide from which of 2 “jars” individually-presented colored “beads” were drawn. One jar contained a ratio of red to blue beads of 60:40 and a second jar contained the same ratio of blue to red beads. The number of trials or “draws” prior to decision was recorded as the dependent variable.
Two aspects of social cognition with demonstrated sensitivity to positive symptoms were also measured. Externalizing attributions for negative events were measured with the Internal, Personal and Situational Attributions Questionnaire (IPSAQ) developed and validated by Kinderman and Bentall (1996). The IPSAQ provides brief situational descriptions and elicits attributions by posing questions and providing responses in a multiple choice format. An Externalizing Bias score is calculated by subtracting the number of negative internal attributions from the number of internal positive attributions. Theory-of-Mind, or reasoning about the mental states of other people, was measured with the Reading the Mind in the Eyes test (Baron-Cohen et al., 2001). This is a visual task that involves inferring thoughts and emotions from photographs of a person’s eyes. The Reading subtest of the Wide Range Achievement Test (WRAT-4) was used to estimate premorbid cognitive ability (Johnstone and Wilhelm, 1996). In addition, a premorbid–current ability discrepancy score was created by transforming the Reading standard score into a T score and subtracting the MCCB composite T score from this value. Similar discrepancy scores have been used to index premorbid–current ability differentials by Ammari et al. (2014), Badcock et al. (2005), Harvey et al. (2006) and O’Connor et al. (2012). Clinical state and symptom severity were measured with the Positive and Negative Syndrome Scale (PANSS; Opler et al., 1999).
1.3. Group assignment procedure and data analysis
Group assignment was based on MCCB composite scores summarizing performance across 7 ability domains, with a T score of 50 ± 10 representing normative mean performance in the community standardization sample and in line with previous studies using this instrument (Kern et al., 2004, Kern et al., 2011, Muharib et al., 2014). Accordingly, the criterion for assignment to cognitively normal-range groups was an overall composite T score from 40 to 60. Application of this performance criterion to the pool of 96 patients yielded 17 with normal-range ability. Given the low prevalence (18%) of normal range MCCB performance and to ensure demographic similarity with comparison subgroups, these patients’ age range and gender proportion along with composite scores were used as criteria in assigning controls to a normal range ability group. Hence, inclusion criteria for the normal range control group were: 1) age 25–50; 2) gender ratio 50%–60% male; and 3) MCCB composite T score between 40 and 60. A total of 24 controls in the pool of 144 community-based non-psychiatric participants met these criteria. Cognitively below-normal range was defined as a composite T score from 20 to 39. Applying this criterion to the pool of community participants yielded n = 20 below normal range control participants. Demographic and psychometric data from this group were used to set inclusion criteria for the below normal range patients as follows: 1) age 20–53; 2) ratio 50%–60% males; and 3) MCCB composite T score between 20 and 39. These requirements were met by n = 19 patients who were therefore assigned to the below normal-range category.
The primary contrasts of interest in the MCCB profile analysis were between cognitively normal patients and controls and between below-normal range patients and controls rather than between normal and below-normal range groups. Therefore, and to economize on statistical testing, group differences (normal range patients versus normal range controls and below normal range patients versus below normal range controls) were assessed with t tests adjusted for multiple comparisons rather than with analysis-of-variances methods. However, analysis of adjunct cognitive measures included all four groups within a multivariate analysis-of-variance (MANOVA) approach (SPSS Version 22). This was done following Box’s tests for covariance matrix inequality and Levene tests for variance inequality.
2. Results
Table 1 shows corresponding descriptive statistics for all 4 study groups. Patient and control subgroups did not differ significantly on any variable except educational achievement. High school completion rates were lower in cognitively below normal-range patients relative to normal-range patients (χ 12 = 6.44; p < .05) and controls (χ 12 = 3.78; p = .05). However, rates were equivalent in below normal-range patients and controls and in normal range patients and controls.
Table 1.
