Abstract
Brain iron has been previously found elevated in the substantia nigra pars compacta (SNpc), but not in other brain regions, of Parkinson’s disease (PD) patients. However, iron in circulation has been recently observed to be lower than normal in PD patients. The regional selectivity of iron deposition in brain as well as the relationship between SNpc brain iron and serum iron within PD patients has not been completely elucidated. In this pilot study we measured brain iron in six regions of interest (ROIs) as well as serum iron and serum ferritin, in 24 PD patients and 27 age- gender- matched controls. Brain iron was measured on magnetic resonance imaging (MRI) with a T2 prime (T2’) method. Difference in brain iron deposition between PD cases and controls for the six ROIs were calculated. SNpc / white matter brain iron ratios and SNpc/ serum iron ratios were calculated for each study participant, and differences between PD patients and controls were tested. PD patients overall had higher brain iron than controls in the SNpc. PD patients had significantly higher SNpc / white matter brain iron ratios than controls, and significantly higher brain SNpc iron / serum iron ratios than controls. These results indicate that PD patients’ iron metabolism is disrupted toward a higher partitioning of iron to the brain SNpc at the expenses of iron in the circulation.
Keywords: Brain iron, magnetic resonance imaging, Parkinson’s disease, serum iron, serum ferritin
1. INTRODUCTION
Parkinson’s disease (PD) is a movement disorder characterized by the loss of dopaminergic neurons in the substantia nigra (SN) of the brain, which regulate voluntary movements [1]. Abnormally elevated iron accumulation in the SN of the brain has been demonstrated in post-mortem PD patients [2, 3]. In-vivo measurements of brain iron by magnetic resonance imaging (MRI) confirmed the presence of increased iron deposition in the SN [4, 5, 6] and especially in the substantia nigra pars compacta (SNpc) [7] in PD patients. MRIs measures using multiple gradient echo sequences to map proton transverse relaxation rates (R2*) were reported to correlate well with brain iron concentration from post-mortem measurements [5, 7].
Iron can produce reactive oxygen species such as hydroxyl radicals by reacting with hydrogen peroxide via the Fenton reaction. In dopaminergic neurons and glia hydrogen peroxide is generated by the action of monoamine oxidases that catabolize dopamine [8]. The oxidative stress resulting from iron and hydroxyl radical formation is believed to contribute to the neuronal degeneration that occurs in PD [9, 10]. A support in the role of iron in neuronal degeneration was observed in the rat model of PD induced by intra-nigral iron injection [11]. Iron chelators on the other hand, have been proven to protect from the neurotoxic effect of 6-hydroxydopamine in the rat model of PD [12]. Recent clinical trials with the iron chelator deferiprone showed that it was possible to achieve a decrease in SN iron in PD patients, which was correlated with a reduction in the Unified Parkinson’s Disease Rating Scale motor symptoms of PD progression, further supporting the crucial role of brain iron in PD [13].
Normally, most of the iron in the brain SN is bound to brain ferritin [14], which has a higher ratio of Heavy chain/Light chain ferritin than liver ferritin [15]. In PD patients there is a decrease of brain Light chain-ferritin as compared to controls [16]. The Light-chains of ferritin are involved in the storage of iron within the iron shell of the protein [17], therefore in PD patients there could be an easier efflux of iron from ferritin, which could trigger the Fenton reaction [18]. In normal subjects, about 20% of the iron in the SN is bound to neuromelanin, and a small amount bound to hemosiderin [19]. There is evidence that a release of iron occurs also from neuromelanin in PD patients [20].
A higher concentration of non-ferritin, labile iron in PD patients’ SN was observed by Wypijewska et al., [21]. Overall, PD patients’ SN generally shows a loss of pigmented dopaminergic neurons, decrease in neuromelanin, an increase in ferritin, and an increase in iron, including free iron [22, 23]. Ceruloplasmin is a ferroxidase enzyme involved in brain iron levels regulation that facilitates cellular iron export; hereditary aceruloplasminemia causes iron accumulation in the astrocytes, and degeneration with motor symptoms similar to parkinsonism [24]. Interestingly, even allelic variants of the ceruloplasmin gene that don’t cause complete absence of ceruloplasmin, but that alter ceruloplasmin activity [25] are associated with elevated SN brain iron content in PD patients [26], suggesting that the low ferroxidase activity that results from these variants contributes to brain iron accumulation. In this respect, peripheral infusion of ceruloplasmin was shown to attenuate neurodegeneration in a mouse model of PD [27], which suggests a therapeutic potential.
The increase in brain iron in the SNpc continues over time in PD patients, as demonstrated by Ulla et al. [28], with a study of follow up of PD patients over three years. On the other hand, the same authors reported that the levels of iron in the brain white matter decreased, over the three-year period in PD patients. Yu et al. [29], observed a significant decrease of iron levels in the temporal cortex of PD patients in the postmortem study, which was also associated with a decreased expression of divalent metal transporter 1, transferrin receptor, and ferropontin.
In spite of higher levels of brain iron in the SNpc, many studies indicate that PD patients have lower than normal levels of serum iron [30, 31]. In the study of Logroscino et al. [30] not only serum iron, but also ferritin, transferrin, total iron binding capacity, and transferrin saturation were lower among PD patients than controls; since transferrin levels were also reduced, this result suggests that in PD patients there is a defect in the systems that regulate the synthesis of major proteins in the liver, implying a systemic defect in iron metabolism [30]. Moreover, a history of recent multiple blood donations, which is a marker of systemic iron stores, was associated with PD among men, in a large longitudinal study [32]. Savica et al. [33], reported that anemia over the lifetime is positively associated with PD risk; anemia is defined as low hemoglobin levels, and iron deficiency is one of the most common causes of anemia.
