Abstract
Schizophrenia is increasingly being viewed as a “whole brain” disorder with deficits affecting widespread cortical and subcortical networks. Within this context, studies of visual cortical function may be particularly important both because visual processing deficits directly affect social and occupational function and because these systems are well characterized at the basic science level, permitting informative translational research. This article summarizes a conference on visual processing dysfunction in schizophrenia held in Lausanne, Switzerland from June 30 to July 1, 2014 and introduces this special issue. Speakers focused on multiple aspects of visual dysfunction in schizophrenia using behavioral, neurophysiological and fMRI-based approaches.
Four main themes emerged. First was a focus on response disturbances within the early visual system, using paradigms such as sensory EEG and MEG-based responses. Second, behavioral deficits were noted in processing related to local interaction within visual regions, using paradigms such as Vernier acuity or contour integration. These deficits provided potential model systems to understand impaired connectivity within the brain in schizophrenia more generally.
Third, several visual measures were found to correlate highly with symptoms and/or neurocognitive processing. Deficits in contour integration, for example, correlated highly with conceptual disorganization, whereas perceptual instability correlated with delusion formation. These findings highlight links between perceptual-level disturbance and clinical manifestation. Finally, the potential involvement of specific neurotransmitter receptors, including N-methyl-D-aspartate (NMDA)-type glutamate receptors and alpha7 nicotinic receptors were discussed as potential etiological mechanisms. Overall, the meeting highlighted the contributions of visual pathway dysfunction to the etiopathogenesis of neurocognitive dysfunction in schizophrenia.
Keywords: Schizophrenia, Visual function, Magnocellular, Glutamate, GABA, Nicotine
1. Introduction
This special issue reviews presentations from a special workshop on “Sensory Perception & Schizophrenia,” held in Lausanne, Switzerland from June 30 to July 1, 2014, and organized by Profs. Michael Herzog and Micah Murray, and Dr. Alublena Shaqiri. The purpose of the workshop was to highlight the considerable progress that has been made in understanding the nature of the visual deficits in schizophrenia, and, perhaps more importantly, using the visual system as an opportunity to probe basic mechanisms in physiological dysfunction in schizophrenia.
An emerging consensus is that although visual system function is probably no more impaired than that of other brain regions in schizophrenia, it is probably also no less impaired. Furthermore, neural mechanisms of basic visual processing are well known and are highly amenable to translational research at least using primate models. Thus, hypotheses developed in the course of clinical investigation can be examined in animal models, whereas basic findings from animal studies can be used to develop strong, testable hypotheses regarding mechanisms underlying local circuit and neurophysiological impairments in schizophrenia.
2. Visual input dysfunction and subcortical visual systems
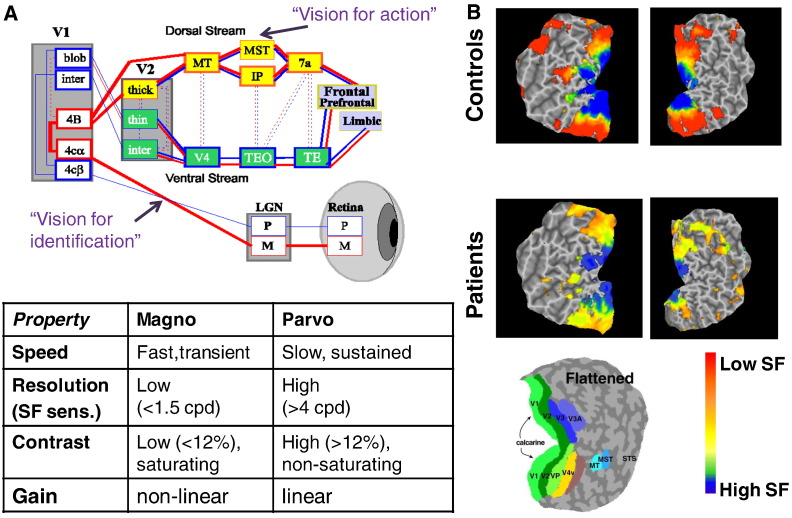
One strong theme of the conference was using neurophysiological approaches to probe basic visual function in schizophrenia. Such studies take advantage of the well-characterized functional properties of the early visual system. The subcortical visual pathways are divided into two main components, the magnocellular and parvocellular systems, which have different functional properties based upon the response properties of magnocellular and parvocellular neurons within retina and lateral geniculate nucleus (Fig. 1A). These subcortical visual pathways project preferentially to the dorsal and ventral visual streams, respectively.
