Abstract
It has been hypothesized that an important function of the cerebellum is predicting the state of the body during movement. Yet, the extent of cerebellar involvement in perception of limb state (i.e. proprioception, specifically limb position sense) has yet to be determined. Here we investigated whether patients with cerebellar damage have deficits when trying to locate their hand in space (i.e. proprioceptive localization), which is highly important for everyday movements. By comparing performance during passive robot-controlled and active self-made multi-joint movements, we were able to determine that some cerebellar patients show improved precision during active movement (i.e. active benefit), comparable to controls, whereas other patients have reduced active benefit. Importantly, the differences in patient performance are not explained by patient diagnosis or clinical ratings of impairment. Furthermore, a subsequent experiment confirmed that active deficits in proprioceptive localization occur during both single-joint and multi-joint movements. As such, it is unlikely that localization deficits can be explained by the multi-joint coordination deficits occurring after cerebellar damage. Our results suggest that cerebellar damage may cause varied impairments to different elements of proprioceptive sense. It follows that proprioceptive localization should be adequately accounted for in clinical testing and rehabilitation of people with cerebellar damage.
Keywords: proprioception, upper extremity, cerebellum, ataxia
Introduction
Accurate movement control depends on our innate knowledge of limb and body position or proprioception [1–4]. Our proprioceptive sense is known to depend on multiple cerebral cortical areas, but historically, has not been thought to involve the cerebellum [5]. The cerebellum is known to receive substantial projections from the periphery reflecting joint and limb position [2,6]. These projections are thought to be important for movement control, but not needed for perception. More recent work, however, has shown cerebellar projections to cerebral sensory areas, bringing into question whether the cerebellum is involved in sensory perception [7,8]. This anatomical data is in line with behavioral and imaging studies that strongly suggest cerebellar involvement in sensory perceptions based on proprioceptive information [9–13].
Recently there have been a few papers showing specific proprioceptive deficits in people with cerebellar damage [13,14]. Cerebellar patients do not perform as well as controls on active proprioceptive discrimination tasks during an elbow joint movement [13]. Yet, these patients can perform normally on passive proprioceptive detection and discrimination tasks [13,15]. Healthy people normally show better discrimination on active tasks versus passive tasks. However, when their movements are perturbed by small forces, thus becoming less predictable, healthy people lose the benefits of active movements [13]. Taken together, these results are consistent with one idea about cerebellar function—namely that it normally acts to predict the sensory consequences of motor commands. Such a prediction would be useful for motor control as well as proprioception as it would enhance the precision with which the nervous system can estimate limb position [13,16,17].
The finding that cerebellar patients do not update sensory predictions following motor adaption (i.e. learning) tasks is also consistent with cerebellar function in predictive control [18,19]. In both Synofzik et al. [18] and Izawa et al. [19], healthy people learned a new visuomotor calibration and also recalibrated their proprioceptive estimates. In contrast, cerebellar patients exhibited a perceptual deficit which resulted in much less proprioceptive recalibration. This is in line with another study which showed that cerebellar patients had comparable proprioceptive realignment to controls during a purely sensory task, but less proprioceptive realignment during a sensorimotor task [20]. Importantly, Synofzik et al. [18] and Izawa et al. [19] both found that cerebellar patients did not have proprioceptive deficits during baseline active movements, accurately assessing the direction of their movements. It is possible that when sensory prediction updates are unnecessary (i.e. during baseline assessments), cerebellar patients do not have proprioceptive deficits when only estimating active movement direction. In contrast, when cerebellar patients have to estimate the length of their active movements, proprioceptive deficits occur [13]. This may suggest that cerebellar damage induces varied deficits in distinct aspects of proprioception, with a greater effect on movement extent than movement direction.
We are interested in further characterizing cerebellar proprioceptive deficits, with a particular focus on the ability to localize the hand in space. Proprioceptive localization requires estimates of both movement direction and movement length to pinpoint hand position. Previous studies in healthy people have shown that localization is more precise after active movements than passive movements [21–23]. Given that the cerebellum is involved in active discrimination, but does not seem to influence passive discrimination [13], it may provide a similar ‘active benefit’ in localization as well. It follows that people with cerebellar damage may exhibit deficits in active localization—specifically, they may not show the active benefit associated with healthy proprioception—yet this has not been tested previously. Knowing where the hand is in space (i.e. localization) is very important for making and calibrating real-life movements, so determining whether localization is affected in cerebellar patients will provide further insight into their motor difficulties. Here we asked if there are deficits in active versus passive proprioceptive localization in people with cerebellar damage and compared their performance to healthy individuals.
Materials and Methods
Subjects
We recruited 18 patients with cerebellar damage for a multi-joint experiment and excluded five patients from testing or analysis. One patient presented with peripheral sensory loss during the clinical examination and did not perform the experiment due to the potential for these symptoms to confound our assessment of central sensory processing. Two patients presented with double vision at the time of testing, which caused uncertainty in whether they were localizing to the correct visual dot throughout the experiment. Two additional patients completed the experiment, but had very poor passive proprioception, as identified by outlier analysis, and thus did not meet control criteria for inclusion. We retained 13 patients with cerebellar damage but no upper-limb sensory loss (6 women; mean age: 61.3 ± 12.1 years) and 13 age-, gender-, and handedness-matched controls with no known neurological impairments (6 women; mean age: 56.5 ± 10.2 years). We subsequently tested a subset of patients on a single-joint experiment, retaining 10 patients (5 women; mean age: 58.8 ± 11.9 years) and 11 matched controls (5 women; mean age: 57.2 ± 11.8 years). All subjects were tested on their dominant hands, unless the cerebellar damage only affected the non-dominant side (e.g. in the case of unilateral stroke). All subjects gave informed consent to the protocols approved by the Johns Hopkins Institutional Review Board.