Conversely, gene variants that are known to increase blood iron levels were found to be associated with a lower risk of developing PD [34].
Lower levels of total serum iron in PD patients than controls were confirmed in our previous study [31], where the drop in serum iron levels was especially pronounced among male PD patients with the haptoglobin Hp 2-1 phenotype [31]. In our previous results, however, ferritin levels were found to be higher in PD patients than controls, in contrast to what previously observed [30].
Some other contradictory results are present in the literature, where serum iron in PD patients were found to be not different from controls [35, 36].
However, the regional distribution of brain iron deposition in PD, or selective vulnerability of SN as compared to other regions of the brain, and relative changes in brain iron versus blood iron in PD patients have not been completely elucidated.
In this study we directly tested whether SNpc/white matter brain iron ratios, SNpc brain iron/ serum iron ratios, and SNpc brain iron/ serum ferritin ratios were different between PD patients and controls. Since these ratios are within-person measures, taking these ratios reduces the across-person variability that would be present by measuring correlations, therefore the ratios are more sensitive than correlations in detecting differences between PD patients and controls. Because PD patients have been shown to have higher levels of iron in the SNpc region of the brain, but not in other brain areas, in several studies (4, 5, 6, 28), we expect that PD patients will have higher SNpc/white matter brain iron ratios than controls.
And since lower blood iron has been shown to be present in PD patients than controls in some studies (30,31), our hypothesis is that SNpc brain iron/serum iron ratios will be higher in PD patients than controls.
2. METHODS
2.1 Study participants
All study participants provided written consent. All study procedures were approved by Institutional Review Boards of Bastyr University and the University of Washington. Study participants were recruited in the Puget Sound area of Washington State between March 2013 and July 2015. Recruitment sources for the PD patients were the Washington Parkinson’s Disease Registry (WPDR), the Michael J. Fox Foundation Fox Trial Finder, Bastyr University campus, the Bastyr Center for Natural Health (BCNH), Senior Centers, and referral from collaborating neurologists in the Seattle area. Control participants were recruited from the Bastyr University campus, the BCNH, Senior Centers, and advertisement over local websites.
All PD patients recruited met the “UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria” (UKBB) for PD [37]. Only patients meeting UKBB clinical criteria of diagnosis were included in the study.
For all PD cases, medical records were obtained from each patient’s neurologist and were reviewed by a second neurologist (S-C Hu) to determine whether each patient met UKBB criteria. Since this study was targeted toward idiopathic PD, patients with more than one relative with PD were excluded, as determined from a screening telephone interview before the study visit and from medical records review.
Control participants were subjects free of PD or other neurodegenerative diseases as determined from chart reviews and subject interviews.
Exclusion criteria for all participants, PD patients and controls, included: the presence of current active cancer under treatment, chronic hepatitis, HIV sero-positivity, blood donation or other reason for severe blood loss within the previous 6 months, as determined from participants’ interviews and charts reviews. Participants willing to participate in the study were carefully screened for safety for magnetic resonance imaging by administering an MRI safety screening form. Those individuals who had or were suspected to have any ferro-magnetic metal objects in their body were excluded from the study because of the risks for MRI. Presence of claustrophobia also constituted exclusion criteria for MRI.
The control group was frequency matched to cases by age in 10 year categories. Demographics and smoking status information from study participants was also collected by in-person administration of a questionnaire to study participants.
Data collection
Blood samples were collected by venipuncture from each study participant in the morning after fasting. All blood draws were performed in the morning in order to avoid possible confounding effects of diurnal variation of blood iron levels [38]. Serum was separated, split in different aliquots and frozen at −70°C. One aliquot was submitted to the LabCorp Clinical laboratory for tests of total serum iron levels and serum ferritin levels.
MRI imaging was performed for quantification of brain iron. Even if changes in brain iron with respect to the diurnal cycles have not been demonstrated [39], all participants had brain MRI in the morning, for consistency with the blood draws that were done in the morning. The MRI method used for brain iron measurements was a T2 prime (T2’) method similar to the one published by Wallis et al. [5], with some modifications. Iron deposits have been shown to shorten T2’ relaxation times as measured by MRI.
MRI imaging was performed using a Phillips 3T Achieva scanner (software version R 3.2). To calculate T2’ values, first eight-echo spin echo images were acquired, with a FOV = 240 mm × 180 mm, acquisition matrix = 288 ×216, for a voxel size of 0.83 mm × 0.83 mm. Forty-two slices were acquired, with a slice thickness of 3 mm and 0 mm slice gap. TR = 2,000 ms, and TE for the nth echo was n*12 ms. These data were fitted on a voxel by voxel basis to a mono-exponential decay model using a non-negative least squares method to create a T2 map. Similarly, a T2* map was created by acquiring eight-echo gradient echo images, with the same slice geometry and resolution as the spin echo images, but with a 3D gradient echo acquisition technique, and TR = 64 ms, and TE = n*6.9 ms. The T2* map was then calculated using the same methodology as above. To account for any possible motion between the acquisition and the T2 and T2* images, the first echo image-volume of the gradient echo acquisition was aligned to the first echo of the spin echo sequence, and the ensuing transformation matrix was used to align the T2* map with the T2 map. This reduces any mis-registration errors when combining the two measures to obtain the parametric T2’ map, using the formula:
Regions of interest (ROIs) were drawn by a board-certified neuroradiologist (YA) with 16 years’ experience, on either one of the spin echo volumes, or the derived T2 map, and transferred to the T2’ map, from which the T2’ values were determined. The neuroradiologist who placed the ROIs was blinded to the PD case or control status of the subjects, since the MRI images for each study participant were labeled with a code that did not include personal identifiers nor identifiers of the PD status.