Fig. 1.
A. Cartoon of the visual system showing that separate magnocellular (M) and parvocellular (P) pathways project differentially to dorsal vs. ventral visual streams. Properties of the pathways are indicated in the table.
B. Flattened map of visual cortex showing primary (V1) and secondary (V2–V4) visual regions (legend shown at bottom). Regions showing preferential response to low, medium and high spatial frequency stimulation are shown in red, yellow and blue, respectively. Schizophrenia patients show significant reductions in response to low spatial frequency stimuli (red areas), whereas response to high spatial frequency stimulation (blue regions) remains relatively intact. From Martinez et al. (2008).
Furthermore, rapid transmission of information through the magnocellular system permits rapid processing of low spatial frequency information by the dorsal stream “vision for action” system. This input both permits rapid orienting of attention to salient stimuli, and provides an organizing input to ventral stream “vision for identification” regions. Slower, higher resolution input through the ventral stream system then permits “filling in” of the detailed information that is responsible for detailed stimulus evaluation (Fig. 1A).
Javitt et al. focused on deficits in magnocellular function that have been detected using both neurophysiological (Friedman et al., 2012, Martinez et al., 2012a) and fMRI-(Martinez et al., 2008, Martinez et al., 2012a, Martinez et al., 2012b) based approaches. For example, in fMRI, highly robust deficits are observed for activation of primary visual cortex by low spatial frequency information, which primarily targets peripheral regions of primary visual cortex consistent with primary magnocellular pathway input (Fig. 1B, red regions). By contrast, activation by high spatial frequency information, which primarily targets foveal regions of primary visual cortex is relatively intact (Fig. 1B, blue regions), suggesting functional dissociation between the two pathways.
Similar neurophysiological disturbances of early function have been highlighted by Uhlhaas et al. (Rivolta et al., 2014), who investigate visual dysfunction in schizophrenia using Mooney faces. Mooney faces are two-tone images of human faces that are known to activate human face areas, but to require extensive local processing to determine whether or not a face is present. These faces induce a characteristic sequence of activations within cortex including.
It has been suggested that such deficits may arise from volumetric decreases in striate visual cortex (area 17), and may specifically reflect impairments in cortico-cortical neurotransmission (Tan et al., 2013). In contrast to first episode patients, individuals with established schizophrenia showing deficits in gamma band activity have also been detected with MEG (Grutzner et al., 2013). Thus, evolution of these findings over the course of the disorder may provide critical insights into underlying pathophysiological mechanisms.
3. Local circuit interaction within the visual system
A second strong theme was the use of visual studies to analyze mechanisms of local circuit processing within the early visual system. Herzog et al. highlighted the use of the Vernier offset paradigm to demonstrate early visual sensory deficits. In the Vernier offset paradigm, vertical lines are presented that are offset either left or right at the midpoint. Relative to controls, schizophrenia patients require a larger offset between halves to detect the direction of the offset. In general, detection of Vernier offset is thought to reflect local processing within ventral stream visual regions, such as lateral occipital cortex, supporting the concept of impaired local processing within ventral, as well as dorsal, visual stream. In EEG analysis of these deficits, reduced activation was observed at about 200 ms after stimulus onset with activity localizing primarily to LOC, supporting the localization to ventral stream, although additional deficits were observed in left posterior parietal cortex, reflecting dorsal stream activation as well. Deficits are interpreted as reflecting impaired top-down regulation of LOC from regions inferior parietal cortex and frontal eye fields (Plomp et al., 2013).
Silverstein et al. also investigated interaction among visual regions as a model for understanding more general features of disorganization in schizophrenia using paradigms involving contour integration and size contrast, and also evaluated the degree to which differences in behavior and brain activation in these paradigms differentiate groups and predict level of disorganization (Feigenson et al., 2014, Silverstein and Keane, 2011, Silverstein et al., 2013).
Murray et al. highlighted the differential visual pathways involved in Kanizsa figure processing and perceptual closure, with particular focus on the P1 potential. Deficits in P1 generation, in particular, were found to localize to left precuneus and medial inferior parietal cortex, suggesting failure of early stage dorsal stream dysfunction in schizophrenia (Knebel et al., 2011).