The monofilament test, a standard clinical exam, assessed cutaneous mechanoreception on subject index fingertips to ensure no peripheral sensory loss [24]. All subjects in both groups were within the normal range (≤ 0.40 g) [25]. The level of cerebellar impairment was assessed with the International Cooperative Ataxia Rating Scale (ICARS) [26], which results in higher scores for more impaired patients in posture and gait, limb control (kinetic), speech, and eye movements. For the patients, the mean total ICARS score was 37.8 ± 16.0 (maximum score = 100). For the kinetic portion of the ICARS, which is relevant for arm movements, the mean subscore was 16.4 ± 6.8 (maximum subscore = 52). Additional details about cerebellar patient characteristics are listed in Table 1.
Table 1.
Subject characteristics
| Subject | Sex | Age (years) | DH | Hand Fine Touch (g) | Diagnosis | ICARS
|
|
|---|---|---|---|---|---|---|---|
| Total (/100) | Kinetic (/52) | ||||||
| CB01 | M | 77 | R | 0.40 | ADCA III | 38 | 19 |
| CB02a | M | 49 | R | 0.07 | Sporadic | 49 | 28 |
| CB03a | M | 42 | R | 0.07 | Right AVM | 19 | 7 |
| CB04a | F | 58 | R | 0.07 | SCA 6 | 40 | 16 |
| CB05a | M | 74 | R | 0.04 | Sporadic | 45 | 15 |
| CB06 | M | 74 | R | 0.07 | SCA 6 | 54 | 20 |
| CB07a | M | 39 | L | 0.07 | SCA 8 | 43 | 16 |
| CB08a | F | 63 | Rb | 0.07 | L stroke | 27 | 15 |
| CB09a | M | 60 | R | 0.16 | ADCA III | 13 | 5 |
| CB10a | F | 64 | R | 0.07 | ADCA III | 55 | 21 |
| CB11 | F | 58 | R | 0.07 | Sporadic | 32 | 17 |
| CB12a | F | 69 | R | 0.07 | ADCA II/III | 14 | 8 |
| CB13a | F | 70 | R | 0.40 | Sporadic | 63 | 26 |
| CB group | F = 6 | 61.3 ± 12.1 | L = 1 | 0.13 ± 0.12 | 37.8 ± 16.0 | 16.4 ± 6.8 | |
| CON group | F = 6 | 56.5 ± 10.2 | L = 1 | 0.25 ± 0.17 | |||
subject also completed the single-joint task;
subject was tested on the L arm due to unilateral damage;
Group data are means ± SD; CB, cerebellar patient; CON, control subject; M, male; F, female; DH, dominant hand; R, right; L, left; g, grams; ADCA, autosomal dominant cerebellar ataxia type II or type III; Sporadic, sporadic adult-onset cerebellar ataxia; AVM, cerebellar arteriovenous malformation; SCA, spinocerebellar ataxia type 6 or type 8; ICARS, International Cooperative Ataxia Rating Scale
Apparatus
For the following tasks, subjects’ proprioceptive localization was measured using the KINARM exoskeleton robot system (BKIN Technologies, Kingston, Canada). The shoulder and elbow joints of the robot were aligned with the corresponding joints on the tested arm. The subject’s arm rested in arm trays with the index finger on a Velcro square. The chair height was adjusted for each subject so arm movements occurred in the shoulder-level horizontal plane. During the test blocks, vision of the subject’s arm was blocked with a metal screen unless otherwise stated. All visual feedback was projected onto the screen surface. All subjects in both groups experienced the same conditions.
Procedure
Experiment 1: Multi-joint
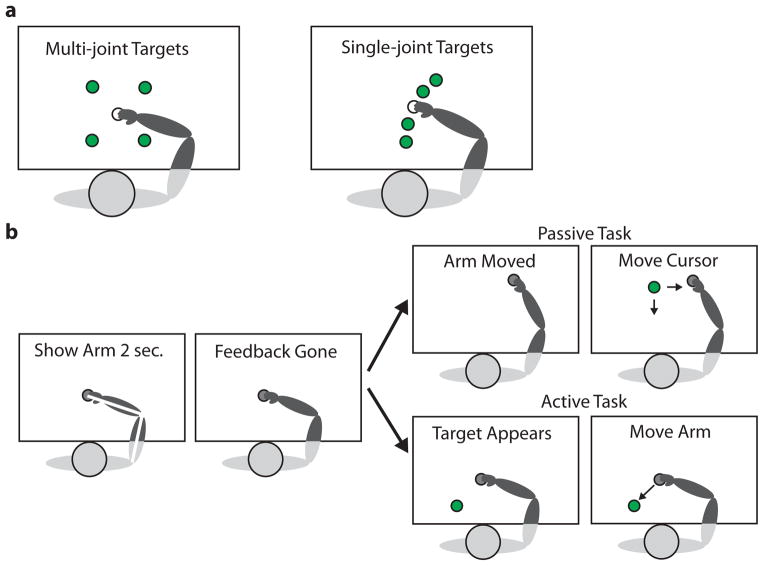
Cerebellar patients and controls completed two multi-joint proprioceptive localization tasks to compare passive robot-controlled movement with active self-movement. We tested localization of fingertip position, which requires knowledge of both shoulder and elbow joint angles and is more precise than joint angle estimation [23]. During both tasks, vision of the arm was blocked by a metal screen. At the beginning of each trial, subjects were moved to the start target, located at a shoulder angle of 60° and elbow angle of 90° for each subject. While at the start target, subjects were shown a depiction of their arm position for 2 seconds, including a white line going from their elbow to the tip of their index finger, to ensure that their estimate of arm position did not drift over time (Figure 1b).