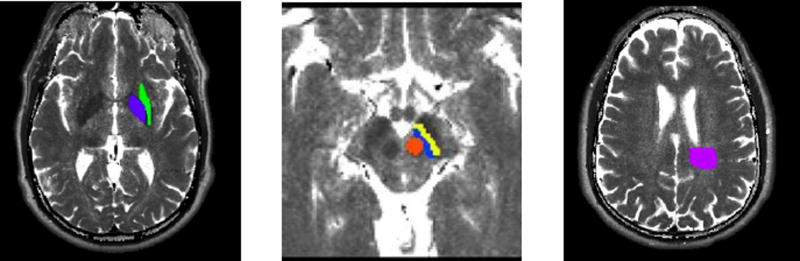
ROIs of the following brain areas were obtained from both sides of brain: Substantia Nigra pars compacta (SNpc), Substantia Nigra pars reticulata (SNpr), Red Nucleus, Globus Pallidus, White Parietal Matter, Putamen (Fig. 1).
Fig. 1.
Brain Regions of Interest (ROIs) where brain iron was measured with theT2’ method:
Calculated T2 image maps showing locations of ROIs for a. Globus pallidus (purple) and putamen (green); b. Substantia nigra pars compacta (SNpc) (blue), substantia nigra pars reticulata (SNpr) (yellow), and red nucleus (red); c. White matter (magenta).
The Parietal White Matter ROI was selected from the right parietal centrum semiovale at the superior level of lateral ventricle, immediately posterolateral to the ventricle, of approximately 3 cm in a diameter. When signal abnormality was present in the Parietal White Matter, the lesion was excluded from ROI.
Consistency of mapping of the White Matter ROI from image to image was ensured by the selection of the area immediately posterolateral to the lateral ventricle.
For the separation between the SNpc and the SNpr, a medial part was considered pars compacta, while a lateral part was considered pars reticulata. We cannot exclude some degree of imprecision in separating the SNpc from the SNpr, since the MRI images were taken at 3 T. However, we believe the degree of imprecision in separating SNpc versus SNpr does not alter the main results of the study.
For post processing analysis we used fslview software - a part of the fMRIB software library (https:fsl.fmrib.ox.ac.uk/fsl/fslwiki/)".
The averages of T2’ values obtained for each voxel of each ROI was calculated and used for statistical data analyses as below. R2’ values were calculated as 1/T2’, and reported in seconds−1. R2’ values have been previously demonstrated to correlate well with the concentration of brain iron [5, 40].
Data analysis
PD cases and controls that underwent MRI and brain iron measurements were carefully matched by age and gender, in order to ensure that the results are not affected by age or gender differences between cases and controls. Statistical analyses were performed after adjustment for age, gender, and packyears of smoking.
Means of the R2’ /sec values of brain iron for the right and left side of each ROI were calculated. The values used for statistical calculations were the means between right and left side R2’/sec for each brain ROI.
Differences in mean R2’/sec values for each ROI between PD cases and controls were tested by one-way Analysis of Covariance (ANCOVA), where the dependent variable was the R2’/sec value for a specific brain ROI, fixed factors were case/control status, and gender, and covariates were age, and packyears of smoking. We included also packyears of smoking as a covariate in the calculations because of the inverse association between smoking and PD risk that was previously observed [41].
The association between SNpc brain iron and age was calculated as the standardized beta coefficient from a regression model with SNpc brain iron as the outcome, and age as predictor variable, as well as gender and packyears of smoking as covariates, for the overall study population and separately for PD cases and controls.
SNpc/white matter brain iron ratios, as well as SNpc/serum iron ratios and SNpc/ serum ferritin ratios were calculated for each subject, and differences in these ratios between PD cases and controls were tested by ANCOVA in models that included age, gender, and packyears of smoking.
Boxplots of ratios between SNpc/white matter ratios were also presented for the PD case/control status groups.
All analyses were performed in the overall study population independently of race. One of the subjects was of Black ethnicity and one of Asian ethnicity. In separate analyses limited to subjects of White race, the results did not change materially, and thus only data for the entire study population are presented.
All data analysis was performed with SPSS 19.0 software.
3. RESULTS
3.1. Demographic characteristics
Fifty-one study participants, of which 24 were PD cases (11 men, 13 women) and 27 were controls (14 men and 13 women) underwent brain MRI for iron measurement in the ROIs and blood test for total serum iron measurement. Table 1 reports the demographic characteristics of the study participants, and the mean levels of serum iron in PD cases and controls.
Table 1.
