Aberrant activation of FGFR1 kinase in human hematopoietic stem cells, resulting from chromosome translocations, leads to myeloproliferative neoplasms (MPN) that almost inevitably progress to acute myeloid leukemia (AML)(Jackson, et al 2010). These patients, however, often also develop T- or B-lymphomas coincident with the AML that carry the same chromosome translocations, suggesting that activation of FGFR1 occurs in hematopoietic precursor cells that can differentiate down different cell lineages (Jackson, et al 2010). These MPN are aggressive and resistant to all current chemotherapies (Jackson, et al 2010). The chromosome translocations lead to the constitutive and ligand independent activation of the FGFR1 kinase through dimerization facilitated by the fusion partner (Baumann, et al 2003). To date, over 14 different fusion partners have been identified, the most common of which involve the ZMYM2 (Baumann, et al 2003), BCR (Demiroglu, et al 2001) and CNTRL (Guasch, et al 2000) genes. Despite the common occurrence of MPN and AML, there are variations in the presentation of the disease depending on the fusion partner that affects the lineage phenotype and its aggressiveness. Of the chimeric variants, BCR-FGFR1 is the most aggressive and, in addition to AML, leads to B-lymphomas (Baldazzi, et al 2010). The same phenotype occurs in syngeneic mouse models where, unlike the ZMYM2-FGFR1 rearrangement that typically leads to development of T-lymphomas (Ren, et al 2009, Roumiantsev, et al 2004), BCR-FGFR1 leads to B-lymphomas (Ren, et al 2012). Neither of these mouse models, however, develops AML and typically the mice are overcome by the lymphomas within 4–12 weeks. In contrast, CNTRL-FGFR1 induces development of T-lymphomas in a sub set of animals but largely leads to the development of AML following a 9–12 month latency period (Ren, et al 2013).
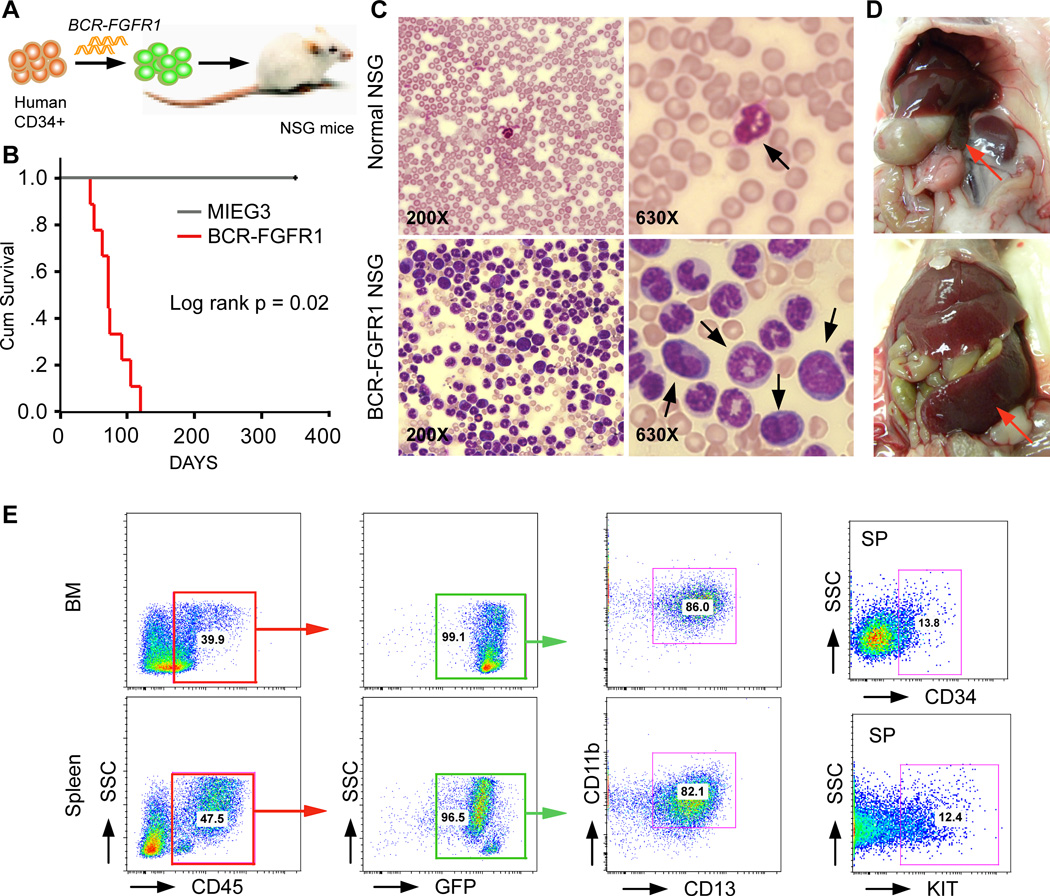
Previously, syngeneic mouse bone marrow transduction/transplantation experiments with BCR-FGFR1 led to CML-like myeloproliferation as well as B-cell leukemia/lymphoma (Ren, et al 2012, Roumiantsev, et al 2004) but, unlike the human disease, did not progress to a highly aggressive AML (Jackson, et al 2010). To develop a human cell model, we retrovirally expressed BCR-FGFR1 in primary, human cord blood-derived, CD34+ cells and transplanted them into NOD/SCID/IL2Rg (NSG) immunodeficient mice (Figure 1A). All engrafted mice (n =10, from 5 biological replicates) developed AML with a median latency of ~3 months (Figure 1B). AML development was accompanied by increased blast cell counts in the peripheral blood (Figure 1C), splenohepatomegaly (Figure 1D), and a hypercellular bone marrow, as well as infiltration of abnormal leukocytes into multiple organs (not shown). Consistent with AML, flow cytometry analyses showed that the majority (>80%) of human CD45+ leukocytes from these mice were GFP+CD13+CD11b+ and >10% expressed CD34+ and KIT+ (Figure 1E).