Descriptive and criterion data for cognitively normal range (CNR) and below normal range (BNR) patients and controls.
| Variable | CNR Patients (n = 17) | CNR Controls (n = 24) | BNR Patients (n = 19) | BNR Controls (n = 20) | Statistic |
|---|---|---|---|---|---|
| Age, yrs (M, SD) | 34.47 (7.71) | 35.54 (9.34) | 38.37 (8.19) | 38.70 (8.19) | F3, 76 = 1.12 |
| High school completion (%) | 100 | 92 | 68 | 80 | χ 32 = 8.32⁎ |
| Skilled parental occupation (%) | 81 | 74 | 56 | 64 | χ 32 = 2.76 |
| Gender (males %) | 59 | 54 | 63 | 50 | χ 32 = 0.77 |
| First language English (%) | 82 | 100 | 89 | 80 | χ 32 = 5.28 |
| MCCB composite T (M, SD) | 46.94 (5.00) | 48.54 (6.11) | 25.58 (5.43) | 24.50 (9.33) | F3, 76 = 77.08⁎⁎ |
Note: MCCB = MATRICS Consensus Cognitive Battery.
p < .05.
p < .001.
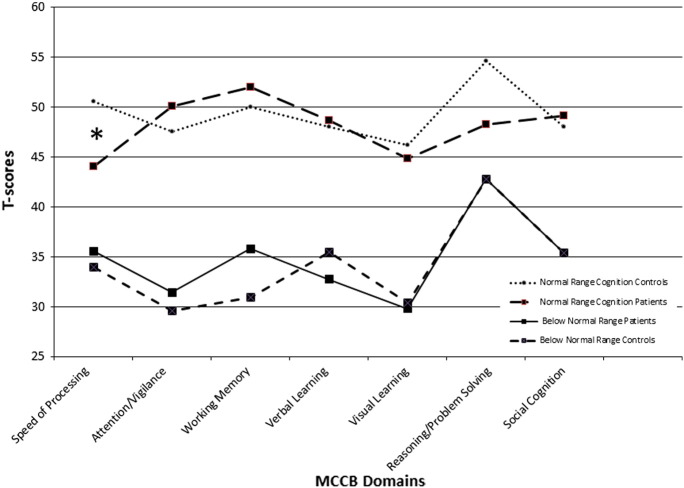
MCCB performance profiles for patient and healthy comparison groups are presented in Fig. 1. First, cognitively normal-range patients and controls were compared on each domain index T score. This revealed a significant group difference on the Processing Speed comparison (t (39) = − 2.83, p < .05) indicating lower patient scores relative to controls. In addition, 11/17 or 65% of the patients obtained T scores < 40 on this index despite meeting the normal range composite score criterion (T ≥ 40). The remaining differences between normal range patients and controls were non-significant across the Reasoning and Problem-Solving, Attention, Working Memory, Verbal Learning, Visual Learning and Social Cognition indices. The next comparison comprised below normal range patients and controls and yielded no significant group differences for any MCCB index.
Fig. 1.
Performance profiles on the MATRICS Consensus Cognitive Battery (MCCB) in schizophrenia patients and healthy control participants with MCCB composite T scores between 40 and 60 (normal range) and < 40 (below normal range). The Processing Speed difference between cognitively normal range patients and controls was significant after Bonferonni correction.
Results for adjunct cognition measures are presented in Table 2. Levene tests for error variance inequality were non-significant for each variable except Reading/MCCB Discrepancy. Therefore, data for this variable were transformed to positive values and further subjected to square root transformation. This yielded a non-significant Levene test. Box’s test of covariance matrix inequality was also non-significant. Accordingly, a multivariate analysis-of-variance (MANOVA) was carried out with group as the independent factor and WRAT Reading, Reading/MCCB Discrepancy, Reading the Mind in the Eyes total correct, “bead” task draws-to-decision and Externalizing Bias measures as dependent variables. The MANOVA indicated a significant main effect of group (F 15, 196 = 10.03, p < 0.001, partial Ƞ2 = .407). Univariate F ratios showed significant group differences for all dependent variables except Externalizing Bias. Additional testing of group means corrected for multiple comparisons revealed that cognitively normal patients and controls did not differ on any measure. Similarly, there were no differences between cognitively below normal range patients and controls. However, there were significant differences on several indices between patient and control normal range relative to both below normal range groups. This included WRAT-4 Reading/MCCB composite discrepancies, which were significantly larger in both below normal range groups. Finally, analysis of the clinical and functional status data (Table 3) for the patient groups showed no significant differences in mean Positive, Negative or General Psychopathology PANSS scores or in terms of illness length and frequency of second generation antipsychotic medication use or in living situation and employment.
Table 2.