Demographics of the study population and serum iron and ferritin levels
| PD cases | Controls | p-value | |
|---|---|---|---|
| Number, n | 24 | 27 | |
| Men, n | 11 | 14 | |
| Women, n | 13 | 13 | |
| Age, mean (SD) | 63.6 (9.0) y/o | 64.0 (9.2) y/o | 0.871 |
| Men | 62.5 (10.3) y/o | 65.4 (10.4) y/o | 0.493 |
| Women | 64.6 (8.0) y/o | 62.6 (7.8) y/o | 0.524 |
| Hoehn & Yahr stage of PD (SD) | 2.1 (0.5413) | 0 | |
| Men | 2.0 (0.522) | 0 | |
| Women | 2.2 (0.563) | 0 | |
| Packyears smoking | 3.53 | 10.47 | 0.061 |
| Men | 0.31 | 12.15 | 0.045 |
| Women | 6.25 | 8.67 | 0.613 |
| Total serum iron, μg/100 ml (SD) | 81.6 (31.9) | 100.0 (26.7) | 0.030 |
| Men | 92.5 (34.7) | 105.1 (29.3) | 0.332 |
| Women | 72.5 (27.3) | 94.5 (23.6) | 0.038 |
| Serum Ferritin, ng/ml (SD) | 110.2 (85.0) | 101.3 (60.4) | 0.665 |
| Men | 151.4 (106.3) | 115.9 (73.6) | 0.335 |
| Women | 75.3 (39.6) | 85.5 (38.9) | 0.516 |
3.2 Differences in brain iron between PD cases and controls
We observed that PD patients have significantly higher levels of brain iron in the SNpc as compared to age-matched controls. In fact PD cases SNpc R2’ was = 25.3 s−1, while SNpc R2’ for controls was = 22.6 s−1 (p=0.02). The levels of brain iron in the substantia nigra pars reticulata (SNpr), red nucleus, globus pallidus, white matter, and putamen were not significantly different between PD cases and controls. In the white matter region brain iron levels were slightly higher among controls than PD patients, however the difference was not statistically significant. The average R2’ values in the ROIs in PD cases and controls are shown in Table 2.
Table 2.
R2’/sec for brain iron (average of left and right) in the different ROIs, in PD patients and controls.
| ROIs | PD (SD) | Controls (SD) | p-Value* | |
|---|---|---|---|---|
| Overall | SNpc | 25.3 (5.4) | 22.6 (4.7) | 0.020 |
| SNpr | 26.2 (6.7) | 24.6 (5.1) | 0.225 | |
| Red nucleus | 23.7 (5.3) | 24.0 (5.5) | 0.912 | |
| Globus pallidus | 24.9 (6.3) | 24.1 (4.5) | 0.390 | |
| White matter | 11.6 (2.5) | 12.7 (2.5) | 0.150 | |
| Putamen | 19.9 (5.6) | 20.8 (5.5) | 0.903 |
p-Values were age - gender - packyears - adjusted
3.3 SNpc/ white matter brain iron ratios and differences between PD cases and controls
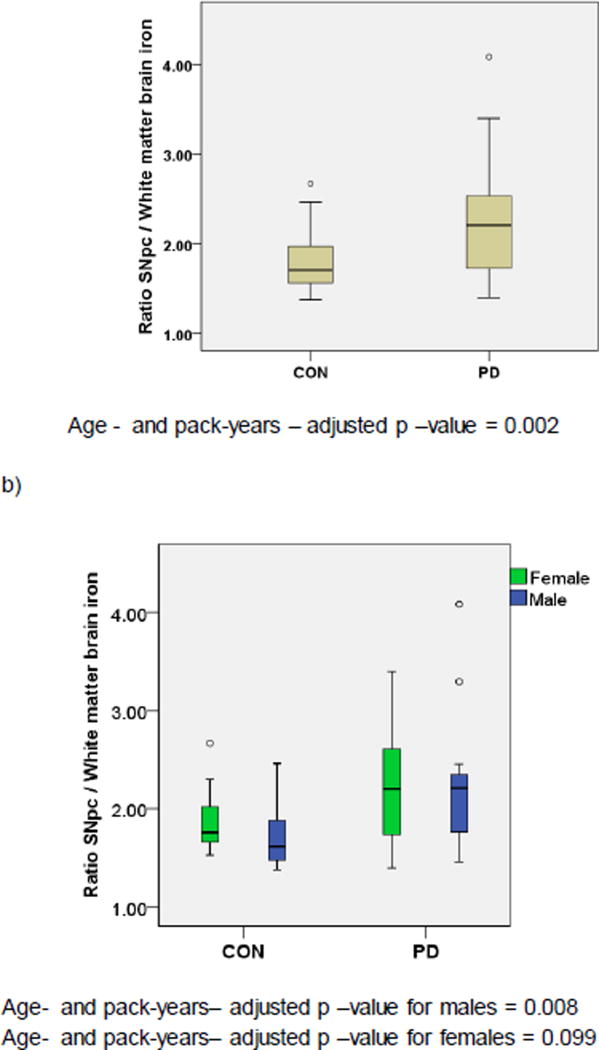
We calculated directly the SNpc/white matter brain iron ratios for each study subject, and tested for differences of these ratios between PD cases and controls. As shown in Table 3, and Fig. 2, SNpc/white matter brain iron ratios were significantly different between PD cases and controls (SNpc/white matter = 2.25 in PD cases, and = 1.80 in controls; age-, gender-, and packyears adjusted p – value = 0.002).
Table 3.
SNpc / White matter brain iron ratios.
| N. | Mean SNpc/WM brain iron |
St. deviation |
p-Value* | |
|---|---|---|---|---|
| Controls | 27 | 1.80 | 0.34 | 0.002 |
| PD | 24 | 2.25 | 0.65 | |
| Total | 51 | 2.01 | 0.55 |
p-Values were age - and packyears - adjusted.
Fig. 2.
SNpc/ white matter brain iron ratios in PD patients and controls in a) overall PD cases and controls, and b) stratified by gender
3.4 Serum iron levels and serum ferritin levels in PD cases and controls
Serum iron levels were significantly lower in PD cases (81.63 μg/100 ml) than controls (100.00 μg/100 ml, p = 0.03) (Table 1), consistently with what we had previously observed in a larger population of PD cases and controls in our previous study [31], as well as in other studies [30].