Figure 1.
Task overview. a. Start targets (white) and end targets (green) for the multi-joint and single-joint proprioceptive localization experiments. The multi-joint start target was at shoulder angle 60° and elbow angle 90°. The end targets were (10 cm, 10 cm) away from the start target. The single-joint start target was at elbow angle 60°, with the shoulder locked at 75°. The end targets were at elbow angles 30, 45, 75, and 90°. b. Task protocol: At the beginning of each trial, the subject’s arm was moved to the unseen start target (gray) and a white stick-figure of the arm was displayed for 2 seconds. Then in the passive task the subject’s arm was moved to an unseen end target (gray) and a green cursor dot appeared. The subject controlled a joystick with the non-dominant hand to move the green cursor dot in Cartesian space as pictured (multi-joint) or along the arc of the fingertip path (single-joint). In the active task, once a green dot appeared at one of the end targets, the subject moved their arm to the visual dot. Subjects clicked a button to indicate that they aligned their fingertip and the green target dot. During the first two sets of each task, vision of the arm was blocked. In the third set, subjects completed the same task while viewing their arm.
During the passive localization task, subjects were told to relax their arm and let the robot move it. EMG signals from the arm muscles were monitored in this task. The robot then moved the subject’s arm along a smooth trajectory (constant max. velocity) to one of the four end targets, arrayed in Cartesian space, 10 cm in X and 10 cm in Y from the center target (Figure 1a). Once the subject’s index fingertip was aligned with the end target, a green visual dot (0.5 cm radius circle) appeared in a pseudo-random location 5.7 – 14 cm away from the target. Subjects used a joystick in their non-dominant hand to move the cursor dot in Cartesian space to their felt fingertip location and clicked a button to indicate their position choice.
During the active localization task, subjects were held at the start position until a green target dot (0.5 cm radius circle) appeared at one of the end targets. Then subjects moved their arm, with zero robot forces, to try to place their unseen fingertip on the target dot. Subjects were told that they could make multiple movements to get their fingertip in the correct location. Once subjects felt that their fingertip was aligned with the target, they clicked a button on the joystick to indicate their decision.
For each task, subjects completed 2 sets of 20 trials. If subjects accidentally clicked the button too soon or showed EMG activity during the passive trials, the trial was discarded. If more than two trials at an end target were discarded, subjects completed an additional set containing only the trials that were previously discarded.
Subjects then completed one last set of 8 trials with full vision of the arm. This set served as a control task, to document any visual biases and to ensure that subjects were able to manipulate the joystick to place the dot over their fingertip (passive task) and move their arm to a seen location (active task). The passive task was always completed before the active task to prevent subject exposure to seen target positions prior to the passive task. Before starting the experimental sets, subjects were given a practice set and completed trials until they understood the task and were correctly completing it.
Experiment 2: Single-joint
A subset of cerebellar patients and controls subsequently completed single-joint proprioceptive localization tasks with the same task protocols as described in Experiment 1. The shoulder was locked at 75°, so subjects only moved their forearm. Four end targets were at elbow angles 30, 45, 75, 90°, with the start target at 60° (Figure 1a). In the passive task, the green cursor dot only moved along the arc of the fingertip path and initially appeared 15–30° from the end target.
EMG Recordings
Electromyographic (EMG) signals were recorded with the Bagnoli EMG system (Delsys, Boston, MA). Surface electrodes were positioned on five muscles in the dominant arm: brachioradialis, biceps brachii, triceps brachii, pectoralis major, and posterior deltoid. An amplifier gain of 10,000 was applied, and the data was sampled at 1 kHz. EMG signals were monitored online during the multi-joint and single-joint passive localization tasks and trials with muscle activity were discarded.
Analysis
We parameterized error in all tasks by measuring both directional accuracy (bias) and precision. In the multi-joint experiment, we worked in a two-dimensional Cartesian coordinate frame and all calculations were done from a right-handed perspective. As a first step, we calculated the visual bias to the 4 targets for each subject, separately for the active and passive conditions, using the reaches where vision was present. Visual bias at each target was calculated as the average vector between the fingertip position, which was visible, and the visual dot. The visual bias was minimal in patients and controls as is reported in the results. We subtracted the visual bias from the active and passive conditions without vision for each target which allowed us to make a purer measurement of proprioceptive localization sense. Second, we calculated the proprioceptive bias to each target as the average vector between the actual fingertip position (unseen) and the perceived fingertip position (i.e. the position of the visual dot). The consistency of the bias between subjects was calculated with the Rayleigh test, which is a circular statistical method for assessing the dispersion of angular direction in polar coordinates. Low dispersion would indicate that subjects’ biases occurred in a single direction, whereas high dispersion would indicate no consistency between the biases of different subjects. In the single-joint experiment, a one-dimensional task, we calculated both visual and proprioceptive bias as the perceived elbow angle (i.e. the elbow angle based on the visual dot position) minus the actual elbow angle, again subtracting the minimal visual bias from the conditions without vision.