Cognitively normal (CNR) and below normal range (BNR) patient and control group comparisons on MCCB and WRAT-4 measures.
| 1.CNR Patients (n = 17) | 2.CNR Controls (n = 24) | 3.BNR Patients (n = 19) | 4.BNR Controls (n = 20) | F (3, 75) | Post hoc Comparisons⁎ | |
|---|---|---|---|---|---|---|
| WRAT-4 Reading | 100.53 (6.17) | 101.08 (8.76) | 85.68 (8.04) | 85.00 (7.79) | 24.24⁎ | 1 > 3,4; 2 > 3,4 |
| Reading/MCCB discrepancy | 3.44 (6.91) | 2.17 (5.06) | 14.79 (6.42) | 15.42 (9.32) | 18.72⁎ | 1 < 3,4; 2 < 3,4 |
| Trials-to-decision (Beads task) | 10.29 (2.42) | 8.67 (4.56) | 6.06 (4.83) | 5.05 (3.30) | 6.86⁎ | 1 > 3,4; 2 > 4 |
| Reading the Mind in the Eyes | 25.71 (3.16) | 26.17 (3.62) | 10.68 (2.54) | 10.95 (3.33) | 12.78⁎ | 1 > 3,4; 2 > 3,4 |
| Externalizing Bias | 3.06 (4.19) | 2.62 (3.84) | 0.50 (3.13) | 2.95 (3.72) | 1.88 |
Note. MCCB = MATRICS Consensus Cognitive Battery composite T score; WRAT-4 = Wide Range Achievement Test-4 (standard score).
p < .05.
Table 3.
Patient characteristics.
| Cognitively Normal Range (n = 17) | Below-Normal Range (n = 19) | |
|---|---|---|
| Duration of illness (yrs), mean (SD) | 10.59 (8.68) | 14.96 (10.48)a |
| 2nd generation antipsychotic drugs | 13 | 11 |
| Diagnosis | ||
| schizophrenia, n (%) | 10 (59) | 12 (63) |
| schizoaffective, n (%) | 7 (41) | 7 (37) |
| PANSS Positive T score, mean (SD) | 38.82 (6.19) | 42.94 (8.12) |
| PANSS Negative T score, mean (SD) | 37.29 (7.73) | 38.50 (6.84) |
| PANSS General T score, mean (SD) | 37.65 (6.68) | 41.94 (8.03) |
| Independent living situation, n (%) | 9 (53) | 8 (42) |
| Paid employment, n (%) | 3 (18) | 4 (23)a |
Note. PANSS = Positive and Negative Syndrome Scale.
n = 17.
3. Discussion
The data presented above suggest that the study of normal range cognition is a valuable complement to the analysis of cognitive impairment in schizophrenia. We found no evidence that estimated premorbid ability distinguishes normal range patients from control participants. In addition, this similarity of estimated premorbid ability also applied to more typical cognitively impaired patients relative to controls falling below the normality criterion. However, both below normal range patients and controls had substantial discrepancies between premorbid and current abilities and differed in this respect from normal range groups. Profile comparisons of component test battery scores revealed no significant differences between cognitively normal-range patients and controls on 6 of 7 indices. The exception was the Processing Speed index, comprising timed paper-and-pencil measures of symbol coding, response generation and sequencing. In contrast, processing speed differences were not found in patients and controls with below normal-range cognition. In terms of social and probabilistic reasoning, we found no differences between patients and control participants at normal range or below normal range ability levels although, again, there were marked differences between ability levels. Finally, despite differing in cognitive performance, patient subgroups did not differ significantly in symptom severity or in other aspects of clinical and functional status.
These results are consistent with earlier reports of slowed processing speed in cognitively normal range patients relative to controls (Holthausen et al., 2002, Vaskinn et al., 2014). However, no other cognitive difference was detected in the normal range groups. In addition, to our knowledge, this is the first comparison of below normal range control participants and schizophrenia patients and the processing speed difference did not occur for these groups. It is possible that the large group differences reported in the literature reflect comparisons between cognitively impaired patients and cognitively normal rather than below normal range control participants. Nevertheless, this does not account for the special sensitivity of processing speed tasks at higher joint ability levels. Recent evidence suggests that these tasks associate specifically with white matter integrity (Karbasforoushan et al., 2015). Therefore, a further possibility is that the general cognitive deficit observed across multiple domains in most schizophrenia patients is absent or virtually absent in a minority, but with reduced connectivity and hence slowed processing persisting as a residual weakness.