Tschacher et al. evaluated the integrity of “binding” in schizophrenia using paradigms in which binding was determined by either visual stimulation alone (type 1) or combined auditory/visual stimulation (type 2). Patients showed deficits only in type 2 binding, permitting refinement of disconnection hypotheses of schizophrenia related to impaired processing of Gestalt information (Tschacher and Bergomi, 2011).
4. Contribution of visual-level dysfunction to symptoms and higher order cognition
In addition to their importance as markers of local circuit dysfunction, visual deficits may contribute directly to symptoms and to cognitive functions necessary for normal social and occupational performance.
Giersch et al. highlighted the importance of temporal sequencing in both visual processing and higher order cognitive function. In the visual system, the ability to detect temporal order can be easily assessed using stimuli presented either simultaneously or with temporal offset. Patients require increased offset to detect asynchronies, suggesting that they may have more general impairments in determining temporal order that may significantly mediate difficulties in engaging in everyday events (Capa et al., 2014).
Silverstein, by contrast, highlighted the relationship between reduced perceptual organization (on 2 different tasks) and reduced conceptual organization (i.e., increased conceptual disorganization). These data support the hypothesis that impaired perceptual organization is an aspect of the disorganization syndrome, and therefore that psychophysical tests of vision may be the most easily and objectively measured biomarkers of this symptom dimension. These data also support the related hypothesis that there is a canonical cortical computation algorithm involving gain control via contextual modulation that is impaired in schizophrenia (owing to widespread circuit dysfunction rooted in changes in NMDA-receptor and GABA-ergic activity) and that account for the significantly correlated manifestations of disorganization across cognitive and behavioral domains (Phillips and Silverstein, 2003, Phillips et al., 2015).
Similarly, Sterzer et al. highlighted the importance of perceptual inference to delusion formation. In the study, response to ambiguous stimulation was used to probe the relationship between lower level perceptual instability and delusion-proneness. Increased sensitivity to delusions was associated with increased perceptual instability but also increased functional connectivity between frontal and occipital visual sensory regions (Schmack et al., 2013).
Javitt et al. have highlighted the importance of early visual functions to such processes as reading (Revheim et al., 2014), face emotion processing (Butler et al., 2009) and perceptual closure (Sehatpour et al., 2010). In all cases, failure of magnocellular input into dorsal stream regions may play a critical role in these impairments, which then lead to impairments in occupational and social function (Javitt and Freedman, 2015).
Mohr et al. investigated the utility of visual measures as endophenotypes in schizophrenia, using the shine-through visual backward masking paradigm and the Wisconsin Card Sorting Test (WCST) to test visual and higher cognitive function, respectively. Backward masking performance differentiated groups scoring high vs. low on the cognitive disorganization factor of the WCST, suggesting potential utility of the shine-through paradigm as an endophenotype in the study of schizotypy (Cappe et al., 2012).
5. Neurochemical models
A final theme touched on by several of the presentations concerned potential neurochemical theories underlying visual cortical dysfunction. Javitt et al. have focused on the importance of dysfunction of N-methyl-D-aspartate (NMDAR) type in the pathogenesis of early sensory impairments in schizophrenia, including visual sensory dysfunction (Javitt and Freedman, 2015). Glutamatergic theories of schizophrenia are based upon the observation that NMDAR antagonists such as phencylidine (PCP) or ketamine induce symptoms and neurocognitive deficits in healthy volunteers that closely resemble those of schizophrenia. Such agents function by blocking neurotransmission at NMDAR type glutamate receptors, supporting the importance of NMDAR in the pathophysiology of schizophrenia (Javitt, 1987, Javitt and Zukin, 1991).