To assess precision in the two-dimensional space (multi-joint), we computed a 95% confidence ellipse (CE) of the average error at each target location. These can be visualized in Figure 2. The CE axes are proportional to the eigenvalues of the variance-covariance matrix, so the area of each CE encompasses the two-dimensional variance at that target [27,28]. To measure each subject’s precision, we calculated the CE area at each end target and then averaged across the four end targets. To assess precision in the one-dimensional space (single-joint), we calculated the variance in elbow angle at each of the four end targets and averaged across targets for each subject.
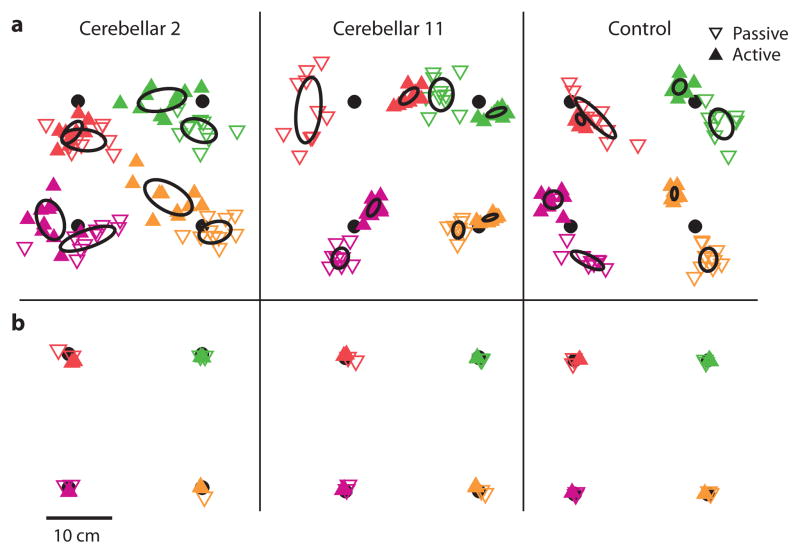
Figure 2.
Representative single subject plots for the multi-joint localization experiment. a. Cerebellar 2, from the impaired subgroup, showed no difference in precision between the passive and active task, as seen by the similar-sized 95% confidence ellipses (CE) of the average error. Cerebellar 11, from the unimpaired subgroup, and the Control had smaller CE for the active task than the passive task, indicating better active precision. b. Importantly, the cerebellar patients performed as well as the control when vision was allowed, suggesting that visual and motor deficits were not limiting factors.
We then determined whether subjects showed active benefit (i.e. improved performance in the active task compared to the passive task), which has been seen in previous proprioceptive studies [13,21–23]. However, in both of our experiments there was large between-subject variability in passive precision. In order to better compare the amount of active benefit between subjects, we normalized each subject’s active precision to their passive precision by taking a ratio of active precision over passive precision. A ratio close to 1 indicated minimal active benefit, whereas smaller ratios indicated more active benefit. To confirm that both patients and controls showed active benefit, we calculated a confidence interval of each group’s average ratio. The confidence intervals were bootstrapped using 100,000 replications, the BCa (bias corrected and accelerated percentile) method, and a 99% confidence level [29]. If the confidence interval did not contain 1, the group showed active benefit. To test whether there was a difference in active benefit between groups, we performed a t-test for independent samples.
To further investigate the differences in performance in the patient population during the multi-joint experiment, we compared each cerebellar subject’s ratio to the upper bound of the control confidence interval. If patients were below the upper bound, they were classified as unimpaired; if they were above the upper bound, they were classified as impaired. These groups were then used to compare performance within the patient group for the single-joint experiment. To determine whether the difference between groups was maintained in the single-joint experiment, we performed a nonparametric Kruskal-Wallis ANOVA (3 groups). Post hoc comparisons were done using the Mann-Whitney U test.
Results
In the first experiment we tested whether cerebellar patients and control subjects performed differently in active compared to passive proprioceptive localization in a multi-joint movement task. In the passive task, subjects had to localize their unseen fingertip by moving a visual cursor to the location where they felt their finger was moved by the robot. In the active task, subjects had to move their unseen fingertip to place it underneath a stationary visual target. Figure 2a shows sample endpoints for three subjects. A CE of the mean error at each target was calculated for individual subjects. Smaller CEs indicate better precision. Note that the CEs for the control subject were smaller for the active endpoints than the passive endpoints. In the cerebellar subjects, some showed CEs comparable to controls (Cerebellar 11), whereas others had similar-sized CEs across the two tasks (Cerebellar 2). Regardless, all subjects were able to perform localization in the full vision condition (i.e. when they could see their finger and the visual cursor; average group error with vision: patients: passive 1.15 ± 0.05 cm SE, active 0.80 ± 0.02 cm; controls: passive 0.79 ± 0.03 cm, active 0.75 ± 0.02 cm). This control condition was tested to make sure that subjects did not have any fundamental motor or visual problem that could have interfered with the passive or active proprioceptive tasks. Sample endpoints with vision are shown in Figure 2b.