Serum ferritin levels were not significantly different between PD cases (110.2 ng/ml) and controls (101.3 ng/ml, p = 0.665). The variability of serum ferritin values was high (SD among PD = 85.0; among controls = 60.4)
3.5 SNpc brain iron/serum iron ratios and SNpc brain iron/serum ferritin ratios
Because we observed in this study that PD patients have lower serum iron than controls, but they have higher iron in the brain SNpc, we tested directly the SNpc brain iron/serum iron levels in each participant, and whether SNpc brain iron/ serum iron ratios were different between PD patients and controls. Overall, among the 51 participants, the ratio SNpc brain iron/serum iron was = 0.2901
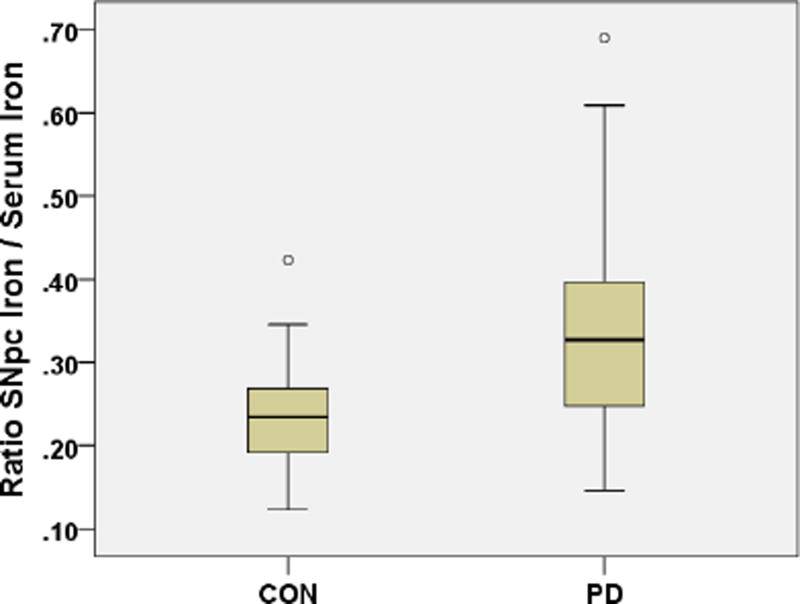
The SNpc brain iron/serum iron ratios were significantly higher in PD patients (= 0.349) as compared to controls (=0.238), p =0.001 (Fig. 3).
Fig. 3.
SNpc brain iron/serum iron ratios in PD cases and controls.
The SNpc brain iron/serum iron ratio for PD patients was = 0.349, for controls = 0.238, age- gender- packyears- adjusted p = 0.001
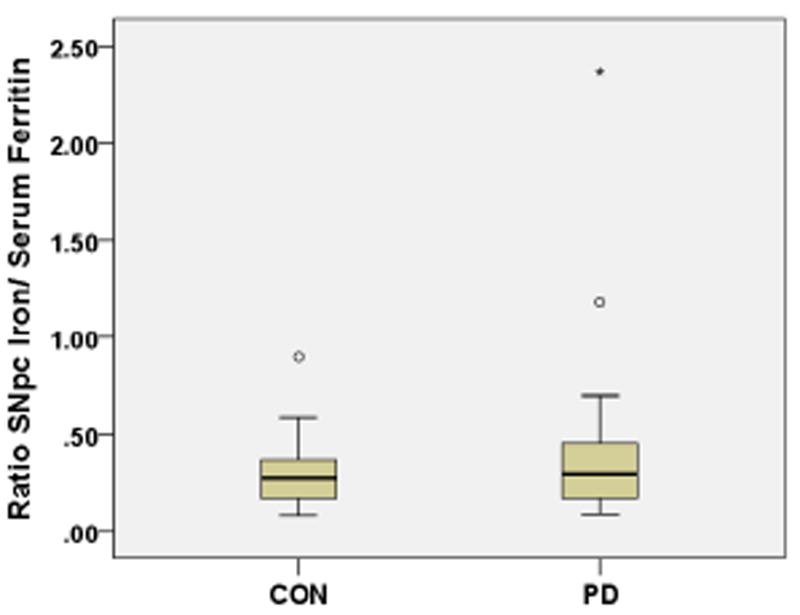
Similarly, we measured directly the ratios between SNpc brain iron/serum ferritin for each participant and differences between PD patients and controls. The SNpc brain iron/serum ferritin ratio was = 0.425 in PD patients and = 0.304 in controls (Fig. 4), however this difference between PD and control group was not statistically significant (p=0.235)
Fig. 4.
SNpc brain iron/serum ferritin ratios in PD cases and controls
The SNpc brain iron/serum ferritin ratio for PD patients was = 0.425, for controls = 0.304, age- gender- packyears- adjusted p = 0.287
4. DISCUSSION
In this study, PD patients had significantly higher iron accumulation in the SNpc than controls, as measured by T2’ MRI imaging. These results are consistent with what found in previous studies, that PD patients have higher brain iron concentrations than controls in the SN [4, 5, 6] and especially in the SNpc region [7]. In the cerebral white matter we actually observed lower levels of iron in PD patients than controls, however the difference was not statistically significant.
No significant differences in brain iron between PD cases and controls were observed in this study in the SNpr, red nucleus, globus pallidus, putamen.