Comparing normal and below-normal ability range patients with corresponding control groups invariably entails sampling from different portions of the respective population distributions and the application of arbitrary, albeit conventional, definitions of “normality.” Different definitions may yield somewhat different results (Heinrichs et al., 2013). Moreover, group comparisons of normal range patients and controls require drawing from the upper end of the patient performance distribution, but from the central tendency of controls. Conversely, comparing below-normal range patients and controls means drawing from the patient central tendency and the lower end of the general population distribution. When these distributions comprise scores from an aggregate performance index like the MCCB, any subsequent statistical comparison using sub-scores is likely to recapitulate the typology. This situation underscores the importance of group comparisons with measures not included in the normality criterion.
Several aspects of cognition not measured by the MCCB, including probabilistic reasoning (“jumping to conclusions”) and Theory of Mind processes, have been hypothesized as mediators of psychotic psychopathology, especially delusions (Garety and Freeman, 2013). Our data suggest that performance on even these specialized tasks fails to distinguish schizophrenia patients from controls at both the below-normal and normal range ability levels. This result thereby supports the validity of our typology. In addition, lack of symptomatic differentiation, especially positive symptom severity, in cognitively normal and impaired patients is reported frequently in the literature (Cobia et al., 2011, González-Blanch et al., 2010). Clinical similarity across cognitive ability is consistent with the weak or absent relations between symptom dimensions and standard cognitive performance measures observed in schizophrenia (Dominguez et al., 2009). These findings imply a psychotic disease process that exists in parallel with cognitive pathology and without substantial attenuation when cognition is relatively proficient. Nonetheless, the high prevalence of cognitive impairment in the disorder suggests that comorbidity of psychotic and cognitive pathologies is the most typical form of illness. Similar kinds of comorbidity, whereby two disorders co-occur at high rates, but vary independently in terms of their severity, appear to exist in neurology and psychiatry (Frías et al., 2014, Shindo et al., 2013).
Our study was limited by the small sample size of cognitively normal-range schizophrenia patients, a subpopulation that may comprise only 15%–20% of outpatients with the disorder. In addition, with upper ages in the 50s it is unclear whether our findings apply to older patients. However, it is notable that Leung et al. (2008) found a normality rate of 18.5% in their study of middle-aged and elderly outpatients, which compares favorably with our rate of 17%. Nevertheless, a possible consequence of small samples is inadequate power in detecting group differences in performance profiles, especially following correction procedures for multiple comparisons. Determining the validity of cognitive normality in schizophrenia highlights the challenge of adequate recruitment from a small portion of the schizophrenia patient population. It is noteworthy that relatively rare subgroups continue to receive study and provide valuable data to schizophrenia research. They include velocardiofacial syndrome (Prasad et al., 2008) and pediatric onset (Clemmensen et al., 2012) patients.
In conclusion, cognitive deficits occur very frequently in schizophrenia, but this occurrence does not prove that psychosis and deficient cognition are causally linked or mutually reducible. The data presented here show that small, but substantial numbers of patients exist with largely normal cognitive profiles, even in terms of tasks selected for their sensitivity to psychotic psychopathology. Information processing speed may be unique in its persisting weakness in this patient subpopulation. At the same time, more typical and hence impaired patients are cognitively indistinguishable from low performing healthy people on these same tasks. Moreover, the symptomatic severity of the clinical illness does not appear to differ in cognitively high and low-performing patients. Therefore our findings support and extend previous reports of cognitive normality in the literature and argue for the careful biobehavioral study of these exceptional individuals.
Role of Funding Sources
This research was supported by the Canadian Institutes of Health Research (FRN102753). The funder had no role in study design, data collection or manuscript preparation.
Contributors
RWH designed the study and wrote the manuscript draft and revision with participation of all authors. FP and EM assisted with data collection and analysis and design of tables and figures. All authors approve the final version of the manuscript.
Conflict of Interest
The authors of this manuscript have no conflicts of interest to report.
Acknowledgements
We thank Jamie Curno, Essi Numminen, Narmeen Ammari and participating clinical sites and staff at the Hamilton Program for Schizophrenia, Schizophrenia Outpatient and Cleghorn Early Intervention Clinics at St. Joseph’s Healthcare Hamilton, as well as the Hamilton chapter of Schizophrenia Services Ontario, Path Employment Services and the Wellington Psychiatric Outreach Program for their cooperation on this project.