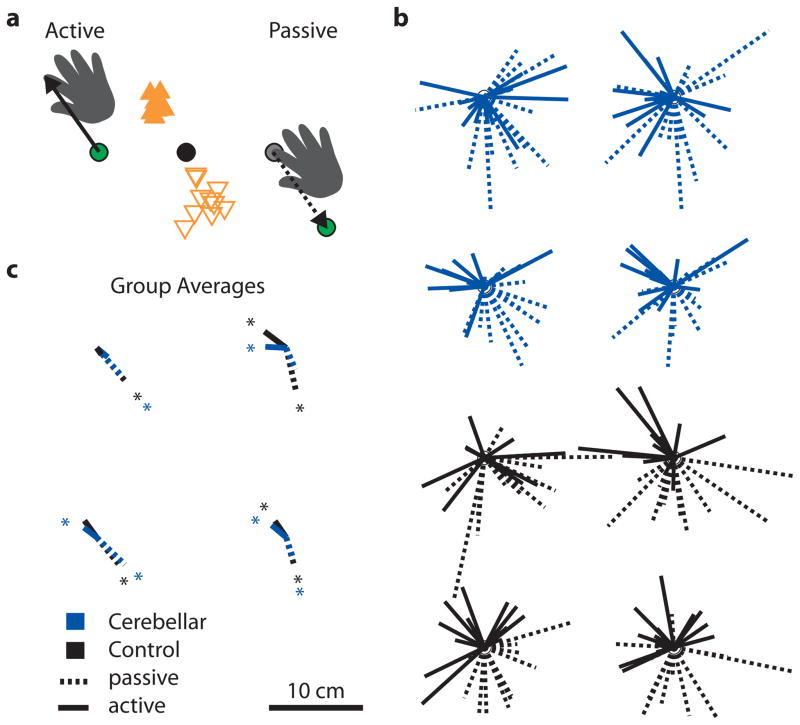
Subject bias, or directional accuracy, varied from subject to subject in both direction and extent (Figure 3b). However, averages showed a consistent directional trend across tasks and groups (Figure 3c). This was measured with the Rayleigh test, with a significant bias indicating that there was consistency between the biases of different subjects. In the passive localization task, controls had significant biases at all 4 targets (Rayleigh test, all P < 0.05), whereas patients had significant biases at 3 of the 4 targets (Rayleigh test, P < 0.05, except top right target). In the active localization task, controls had significant biases at 2 of the 4 targets (Rayleigh test, P < 0.05, trending for the bottom left target at P = 0.061), whereas patients had significant biases at 3 of the 4 targets (Rayleigh test, P < 0.05, except top left target). Furthermore, there was no substantial difference in directional biases between cerebellar patients and controls for any of the four targets in either the passive or active localization tasks (Figure 3c). Note that all bias was calculated from the right-handed perspective. In addition, bias vectors ran in opposite directions for the passive and active tasks—subjects moved the index finger too far and to the left in the active case, and moved the cursor too short and to the right in the passive case (Figure 3a). This means that subjects tended to think their fingertip was closer to them and rightward of their actual fingertip position (Figure 3c). This is consistent with previous studies reporting bias [30,31].
Figure 3.
Directional accuracy (bias) across targets for the multi-joint localization experiment. a. Bias was calculated as the average vector between the actual fingertip position (unseen) and the perceived fingertip position (i.e. the position of the green visual dot— target or cursor depending on the task). In the active task, subjects were asked to move their fingertip directly under the green target dot. They tended to move the index finger too far and further left of the green target dot, which suggests that they thought their hand was closer to them and to the right. In the passive task, subjects moved the green cursor dot too short and further to the right of their stationary fingertip. This also shows that they thought their hand was closer to them and to the right. Thus, the bias is the same for the passive and active tasks, even though the bias vectors point in opposite directions. b. Each vector represents an individual cerebellar patient’s (blue) or control subject’s (black) average bias for that target. c. Average group bias was consistent across the target workspace and across patients and controls. On average, subjects thought their fingertip was closer to them and rightward of its actual position (right-handed perspective). *P < 0.05.
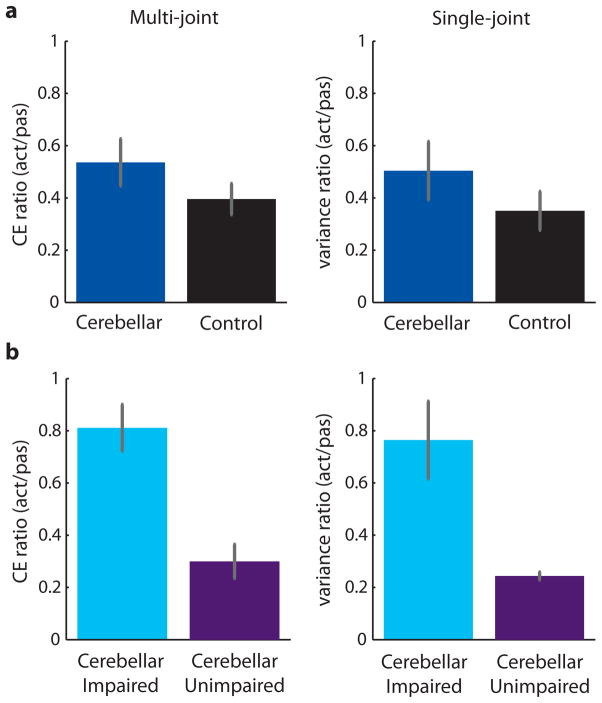
Figure 4 shows the precision measurement for every subject tested in the passive (Figure 4a) and active (Figure 4b) conditions. In the passive precision (open bars) there was no significant difference between cerebellar patients and controls (t-test, t(24) = 1.14, P > 0.26). However, there was a significant difference in active precision (solid bars) between cerebellar patients and controls (t-test, t(24) = 2.38, P < 0.05). Given that our experimental aim was to assess whether patients and controls exhibited differences in the amount of active benefit, their localization performance in the active task was normalized to their passive performance by taking a ratio of the average CE area for the active task compared to the passive task (Figure 4c). All but two control subjects had a ratio below 0.50—they had much better precision on the active task, as expected [22,23]. The two subjects with higher CE ratios also had the best passive precision, so it is possible that they hit a ceiling on their performance. In other words, rather than indicating lower active benefit, the higher CE ratios reflected better passive precision. The cerebellar patients had more variable CE ratios, ranging from 0.15 to 1.17, with 11 of the 13 patients below 0.82. Thus, both groups showed clear active benefit, with the upper bound of the confidence interval below 1 (controls: 0.60, patients: 0.78). In addition, there was no significant difference in CE ratio between the patients and controls (t-test, t(24) = 1.30, P > 0.20; Figure 5a). We did not expect any patients to show active benefit, yet the majority of patients had at least some active benefit. However, this did not seem to fully quantify what occurred in the patients. While it is clear that about half of the patients had active benefit to the same level as controls, other patients did not have as much active benefit.