In this study, the SNpc/white matter brain iron ratios were higher in PD patients than controls. Our findings that PD patients have higher level of iron in SNpc as compared to white matter suggests that a process of iron redistribution may occur in PD from serum to SNpc at the expenses of other brain areas such as cerebral white matter.
These results are consistent with the emerging evidence that other brain areas apart for the SN, may actually have lower iron deposition in PD patients than controls. In this respect the study of Yu et al. [29], reported a significant decrease in iron levels in the temporal cortex of postmortem brains of PD patients. Our results are also in agreement with what was found by Ulla et al. [28], where they observed an increase in brain iron in the SNpc in PD patients, but not in controls, over a three-year period, while the levels of iron in the brain white matter in PD patients decreased over this same three-year period.
The result of a lower brain iron in the white matter in PD is also consistent with what reported by Graham et al. [4] that observed higher levels of SNpc iron and (non-significantly) lower levels of iron in the brain white matter in PD patients.
In this study we tested direct ratios between SNpc brain iron and serum iron levels for each PD patient and controls, and showed that the ratios SNpc brain iron/serum iron were significantly higher in PD patients than controls. SNpc brain iron/serum ferritin ratios were not significantly different between the PD and control group. The high variability of ferritin levels among subjects may be at the base of the fact that the results for ferritin are not statistically significant, and a large study population will be needed to determine if SNpc/serum ferritin ratios are higher in PD patients.
To our knowledge, the SNpc brain iron/ serum iron ratios differences between PD cases and controls have never been reported before. The results from this study suggest a progressive partitioning of iron to the SNpc of the brain at the expense of the iron in circulation. This also suggests that within each PD patient, there is a disruption of the normal homeostasis between blood and brain iron, in favor of an accumulation of iron in the brain SNpc region.
Many studies point toward the direct role of iron in causing neurodegeneration. It was demonstrated that intra-cranial injection of iron can cause neuronal cell death [11, 12]. Iron has been shown to produce reactive oxygen species through the Fenton reaction in presence of hydrogen peroxide that is produced during dopamine catabolism, that can induce neuronal degeneration [10, 22]. However, the cause/effect relationship of SNpc brain iron accumulation in PD is not completely established, since there is some evidence toward brain iron accumulation following toxic insults, which could imply that iron accumulation is a consequence of the neuronal cell death in those cases. In example, dopaminergic cell death precedes iron deposition in animals treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [42]. Abnormally high levels of brain iron are observed not only in PD, but also in other neurodegenerative diseases such as in the genetic syndromes of neurodegeneration with brain iron accumulation (NBIA) [43, 24]. Several genetic causes have been recently identified for various forms of NBIA, and the mutations functionally characterized. Similarities exist between some of the forms of NBIA and the more common idiopathic PD, with the common feature being iron metabolism abnormalities that trigger oxidative damage. For two of the NBIA forms, neuroferritinopathy and aceruloplasminemia, the defective genes are directly involved in iron metabolism [44]. The study of NBIA forms may provide insights as to the link between brain iron metabolism and neurodegeneration that may have relevance also for the more common idiopathic PD [24].
Taken together, our results suggest that a process of repartitioning or redistribution of iron preferentially to the SNpc occurs in PD, that is associated with a decrease of iron levels in circulation and a loss of brain iron in regions such as the white matter. Given the relatively small number of subjects in our study, this should be regarded as a pilot study, and it will be important to follow up with further studies in larger populations of PD patients and controls.
The elucidation of the extent of the disruption in the homeostasis between brain iron and systemic iron in PD will be important for what concerns possible effects of iron supplementation in the diet for PD patients, or, conversely, the effects of systemic iron chelation therapies in PD.
Overall, the measurement brain iron by MRI as well as peripheral iron status and SNpc/ total serum iron ratios could become useful in the future to monitor relative changes in brain iron levels as compared to peripheral iron levels in PD patients. In addition to serum iron measurements as markers of peripheral iron levels, non-invasive testing of peripheral iron levels in PD patients could potentially be based on testing of saliva iron, since levels of saliva iron correlate well with levels of serum iron [45].
It is interesting to note that low levels of iron were observed also in the hair of PD patients as compared to controls by Forte et al. [46]. Since hair iron content reflects extended metal exposure [46], rather than transient levels as those found in the blood or biological fluids, the levels of hair iron content could also be investigated in the future as a potential non-invasive biomarker of peripheral iron status in PD.
In this study we did not have a large enough population of PD patients and control participants to perform meaningful associations between genetic markers of iron status and brain and serum iron levels in PD patients and controls. However, iron levels are strongly influenced by genetic factors. Interestingly, genetic variants of iron-related proteins that are associated with higher levels of serum iron seem to protect from the risk of developing PD [34]. For example, the C282Y mutation of the hemochromatosis gene HFE confers higher serum iron levels, and has been observed to reduce PD risk [47]. These results are consistent with the fact that low levels of serum iron were observed in this study as well as in previous studies, in PD patients [30, 31].
Further studies will be needed to determine the mechanisms responsible for the observed iron levels changes in the different brain areas and in circulation in PD, to distinguish between cause/effect relationships, and elucidate possible genetic influences on iron metabolism in PD.
Highlights.
PD patients have higher SNpc brain iron than controls
PD patients have higher SNpc/White Matter brain iron ratio than controls
PD patients have higher SNpc/serum iron ratio than controls
Acknowledgments
The authors gratefully acknowledge all the study participants.