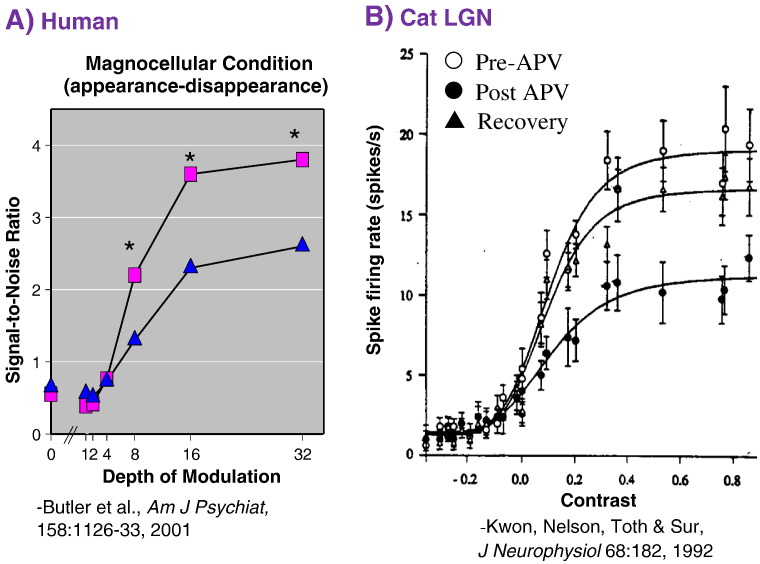
Javitt et al. highlighted the potential role of NMDAR dysfunction in the pathophysiology of early visual deficits, particularly in the reduction of non-linear gain functions within the magnocellular visual pathway (Fig. 2). These findings suggest that the differential deficits in magnocellular vs. parvocellular system function in schizophrenia may be due to the increased non-linear gain of the magnocellular vs. parvocellular visual systems, and further that failure of non-linear gain mechanisms may be a generalized failure throughout cortex in schizophrenia (Javitt and Freedman, 2015).
Fig. 2.
A. Steady-state visual evoked response (ssVEP) as a function of visual contrast in healthy volunteers.
B. Firing rate of neurons in cat lateral geniculate nucleus (LGN) as a function of contrast before and after infusion of the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV).
Uhlhaas et al. also highlighted the potential contributions of glutamatergic dysfunction to the disturbances in M100/M170 responses observed during Mooney face processing, likely through a shift in the excitatory/inhibitory balance toward excitation, potentially through NMDAR dysfunction on inhibitory interneurons (Rivolta et al., 2014).
Herzog et al. focused on the potential role of cholinergic dysfunction. Cholinergic dysfunction has been linked to schizophrenia both through the hypothesis of nicotine smoking as self-medication, as well as through observations that impairments in sensory (e.g. auditory P50) gating are associated with genetic abnormalities of the nicotinic alpha-7 receptor. Links to early visual function are supported by finding of small negative effects of smoking cessation on visual backward masking performance in habitual smokers (Kunchulia et al., 2014), as well as theoretical models.
6. Summary
Deficits in early visual function are now a consistent finding across paradigms and regions in schizophrenia. Although ideal findings are still being developed, behavioral, neurophysiological and fMRI-based approaches have yielded convergent information and critical insights into the nature of physiological impairments in schizophrenia. These deficits provide important targets as well for future interventional research. Although sensory processing was once considered an “intact simple function” in schizophrenia (Javitt, 2009), a confluence of findings continues to show deficits that are as severe as those observed in other types of cognitive function, and that contribute directly to symptoms and impaired psychosocial outcome in schizophrenia.
Role of Funding Source
Preparation of this article was funded in part by R01 MH049334 and P50 MH086385 to DCJ.
Contributors
None
Conflict of Interest
No relevant conflicts
Acknowledgements
None
References
- Butler P.D., Abeles I.Y., Weiskopf N.G., Tambini A., Jalbrzikowski M., Legatt M.E. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr. Bull. 2009;35(6):1095–1107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capa R.L., Duval C.Z., Blaison D., Giersch A. Patients with schizophrenia selectively impaired in temporal order judgments. Schizophr. Res. 2014;156(1):51–55. doi: 10.1016/j.schres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Cappe C., Herzog M.H., Herzig D.A., Brand A., Mohr C. Cognitive disorganisation in schizotypy is associated with deterioration in visual backward masking. Psychiatry Res. 2012;200(2-3):652–659. doi: 10.1016/j.psychres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Feigenson K.A., Gara M.A., Roche M.W., Silverstein S.M. Is disorganization a feature of schizophrenia or a modifying influence: evidence of covariation of perceptual and cognitive organization in a non-patient sample. Psychiatry Res. 2014;217(1-2):1–8. doi: 10.1016/j.psychres.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman T., Sehatpour P., Dias E., Perrin M., Javitt D.C. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol. Psychiatry. 2012;71(6):521–529. doi: 10.1016/j.biopsych.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzner C., Wibral M., Sun L., Rivolta D., Singer W., Maurer K., Uhlhaas P.J. Deficits in high- (> 60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front. Hum. Neurosci. 2013;7:88. doi: 10.3389/fnhum.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J. Clin. Psychiatry. 1987;9(1):12–35. [PubMed] [Google Scholar]