Figure 4.
Individual subject precision for the multi-joint localization experiment. a. Subject precision (average CE area) for the passive task. Both the cerebellar patients (blue) and the control subjects (black) showed varying passive precision. b. Subject precision for the active task. Most subjects showed some improvement when moving actively compared to being moved passively. c. Normalized active benefit to each subject’s passive precision. The CE ratio was calculated as active precision over passive precision, with values close to 1 indicating no active benefit and smaller values indicating more active benefit. Patients showed more variability in CE ratio than controls. The upper bound of the control group’s 99% confidence interval (red dotted line) was used to split patients into two subgroups. Patients over the line were impaired (n = 6), whereas patients under the line were unimpaired (n = 7).
Figure 5.
Group averages of normalized active benefit for the multi-joint and single-joint localization experiments. a. Normalized active benefit with all cerebellar patients grouped together. There was no significant difference between patients and controls for either the multi-joint or single-joint experiments (all P > 0.20). In addition, all confidence intervals of the group averages had an upper bound below 1, indicating that patients and controls showed active benefit in both experiments. b. Normalized active benefit for the cerebellar patients when split into two subgroups. In the multi-joint experiment, the impaired subgroup (n = 6) was outside the control confidence interval for normalized active benefit, whereas the unimpaired subgroup (n = 7) was inside the confidence interval. In the single-joint experiment, subjects were grouped according to the multi-joint split. The impaired subgroup (n = 5) was significantly worse than the unimpaired subgroup (n = 5) and the controls (all P < 0.03). Error bars indicate SEM.
To look at this within group difference, we classified patients into two subgroups by comparing them to the upper bound of the control subject confidence interval (red dotted line at 0.60) around the average CE ratio (Figure 4c). Seven patients had a ratio below the upper bound—they had active benefit comparable to controls and were unimpaired. Six patients had a ratio above the upper bound—they had reduced active benefit compared to controls and were impaired. The split group averages showed that there were distinct differences in performance within the patient group (Figure 5b). Despite differences in active benefit among the patients, there was no particular diagnosis or distinguishing symptom identifying those with reduced active benefit. Specifically, ICARS scores (total and kinetic) were not correlated with CE ratio (all P > 0.81). In addition, CE ratio was not correlated with age for either the patients or the control subjects (all P > 0.15).
To verify that these results were not explained by the difficulty cerebellar patients have with inter-joint coordination [32], we did a second experiment using single-joint movement. Here, the shoulder was locked in place, leaving only the elbow joint free to move. Subjects were again asked to localize their fingertip during both passive and active movement. In the passive task, subjects moved a cursor constrained along an arc to their unseen fingertip. In the active task, subjects moved their forearms to a stationary visual target. As in the multi-joint task, all subjects were able to perform the single-joint localization task with full vision (Average group error with vision: patients: passive 0.88 ± 0.09° SE, active −0.76 ± 0.08°; controls: passive 0.21 ± 0.07°, active 0.05 ± 0.07°). Single-joint bias varied across targets, which is consistent with a previous localization study [23]. Control subjects exhibited the expected bias (passive bias at elbow angle targets 30, 45, 75, and 90°: −5.76 ± 0.58° SE, −5.20 ± 0.46°, 3.58 ± 0.49°, 3.87 ± 0.51°; active: −4.52 ± 0.26°, −1.42 ± 0.24°, 0.99 ± 0.45°, 4.20 ± 0.55°), with overextension to extension targets (i.e. negative error to target angles 30° and 45°) and overflexion to flexion targets (i.e. positive error to target angles 75° and 90°). While cerebellar patients presented a similar bias trend, they had a small offset in extension (~3.5°) at all targets (passive bias at elbow angle targets 30, 45, 75, and 90°: −8.47 ± 0.84° SE, −8.47 ± 0.83°, −2.38 ± 0.64°, −1.09 ± 0.66°; active: −8.22 ± 0.61°, −4.43 ± 0.45°, −1.52 ± 0.40°, 0.97 ± 0.53°).