This study was supported by the NIH grant # R21NS070202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, et al. Increased nigral iron content in postmorterm Parkinsonian brain. Lancet. 1987;2:1219–1220. doi: 10.1016/s0140-6736(87)91361-4. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths PD, Dobson BR, Jones GR, Clarke DT. Iron in the basal ganglia in Parkinson’s disease. An in vitro study using extended X-ray absorption fine structure and cryo-electron microscopy. Brain. 1999;122:667–673. doi: 10.1093/brain/122.4.667. [DOI] [PubMed] [Google Scholar]
- 4.Graham JM, Paley MNJ, Grünewald RA, Hoggard N, Griffiths PD. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain. 2000;123:2423–2431. doi: 10.1093/brain/123.12.2423. [DOI] [PubMed] [Google Scholar]
- 5.Wallis LI, Paley MNJ, Graham JM, Grünewald RA, Vignall EL, Joy HM, Griffiths PD. MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J. Magn. Reson. Imaging. 2008;28:1061–1067. doi: 10.1002/jmri.21563. [DOI] [PubMed] [Google Scholar]
- 6.Rossi M, Ruottinen H, Soimakallio S, Elovaara I, Dastidar P. Clinical MRI for iron detection in Parkinson’s disease. Clin. Imaging. 2013;7:631–636. doi: 10.1016/j.clinimag.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology. 2008;70:1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- 8.Götz ME, Double K, Gerlach M, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- 9.Gerlach M, Ben-Shachar D, Riederer P, Youdim MBH. Altered brain metabolism of iron as a cause of neurodegenerative disease? J. Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Shachar D, Youdim MB. Intranigral iron injection induces behavioural and biochemical “parkinsonism” in rats. J. Neurochem. 1991;57:2133–2135. doi: 10.1111/j.1471-4159.1991.tb06432.x. [DOI] [PubMed] [Google Scholar]
- 12.Youdim MB, Stephenson G, Ben Shachar D. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann. N. Y. Acad. Sci. 2004;1012:306–325. doi: 10.1196/annals.1306.025. [DOI] [PubMed] [Google Scholar]
- 13.Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garçon G, Rouaix N, et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014;21(2):195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galazka-Friedman J, Bauminer ER, Friedman A, Barcikowska M, Hechel D, Nowik I. Iron in parkinsonian and control substantia nigra – Mössbauer spectroscopy study. Mov. Disord. 1996;11:8–16. doi: 10.1002/mds.870110104. [DOI] [PubMed] [Google Scholar]
- 15.Galazka-Friedman J, Bauminger ER, Friedman A, Koziorowski D, Szlachta K. Human nigral an liver iron-comparison by Mössbauer spectroscopy, electron microscopy, and ELISA. Hyperfine Interact. 2005;165:285–288. [Google Scholar]
- 16.Connor JR, Snyder BS, Arosio P, Loeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian and Alzheimer’s diseased brains. J. Neurochem. 1995;65:717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- 17.Levi S, Santambrogio P, Cozzi A, Rovida E, Corsi B, Tamborini E, Spada S, Albertini A, Arosio P. The role of the L-chain in ferritin iron incorporation. J. Mol. Biol. 1994;238:649–654. doi: 10.1006/jmbi.1994.1325. [DOI] [PubMed] [Google Scholar]
- 18.Koziorowski D, Friedman A, Arosio P, Santambrogio P, Dziewulska D. ELISA reveals a difference in the structure of substantia nigra ferritin in Parkinson’s disease and incidental Lewy body compared to control. Park. Relat. Disord. 2007;13:214–218. doi: 10.1016/j.parkreldis.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Fasano M, Bergamasco B, Lopiano L. Modifications of the iron-neuromelanin system in Parkinson’s disease. J. Neurochem. 2006;96:909–916. doi: 10.1111/j.1471-4159.2005.03638.x. [DOI] [PubMed] [Google Scholar]
- 21.Wypijewska A, Galazka-Friedman J, Bauminger ER, Wszolek ZK, Schweitzer KJ, Dickson DW, Jaklewicz A, Elbaum D, Friedman A. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Park. Relat. Disord. 2010;16:329–333. doi: 10.1016/j.parkreldis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Gerlach M, Double KL, Youdim MBH, Riederer P. Potential sources of increased iron in the substantia nigra of parkinsonian patients. J. Neural Transm. Suppl. 2006;70:133–142. doi: 10.1007/978-3-211-45295-0_21. [DOI] [PubMed] [Google Scholar]
- 23.Lotfipour AK, Wharton S, Schwarz ST, Gontu V, Schäfer A, Peters AM, Bowtell RW, Auer DP, Gowland PA, Bajaj NP. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson’s disease. J. Magn. Reson. Imaging. 2012;35:48–55. doi: 10.1002/jmri.22752. [DOI] [PubMed] [Google Scholar]
- 24.Levi S, Finazzi D. Neurodegeneration with brain iron accumulation: update on pathogenic mechanisms. Front. Pharmacol. 2014;5:99. doi: 10.3389/fphar.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochstrasser H, Tomiuk J, Walter U, Behnke S, Spiegel J, Krüger R, Becker G, Riess O, Berg D. Functional relevance of ceruloplasmin mutations in Parkinson’s disease. FASEB J. 2005;19(13):1851–1853. doi: 10.1096/fj.04-3486fje. [DOI] [PubMed] [Google Scholar]
- 26.Hochstrasser H, Bauer P, Walster U, Behnke S, Spiegel J, Csoti I, Zeiler B, Bornemann A, Pahnke J, Becker G, Riess O, Berg D. Ceruloplasmin gene variation and substantia nigra hyperechogenicity in Parkinson disease. Neurology. 2004;63(10):1912–1917. doi: 10.1212/01.wnl.0000144276.29988.c3. [DOI] [PubMed] [Google Scholar]