- Javitt D.C. Sensory processing in schizophrenia: neither simple nor intact. Schizophr. Bull. 2009;35(6):1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C., Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am. J. Psychiatry. 2015;172(1):17–31. doi: 10.1176/appi.ajp.2014.13121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C., Zukin S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Knebel J.F., Javitt D.C., Murray M.M. Impaired early visual response modulations to spatial information in chronic schizophrenia. Psychiatry Res. 2011;193(3):168–176. doi: 10.1016/j.pscychresns.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunchulia M., Pilz K.S., Herzog M.H. Small effects of smoking on visual spatiotemporal processing. Sci. Rep. 2014;4:7316. doi: 10.1038/srep07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Hillyard S.A., Dias E.C., Hagler D.J., Jr., Butler P.D., Guilfoyle D.N. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J. Neurosci. 2008;28(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Hillyard S.A., Bickel S., Dias E.C., Butler P.D., Javitt D.C. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb. Cortex. 2012;22(6):1282–1293. doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Revheim N., Butler P.D., Guilfoyle D.N., Dias E.C., Javitt D.C. Impaired magnocellular/dorsal stream activation predicts impaired reading ability in schizophrenia. NeuroImage Clin. 2012;2:8–16. doi: 10.1016/j.nicl.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W.A., Silverstein S.M. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav. Brain Sci. 2003;26(1):65–82. doi: 10.1017/s0140525x03000025. (discussion 82–137) [DOI] [PubMed] [Google Scholar]
- Phillips W.A., Clark A., Silverstein S.M. On the functions, mechanisms, and malfunctions of intracortical contextual modulation. Neurosci. Biobehav. Rev. 2015;52:1–20. doi: 10.1016/j.neubiorev.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Plomp G., Roinishvili M., Chkonia E., Kapanadze G., Kereselidze M., Brand A., Herzog M.H. Electrophysiological evidence for ventral stream deficits in schizophrenia patients. Schizophr. Bull. 2013;39(3):547–554. doi: 10.1093/schbul/sbr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N., Corcoran C.M., Dias E., Hellmann E., Martinez A., Butler P.D. Reading deficits in schizophrenia and individuals at high clinical risk: relationship to sensory function, course of illness, and psychosocial outcome. Am. J. Psychiatry. 2014;171(9):949–959. doi: 10.1176/appi.ajp.2014.13091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta D., Castellanos N.P., Stawowsky C., Helbling S., Wibral M., Grutzner C. Source-reconstruction of event-related fields reveals hyperfunction and hypofunction of cortical circuits in antipsychotic-naive, first-episode schizophrenia patients during Mooney face processing. J. Neurosci. 2014;34(17):5909–5917. doi: 10.1523/JNEUROSCI.3752-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack K., Gomez-Carrillo de Castro A., Rothkirch M., Sekutowicz M., Rossler H., Haynes J.D. Delusions and the role of beliefs in perceptual inference. J. Neurosci. 2013;33(34):13701–13712. doi: 10.1523/JNEUROSCI.1778-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P., Dias E.C., Butler P.D., Revheim N., Guilfoyle D.N., Foxe J.J., Javitt D.C. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch. Gen. Psychiatry. 2010;67(8):772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Keane B.P. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr. Bull. 2011;37(4):690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S.M., Keane B.P., Wang Y., Mikkilineni D., Paterno D., Papathomas T.V., Feigenson K. Effects of short-term inpatient treatment on sensitivity to a size contrast illusion in first-episode psychosis and multiple-episode schizophrenia. Front. Psychol. 2013;4:466. doi: 10.3389/fpsyg.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.R., Lana L., Uhlhaas P.J. High-frequency neural oscillations and visual processing deficits in schizophrenia. Front. Psychol. 2013;4:621. doi: 10.3389/fpsyg.2013.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschacher W., Bergomi C. Cognitive binding in schizophrenia: weakened integration of temporal intersensory information. Schizophr. Bull. 2011;37(Suppl. 2):S13–S22. doi: 10.1093/schbul/sbr074. [DOI] [PMC free article] [PubMed] [Google Scholar]