Similar to experiment 1, both the control and patient groups again showed a clear active benefit, with the upper bound of the confidence interval below 1 (controls: 0.59, patients: 0.85). Normalized active benefit in the single-joint experiment did not differ significantly between patients and controls (t-test, t(19) = 1.17, P > 0.25; Figure 5a). The patient group was then split based on the Experiment 1 classification, with five patients in the unimpaired subgroup and five patients in the impaired subgroup. Within each subgroup, three patients showed either the same or a slightly worse variance ratio (max decrease 0.15) in the single-joint experiment compared to the multi-joint experiment. However, two patients in each subgroup showed more active benefit in the single-joint experiment compared to the multi-joint experiment, improving their ratio by 0.20 or more. ANOVA of variance ratios in the single-joint task between the two patient subgroups as well as controls resulted in a significant main effect of group (Kruskal-Wallis ANOVA H(2,21) = 7.45, P < 0.03). Post-hoc analysis showed this effect to be driven by the impaired patient subgroup performing worse than both the unimpaired patient subgroup and controls (Mann-Whitney U test, P < 0.03). Thus, it is unlikely that inter-joint coordination deficits were the main contributor to the impairments exhibited by cerebellar patients in the multi-joint localization task.
Discussion
In two experiments, we compared proprioceptive localization during both active and passive movement between individuals with cerebellar damage and healthy controls. Our aim was to determine the extent of cerebellar involvement in the localization process. We developed a test of the proprioceptive ability necessary for everyday movements by using a localization task that involved both multi-joint movements and vision. The ability to correctly estimate limb state is essential for the generation of appropriately calibrated motor commands as well as the updating of those commands in response to changing task requirements. Our results showed that while some cerebellar patients had deficits in proprioception during active, multi-joint movements (i.e. they did not show the active benefit that is characteristic of healthy proprioception), others did not. Specifically, the patients who did show proprioceptive deficits did so in the precision of localization rather than bias. Importantly, this within group distinction could not be explained by patient diagnosis or clinical ratings of impairment.
The division we found in the patient group’s performance is interesting. The tendency for cerebellar patients to exhibit localization impairments in active, multi-joint movements was not correlated with clinical ataxia ratings as assessed by the ICARS total score and kinetic function (limb coordination) subscore. Furthermore, the presence or absence of localization impairment could not be explained by differential diagnoses (e.g. degenerative disease vs. cerebellar stroke). Importantly, all patients were pre-screened using standardized clinical tests of fine-touch sensation as well as proprioception in the upper extremities [24]. These findings indicate the presence of sensory deficits following cerebellar damage that are not adequately detected by current clinical measures of disease type and progression. This is a significant finding because, while cerebellar damage is well known to impair motor coordination, the precise mechanism underlying these impairments remains unclear. The ability to accurately sense limb position is important for the selection and calibration of motor commands [17]. Thus, impairments in limb localization are likely to contribute to the motor symptoms of ataxia.
Bastian et al. [32] showed that cerebellar damage results in an impaired ability to compensate for the interaction torques generated in multi-joint movements. These impairments could have reasonably contributed to the proprioceptive deficits seen in our patients in the multi-joint localization task. To investigate this possibility, we examined patient performance in a single-joint localization task that had participants estimate fingertip position moving only the forearm (i.e. the shoulder angle was locked at 75°). The performance of some patients improved in the single-joint task (i.e. these patients showed more active benefit in the single-joint compared to the multi-joint task). However, when the patient group was split into the impaired and unimpaired subgroups based on their performance in the multi-joint task, we found that the impaired subgroup continued to show less active benefit in the single-joint task. Therefore, it is unlikely that the localization impairment found in some patients resulted from multi-joint coordination deficits following cerebellar damage.
Our work provides important insights into cerebellar contributions to the proprioceptive localization literature not previously seen in other studies. Studies by Synofzik et al. [18] and Izawa et al. [19] focused on cerebellar involvement in updating the sensory consequences of movement. When establishing baseline proprioceptive abilities in cerebellar patients, these authors tested proprioception in terms of movement direction only, and not movement extent. It was clear from these studies that patients did not have deficits in identifying the direction of their movements, as the bias and precision of their directionality estimates was comparable to age-matched controls [18,19]. Here we have shown that patients do not have difficulties with directionality in proprioceptive localization. However, examining performance in a localization task that required subjects to pinpoint their fingertip position (vs. movement direction only) allowed us to calculate a two-dimensional measure of precision. This analysis revealed that a subset of cerebellar patients exhibited deficits in active proprioceptive precision despite showing localization biases comparable to control participants. Overall, this suggests that cerebellar damage may differentially impair component elements of proprioceptive sense.
Previous work from our lab by Bhanpuri et al. [13] found that cerebellar patients showed clear impairments in a single-joint proprioceptive discrimination task. While these results may seem to contradict those of the present study, distinctions in the experimental protocols used may explain these differences. Our task involved matching the positions of a visual stimulus to a sensed limb position. In other words, our task used a combination of visual and proprioceptive information. In contrast, the task used by Bhanpuri et al. [13] relied solely on proprioception. Block and Bastian [20] showed that cerebellar patients are not impaired in their ability to integrate visual and proprioceptive signals. Thus, the use of vision in the present experiment may have allowed other brain areas to compensate for any cerebellar dysfunction. Given that integration of visual and proprioceptive information improves position estimates [33,34], this could reasonably explain the varying results in different proprioceptive tasks.
However, a more likely possibility for the difference in findings of the present study and those of Bhanpuri et al. [13] comes from the proprioceptive assessments used. Previous literature has shown that cerebellar patients exhibit proprioceptive recalibration deficits in tasks where updating of sensory predictions is required [18,19]. In tasks assessing baseline proprioceptive ability, cerebellar patients show deficits when estimating movement length [13], but not movement direction [18,19]. This suggests that the cerebellum may be more involved in proprioceptive estimates that have a temporal or predictive component (e.g. movement length), but that it may be less involved in generating estimates with only a spatial component (e.g. movement direction). The proprioceptive localization assessed in our task required estimating both movement direction and length. In other words, our task tested proprioceptive estimates with both spatial and temporal components. It is possible that some of our patients relied more heavily on the spatial component when generating their proprioceptive estimates and this could have improved their performance. Conversely, the task used by Bhanpuri et al. [13] tested proprioceptive estimates that were largely reliant on the temporal component, which could underlie their finding of widespread proprioceptive deficits in the cerebellar patient group. We are currently investigating whether relying on spatial or temporal information differentially influences proprioceptive estimates of limb position as well as the precise role of the cerebellum in processing this information.