Figure 1. A humanized BCR-FGFR1 AML mouse model.
(A) Scheme of the experimental approach to generate the human CD34+ progenitor mouse model carrying the BCR-FGFR1 fusion gene. (B) Kaplan-Meier survival analysis of primary recipients following engraftment of either the MIEG3 control vector or BCR-FGFR1-transduced human CD34+ progenitor cells. (C) Peripheral blood smears show a remarkable increase in the number of blast cells (arrows). (D) The enlarged spleen and liver is shown in a representative diseased mouse. (E) Representative flow cytometry analysis of bone marrow (BM) and spleen cells from a primary recipient mouse.
In our previous analysis of molecular genetic changes associated with AML driven by the CNTRL-FGFR1 fusion kinase, we demonstrated abnormal activation of several signaling pathways that are related to both development and differentiation of myeloid lineage cells. These changes contributed to the etiology of the AML in both syngeneic and humanized mouse models (Ren, et al 2013). In the current BCR-FGFR1 model, quantitative RT-PCR analysis of leukemic cells showed up-regulation of GFI1 and MYC, which promote stemness and self-renewal, and down-regulation of differentiation associated genes IRF8, CSF1R and SPI1 (Figure 2A). In an analysis of the signaling cascades downstream of FGFR1, Western blot analysis showed (Figure 2B) remarkably increased phosphoactivation of FLT3, ERK1/2 and STAT3/5 in the spleen cells from BCR-NSG mice. Furthermore, the anti-apoptotic BCL2 and BCL-xL proteins were also dramatically increased in the tumor samples (Figure 2B). Thus, BCR-FGFR1-induced AML appears to be related to dysregulation of multiple signaling pathways associated with myeloid cell development and differentiation (Figure 2C). These observations are consistent with those seen in the CNTRL-FGFR1 mouse models (Ren, et al 2013), indicating that chimeric FGFR1 genes function through a common pathogenetic mechanism to induce AML.
Figure 2. BCR-FGFR1 dysregulates multiple signaling pathways related to myeloid cell development and differentiation.
(A) Real-time RT-PCR analysis shows transcription level changes for selected genes related to myeloid development and differentiation in the primary recipients (primers specifically recognize human genes, left panel). (B) Western blot analysis shows the changes of downstream components of the FGFR1 signaling pathway as well as protein levels of GFI1, MYC, and BCL-2/BCL-xL in leukemic mouse spleens compared with normal human peripheral blood mononuclear cells (PBMC). (C) Scheme of the molecular mechanism of BCR-FGFR1-induced AML in the mouse model.
This report demonstrates the development of an aggressive, human cell, BCR-FGFR1 driven AML which is in contrast with a previous report, which showed only development of a chronic myeloproliferation. In this study most mice died within the first month, without developing overt AML (Agerstam, et al 2010). In our series of mice, AML only developed after ~100 days on average, which may be why the previous model failed to demonstrate progression. Even 100 days is a relatively short latency period compared with other chimeric FGFR1 driven MPN/AML. In our recent report of the CNTRL-FGFR1 human model of AML in NSG mice (Ren, et al 2013), for example, latency periods ranged between 9–12 months, highlighting the more aggressive nature of the disease induced by the BCR variant. This rapid progression for BCR-FGFR1-driven AML has also been seen in syngeneic mouse models of FGFR1 related neoplasms (Ren, et al 2012, Roumiantsev, et al 2004), where latency for this chimeric kinase was only 3–4 weeks compared with 8–20 weeks for the ZMYM2-FGFR1 induced disease (Ren, et al 2009). Although the latency period in the syngeneic CNTRL-FGFR1 disease (Ren, et al 2013) was much longer (9–12 months), these mice predominantly developed AML rather than lymphomas, unlike the other mouse models. The preferential development of lymphomas, however, was seen in mice that have a completely functional immune system, in contrast to the NSG model where functional B and T cells are suppressed, which likely accounts for the dominance of myeloid disease in these models.