- 27.Ayton S, Lei P, Duce JA, Wong BXW, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI. Ceruloplasmin dysfunction and the therapeutic potential for Parkinson disease. Ann. Neurol. 2013;73(4):554–559. doi: 10.1002/ana.23817. [DOI] [PubMed] [Google Scholar]
- 28.Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F. Is R2* a new MRI biomarker for the progression of Parkinson’ s disease? A longitudinal follow-up. PLoS One. 2013;8:3:e57904. doi: 10.1371/journal.pone.0057904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Du T, Song N, He Q, Shen Y, Jiang H, Xie J. Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease. Neurology. 2013;80:492–495. doi: 10.1212/WNL.0b013e31827f0ebb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logroscino G, Marder K, Graziano J, Freyer G, Slavkovich V, LoIacono N, Cote L, Mayeux R. Altered systemic iron metabolism in Parkinson’s disease. Neurology. 1997;49:714–717. doi: 10.1212/wnl.49.3.714. [DOI] [PubMed] [Google Scholar]
- 31.Costa-Mallen P, Zabetian CP, Agarwal P, Hu S-C, Yearout D, Samii A, Leverenz JB, Roberts JW, Checkoway H. Haptoglobin phenotype modifies serum iron levels and the effect of smoking on Parkinson disease risk. Parkinsonism Relat. Disord. 2015;21:1087–1092. doi: 10.1016/j.parkreldis.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logroscino G G, Chen HC, Wing A, Ascherio A. Blood donations, iron stores, and risk of Parkinson’s disease. Mov. Disorders. 2006;21:835–838. doi: 10.1002/mds.20826. [DOI] [PubMed] [Google Scholar]
- 33.Savica R, Grossardt BR, Carlin JM, Icen M, Bower JH, Ahlskog JE, et al. Anemia or low hemoglobin levels preceding Parkinson disease: a case-control study. Neurology. 2009;73:1381–1387. doi: 10.1212/WNL.0b013e3181bd80c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichler I, Del Greco M, Gögele M, et al. Serum iron levels and risk of Parkinson’s disease: a Mendelian randomization study. PLoS Med. 2013;10:e1001462. doi: 10.1371/journal.pmed.1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabrera-Valdivia F, Jimenez-Jimenez FJ, Molina JA, et al. Peripheral iron metabolism in patients with Parkinson’s disease. J. Neurol. Sci. 1994;125:82–86. doi: 10.1016/0022-510x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 36.Jiménez-Jiménez FJ, Molina JA, Aguilar MV, Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F, Martinez-Salio A, Ortí-Pereja M, Zurdo M, Martínez-Para MC. Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. J. Neural Transm. 1998;105:497–505. doi: 10.1007/s007020050073. [DOI] [PubMed] [Google Scholar]
- 37.Gibb WR, Lees AJ. A comparison of clinical pathological features of young- and old- onset Parkinson’s disease. Neurology. 1988;38:1402–1406. doi: 10.1212/wnl.38.9.1402. [DOI] [PubMed] [Google Scholar]
- 38.Scales WE, Vander AJ, Brown MB, Kluger MJ. Human circadian rhythms in termperature, trace metals, and blood variables. J. Appl. Physiol. 2005;65:1840–1846. doi: 10.1152/jappl.1988.65.4.1840. [DOI] [PubMed] [Google Scholar]
- 39.Allen RP, Baker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–265. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, Ruottinen H, Elovaara I, Ryymin P, Soimakallio S, Eskola H, Dastidar P. Brain Iron Deposition and Sequence Characteristics in Parkinsonism Comparison of SWI, T2* Maps, T2-Weighted-, and FLAIR-SPACE. Invest. Radiol. 2010;45:795–802. doi: 10.1097/RLI.0b013e3181ec9c96. [DOI] [PubMed] [Google Scholar]
- 41.Ritz B, Ascherio A A, Checkoway H, Marder KS, Nelson LM, Rocca WA, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch. Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 42.He Y, Thong PS, Lee T, Leong SK, Mao BY, Dong F, Watt F. Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free Radic. Biol. Med. 2003;35(5):540–547. doi: 10.1016/s0891-5849(03)00385-x. [DOI] [PubMed] [Google Scholar]
- 43.Gozzelino R, Arosio P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016;17:E130. doi: 10.3390/ijms17010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levi S, Cozzi A, Arosio P. Neuroferritionopathy: a neurodegenerative disorder associated with L-ferritin mutation. Best Pract. Res. Clin. Haematol. 2005;18:265–276. doi: 10.1016/j.beha.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Canatan D, Akdeniz SK. Iron and ferritin levels of patients with thalassemia and iron deficiency anemia. Mediter. J. Hematol. Infect. Dis. 2012;4:e2012051. doi: 10.4084/MJHID.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forte G, Alimonti A, Violante N, Di Gregorio M, Senofonte O, Petrucci F, Sancesario G, Bocca B. Calcium, copper, iron, magnesium, silicon and zinc content of hair in Parkinson’s disease. J. Trace Elem. Med. Biol. 2005;19:195–201. doi: 10.1016/j.jtemb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Xia J, Xu H, Jiang H, Xie J. The association between the C282Y and H63D polymorphism of HFE gene and the risk of Parkinson’s disease: a meta-analysis. Neurosci. Lett. 2015;595:99–103. doi: 10.1016/j.neulet.2015.04.010. [DOI] [PubMed] [Google Scholar]