References
- Allen D.N., Goldstein G., Warnick E. A consideration of neuropsychologically normal schizophrenia. J. Int. Neuropsychol. Soc. 2003;9:56–63. doi: 10.1017/s135561770391006x. [DOI] [PubMed] [Google Scholar]
- Ammari N., Heinrichs R.W., McDermid Vaz S., Miles A.A., Muharib E., Pinnock F. Preserved, deteriorated and premorbidly impaired patterns of intellectual ability in schizophrenia. Neuropsychology. 2014;28:353–358. doi: 10.1037/neu0000026. [DOI] [PubMed] [Google Scholar]
- Badcock J.C., Dragovic M., Waters F.A., Jablensky A. Dimensions of intelligence in schizophrenia: evidence from patients with preserved, deteriorated and preserved intellect. J. Psychol. Res. 2005;399:11–19. doi: 10.1016/j.jpsychires.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The "Reading the Mind in the Eyes" test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Bentall R.P., Kinderman P., Kaney S. The self, attributional processes and abnormal beliefs: towards a model of persecutory delusions. Behav. Res. Ther. 1994;32:331–341. doi: 10.1016/0005-7967(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Clemmensen L., Vernal D.L., Steinhausen H.C. A systematic review of the long-term outcome of early onset schizophrenia. BioMed Central Psychiatry. 2012;12:150. doi: 10.1186/1471-244X-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobia D.J., Csernansky J.G., Wang L. Cortical thickness in neuropsychologically near normal schizophrenia. Schizophr. Res. 2011;133:68–76. doi: 10.1016/j.schres.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D., Ramsey M.E., Gold J.M. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dominguez M.G., Viechtbauer W., Simons C.J.P., Van Os J., Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol. Bull. 2009;135:157–171. doi: 10.1037/a0014415. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. 1996. Structured clinical interview for DSM-IV axis I disorders: non-patient edition SCID-I/NP. New York. [Google Scholar]
- Frías Á., Palma C., Farriols N. Comorbidity in pediatric bipolar disorder: prevalence, clinical impact, etiology and treatment. J. Affect. Disord. 2014;174:378–389. doi: 10.1016/j.jad.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Garety P.A., Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br. J. Psychiatry. 2013;203:327–333. doi: 10.1192/bjp.bp.113.126953. [DOI] [PubMed] [Google Scholar]
- Gold J.M., Hahn B., Strauss G.P., Waltz J.A. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychol. Rev. 2009;19:294–311. doi: 10.1007/s11065-009-9098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg T.E., Torrey E.F., Gold J.M. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophr. Res. 1995;17:77–84. doi: 10.1016/0920-9964(95)00032-h. [DOI] [PubMed] [Google Scholar]
- González-Blanch C., Rodríguez-Sánchez J.M., Pérez-Iglesias R. First-episode schizophrenia patients neuropsychologically within the normal limits: evidence of deterioration in speed of processing. Schizophr. Res. 2010;1191–3:18–26. doi: 10.1016/j.schres.2010.02.1072. [DOI] [PubMed] [Google Scholar]
- Gray B.E., Mcmahon R.P., Gold J.M. General intellectual ability does not explain the general deficit in schizophrenia. Schizophr. Res. 2013;147:315–319. doi: 10.1016/j.schres.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D., Friedman J.I., Bowie C. Validity and stability of performance based estimates of premorbid educational functioning in older patients with schizophrenia. J. Clin. Exp. Neuropsychol. 2006;28:178–192. doi: 10.1080/13803390500360349. [DOI] [PubMed] [Google Scholar]
- Heinrichs R.W. The primacy of cognition in schizophrenia. Am. Psychol. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- Heinrichs R.W., Miles A., Smith D. Cognitive, clinical, and functional characteristics of verbally superior schizophrenia patients. Neuropsychology. 2008;22:321–328. doi: 10.1037/0894-4105.22.3.321. [DOI] [PubMed] [Google Scholar]
- Heinrichs R.W., Miles A.A., Ammari N., Muharib E. Cognition in schizophrenia as a central illness feature. In: Harvey P.D., editor. Cognitive impairment in schizophrenia: characteristics, assessment, and treatment. Cambridge University Press; New York: 2013. pp. 1–23. [Google Scholar]
- Holthausen E.A., Wiersma D., Sitskoorn M.M. Schizophrenic patients without neuropsychological deficits: subgroup, disease severity or cognitive compensation? Psychiatry Res. 2002;112:1–11. doi: 10.1016/s0165-1781(02)00184-1. [DOI] [PubMed] [Google Scholar]