Conclusion
In summary, we have shown that some people with cerebellar damage have increased variability in proprioceptive localization during active movements and that this cannot be explained by current tests of clinical impairment. Given that precise estimates of limb position are important for keeping movements well calibrated, deficits in localization likely affect movement ability in daily life. It follows that localization ability should not only be an important consideration during clinical testing of people with cerebellar damage, but should also be taken into account when developing rehabilitation strategies.
Acknowledgments
We thank the members of the Center for Movement Studies at the Kennedy Krieger Institute for helping to coordinate experiments and the patients for volunteering their time to participate in these studies. This research was supported by National Institutes of Health grants R01 HD040289 to AJ Bastian, T32 EB003383 to N Thakor, and F31 NS086399 to HM Weeks.
Footnotes
Compliance with Ethical Standards
The authors declare that they have no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
This article does not contain any studies with animals performed by any of the authors.
References
- 1.Ghez C, Sainburg R. Proprioceptive control of interjoint coordination. Can J Physiol Pharmacol. 1995;73:273–84. doi: 10.1139/y95-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–68. doi: 10.1152/physrev.2001.81.2.539. [DOI] [PubMed] [Google Scholar]
- 3.Proske U, Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587:4139–46. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–97. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 5.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–42. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 6.Oscarsson O. Functional organization of the spino-and cuneocerebellar tracts. Physiol Rev. 1965;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- 7.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–9. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- 8.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 9.Paulin MG. The role of the cerebellum in motor control and perception. Brain Behav Evol. 1993;41:39–50. doi: 10.1159/000113822. [DOI] [PubMed] [Google Scholar]
- 10.Grill SE, Hallett M, Marcus C, McShane L. Disturbances of kinaesthesia in patients with cerebellar disorders. Brain. 1994;117:1433–47. doi: 10.1093/brain/117.6.1433. [DOI] [PubMed] [Google Scholar]
- 11.Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–7. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 12.Hagura N, Oouchida Y, Aramaki Y, Okada T, Matsumura M, Sadato N, et al. Visuokinesthetic perception of hand movement is mediated by cerebro-cerebellar interaction between the left cerebellum and right parietal cortex. Cereb Cortex. 2009;19:176–86. doi: 10.1093/cercor/bhn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci. 2013;33:14301–6. doi: 10.1523/JNEUROSCI.0784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhanpuri NH, Okamura AM, Bastian AJ. Active force perception depends on cerebellar function. J Neurophysiol. 2012;107:1612–20. doi: 10.1152/jn.00983.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–22. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 16.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 17.Miall RC, Christensen LOD, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5:e316. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one’s behavior. Curr Biol. 2008;18:814–8. doi: 10.1016/j.cub.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 19.Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32:4230–9. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block HJ, Bastian AJ. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia. 2012;50:1766–75. doi: 10.1016/j.neuropsychologia.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. In: Freedman SJ, editor. The Neuropsychology of spatially oriented behavior. Homewood, III: Dorsey Press; 1968. pp. 37–55. [Google Scholar]
- 22.Adamovich SV, Berkinblit MB, Fookson O, Poizner H. Pointing in 3D space to remembered targets. I. Kinesthetic versus visual target presentation. J Neurophysiol. 1998;79:2833–46. doi: 10.1152/jn.1998.79.6.2833. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol. 2010;103:164–71. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell W. DeJong’s the neurologic examination. Baltimore, MD: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 25.Thornbury JM, Mistretta CM. Tactile sensitivity as a function of age. J Gerontol. 1981;36:34–9. doi: 10.1093/geronj/36.1.34. [DOI] [PubMed] [Google Scholar]
- 26.Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 27.Desmurget M, Vindras P, Grea H, Viviani P, Grafton ST. Proprioception does not quickly drift during visual occlusion. Exp Brain Res. 2000;134:363–77. doi: 10.1007/s002210000473. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RA, Wichern DW. Applied multivariate statistical analysis. 6. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. [Google Scholar]
- 29.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. 1996;11:189–228. [Google Scholar]
- 30.Crowe A, Keessen W, Kuus W, Van Vliet R, Zegeling A. Proprioceptive accuracy in two dimensions. Percept Mot Skills. 1987;64:831–46. doi: 10.2466/pms.1987.64.3.831. [DOI] [PubMed] [Google Scholar]
- 31.Wilson ET, Wong J, Gribble PL. Mapping proprioception across a 2D horizontal workspace. PLoS One. 2010;5:e11851. doi: 10.1371/journal.pone.0011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 33.van Beers RJ, Sittig AC, Gon JJ. Integration of proprioceptive and visual position-information: An experimentally supported model. J Neurophysiol. 1999;81:1355–64. doi: 10.1152/jn.1999.81.3.1355. [DOI] [PubMed] [Google Scholar]
- 34.van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Curr Biol. 2002;12:834–7. doi: 10.1016/s0960-9822(02)00836-9. [DOI] [PubMed] [Google Scholar]