Therapies for patients with FGFR1-driven disease have so far followed those traditionally used for other ALL, AML or other MPN, but none of these include targets identified by molecular studies (Jackson, et al 2010). Overall, these therapies have been inadequate, leading to no response, partial response or short term clinical remission with persistent cytogenetic abnormalities (Jackson, et al 2010). Only relatively few survivors have been reported, usually as a result of bone marrow transplantation. The development of the BCR-FGFR1 model, in particular, as well as the other FGFR1-driven AML syndromes, provides an opportunity to develop more targeted therapies, and provides a preclinical in vivo model to assess the effectiveness of anti-FGFR1, anti-FLT3 and anti-BCL2 agents, for example, to provide a more effective therapeutic strategy.
Acknowledgments
This work was supported by Grant CA076167 from the National Institutes of Health.
Footnotes
Author contributions:
M.R., J.K.C. designed the study. M.R., H.Q. C.C. and E.K. carried out experiments. M.R. and J.K.C. wrote the paper.
Conflict of Interest Statement: the authors declare no conflict of interest
REFERENCES
- Agerstam H, Jaras M, Andersson A, Johnels P, Hansen N, Lassen C, Rissler M, Gisselsson D, Olofsson T, Richter J, Fan X, Ehinger M, Fioretos T. Modeling the human 8p11-myeloproliferative syndrome in immunodeficient mice. Blood. 2010;116:2103–2111. doi: 10.1182/blood-2009-05-217182. [DOI] [PubMed] [Google Scholar]
- Baldazzi C, Iacobucci I, Luatti S, Ottaviani E, Marzocchi G, Paolini S, Stacchini M, Papayannidis C, Gamberini C, Martinelli G, Baccarani M, Testoni N. B-cell acute lymphoblastic leukemia as evolution of a 8p11 myeloproliferative syndrome with t(8;22)(p11;q11) and BCR-FGFR1 fusion gene. Leukemia Reserch. 2010;34:e282–e285. doi: 10.1016/j.leukres.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Baumann H, Kunapuli P, Tracy E, Cowell JK. The oncogenic fusion protein-tyrosine kinase ZNF198/fibroblast growth factor receptor-1 has signaling function comparable with interleukin-6 cytokine receptors. Journal of Biolohical Chemistry. 2003;278:16198–16208. doi: 10.1074/jbc.M300018200. [DOI] [PubMed] [Google Scholar]
- Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, Koduru P, Brody JP, Hawson G, Rodwell R, Doody ML, Carnicero F, Reiter A, Goldman JM, Melo JV, Cross NC. The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: transforming activity and specific inhibition of FGFR1 fusion proteins. Blood. 2001;98:3778–3783. doi: 10.1182/blood.v98.13.3778. [DOI] [PubMed] [Google Scholar]
- Guasch G, Mack GJ, Popovici C, Dastugue N, Birnbaum D, Rattner JB, Pebusque MJ. FGFR1 is fused to the centrosome-associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33) Blood. 2000;95:1788–1796. [PubMed] [Google Scholar]
- Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–476. doi: 10.1016/j.humpath.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ren M, Li X, Cowell JK. Genetic fingerprinting of the development and progression of T-cell lymphoma in a murine model of atypical myeloproliferative disorder initiated by the ZNF198-fibroblast growth factor receptor-1 chimeric tyrosine kinase. Blood. 2009;114:1576–1584. doi: 10.1182/blood-2009-03-212704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Qin H, Kitamura E, Cowell JK. Dysregulated signaling pathways in the development of CNTRL-FGFR1-induced myeloid and lymphoid malignancies associated with FGFR1 in human and mouse models. Blood. 2013;122:1007–1016. doi: 10.1182/blood-2013-03-489823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Tidwell JA, Sharma S, Cowell JK. Acute progression of BCR-FGFR1 induced murine B-lympho/myeloproliferative disorder suggests involvement of lineages at the pro-B cell stage. PLoS One. 2012;7:e38265. doi: 10.1371/journal.pone.0038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumiantsev S, Krause DS, Neumann CA, Dimitri CA, Asiedu F, Cross NC, Van Etten RA. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell. 2004;5:287–298. doi: 10.1016/s1535-6108(04)00053-4. [DOI] [PubMed] [Google Scholar]