- Johnstone B., Wilhelm K.L. The longitudinal stability of the WRAT-R Reading subtest: is it an appropriate estimate of premorbid intelligence? J. Int. Neuropsychol. Soc. 1996;2:282–285. doi: 10.1017/s1355617700001296. [DOI] [PubMed] [Google Scholar]
- Karbasforoushan H., Duffy B., Blackford J.U., Woodward N.D. Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychol. Med. 2015;45(1):109–120. doi: 10.1017/S0033291714001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern R.S., Green M.F., Nuechterlein K.H., Deng B. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr. Res. 2004;72:11–19. doi: 10.1016/j.schres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kern R.S., Gold J.M., Dickinson D., Green M.F., Nuechterlein K.H., Baade L.E.…Marder S.R. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr. Res. 2011;126:124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderman P., Bentall R. A new measure of causal locus: the internal, personal and situational questionnaire. Pers. Individ. Differ. 1996;20:261–264. [Google Scholar]
- Kremen W.S., Seidman L.J., Faraone S.V., Toomey R., Tsuang M.T. The paradox of normal neuropsychological function in schizophrenia. J. Abnorm. Psychol. 2000;109:743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- Leung W.W., Bowie C.R., Harvey P.D. Functional implications of neuropsychological normality and symptom remission in old outpatients with schizophrenia. J. Int. Neuropsychol. Soc. 2008;14(3):479–488. doi: 10.1017/S1355617708080600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCabe J.H., Brebion G., Reichenberg A. Superior intellectual ability in schizophrenia: neuropsychological characteristics. Neuropsychology. 2012;26:181–190. doi: 10.1037/a0026376. [DOI] [PubMed] [Google Scholar]
- Mehl S., Rief W., Lüllmann E., Ziegler M., Kesting M.L., Lincoln T.M. Are theory of mind deficits in understanding intentions of others associated with persecutory delusions? J. Nerv. Ment. Dis. 2010;198(7):516–519. doi: 10.1097/NMD.0b013e3181e4c8d2. [DOI] [PubMed] [Google Scholar]
- Muharib E., Heinrichs R.W., Miles A.A., Pinnock F., McDermid Vaz S., Ammari N. Community outcome in cognitively normal schizophrenia patients. J. Int. Neuropsychol. Soc. 2014;20:805–811. doi: 10.1017/S1355617714000629. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- O’Connor J.A., Wiffen B.D., Reichenberg A. Is deterioration of IQ a feature of first-episode psychosis and how do we measure it? Schizophr. Res. 2012;137:104–109. doi: 10.1016/j.schres.2012.01.041. [DOI] [PubMed] [Google Scholar]
- Opler L.A., Kay S.R., Lindenmayer J.P., Fiszbein A. Multi-Health Systems Inc.; North Tonawanda, NY: 1999. Structured clinical interview: the positive and negative syndrome scale SCI-PANSS. [Google Scholar]
- Palmer B.W., Keaton R.K., Paulsen J.S., Kuck J., Braff D., Harris M.J. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Prasad S.E., Howley S., Murphy K.C. Candidate genes and the behavioral phenotype in 22q11.2 deletion syndrome. Dev. Disabil. Res. Rev. 2008;14:26–34. doi: 10.1002/ddrr.5. [DOI] [PubMed] [Google Scholar]
- Schretlen D.J., Testa S.M., Winicki J.M., Pearlson G.D., Gordon B. Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. J. Int. Neuropsychol. Soc. 2008;14:436–445. doi: 10.1017/S1355617708080387. [DOI] [PubMed] [Google Scholar]
- Shindo A., Satoh M., Naito Y. Global aphasia without hemiparesis: the underlying mechanism examined by transcranial magnetic stimulation. Neurologist. 2013;19:11–14. doi: 10.1097/NRL.0b013e31827c6b95. [DOI] [PubMed] [Google Scholar]
- Torrey E.F., Bowler A.E., Taylor E.H., Gottesman I.I. Harper Collins; New York: 1994. Schizophrenia and manic–depressive disorder: the biological roots of mental illness as revealed by the landmark study of identical twins. [Google Scholar]
- Vaskinn A., Ueland T., Melle I., Agartz I., Andreassen O.A., Sundet K. Neurocognitive decrements are present in intellectually superior schizophrenia. Front. Psychiatry. 2014;5:1–9. doi: 10.3389/fpsyt.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. fourth edition. Psychological Corporation; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale. [Google Scholar]
- Weickert T.W., Goldberg T.E., Gold J.M., Bigelow L.B., Egan M.F., Weinberger D.R. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch. Gen. Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Wilk C.M., Gold J.M., McMahon R.P., Humber K., Iannone V.N., Buchanan R.W. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19:778–786. doi: 10.1037/0894-4105.19.6.778. [DOI] [PubMed] [Google Scholar]