Abstract
Astrocytes play a critical role in supporting the normal physiological function of neurons. Recent studies have revealed that astrocyte transplantation can promote axonal regeneration and functional recovery after spinal cord injury. Biomaterial can be designed as a growth-permissive substrate and serve as a carrier for astrocyte transplantation into injured spinal cord. In this study, we developed a method to generate collagen microspheres encapsulating astrocytes by injecting a mixture of collagen and astrocytes into a cell culture medium with a syringe controlled by a syringe pump. The collagen microspheres were crosslinked with poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-StarPEG) to reduce the degradation rate. The viability of cells in the crosslinked microspheres was higher than 90%. Astrocytes were transfected with plasmids encoding nerve growth factor (NGF)-ires-enhanced green fluorescent protein (EGFP) genes by electroporation and encapsulated in crosslinked microspheres. The level of NGF released into the cell culture medium was higher than that remaining in the microspheres or astrocytes. When microspheres encapsulating astrocytes transfected with plasmids encoding NGF-ires-EGFP genes were added into the cultured rat dorsal root ganglion, the axonal growth was significantly enhanced. This study shows that the microspheres can be potentially used as a carrier of astrocytes to promote nerve regeneration in injured neural tissue.
Keywords: astrocyte, microsphere, encapsulation, neurite growth, transfection, crosslinking
Graphical Abstract
INTRODUCTION
Cell transplantation can potentially restore the function of injured neural tissue by replacing neural cell loss and generating functional molecules. However, the microenvironment of the neural tissue lesion encountered by the transplanted cells is unfavorable for cell survival. A cell-delivery vehicle may generate a permissive environment for the growth of grafted cells. Micro- and nanoparticles have demonstrated their versatility to reliably encapsulate drug particles, growth factors, plasmid DNA, and other biomolecules for delivery to targeted tissues1–6. Microspheres are also a promising carrier for therapeutic cell delivery in order to regenerate injured neural tissue. Natural polymers are strongly favored for the synthesis of microspheres because they are usually biodegradable, biocompatible, nontoxic, and non-immunogenic.
Astrocytes are the most abundant glial cells in the central nervous system, and they play a critical role in supporting the normal physiological function of neurons in the spinal cord. Astrocytes are associated with synapses and regulate the connectivity of neuronal circuits by controlling the formation, maturation, and maintenance of synapses7. Astrocytes can maintain ionic balance in the extracellular matrix and provide nutrients for nervous system tissue. Astrocytes generate multiple neurotrophic factors that regulate the survival and physiological function of neurons. It has been reported that astrocyte transplantation into injured neural tissue can promote axonal regeneration and functional recovery8–14.
Type I collagen, the main component of the extracellular matrix, has been widely used to fabricate biomaterial scaffolds for tissue repair. It has been shown that transplantation of collagen scaffolds can support axonal regrowth in wounded neural tissue of peripheral and central nervous systems15, 16. The crosslinking of collagen scaffolds improves their mechanical strength and the ability to resist enzyme degradation. Previous studies have demonstrated the potential of microspheres for the support of stem cell growth17–19. We found that oligodendrocyte progenitor cells (OPCs) can grow and differentiate on the surface of collagen microspheres. Collagen microspheres crosslinked with 1-ethyl-3-(3-dimethylaminopropryl) carbodiimide (EDC) served as a carrier to transfer OPCs to the cultured dorsal root ganglion (DRG). OPCs delivered by microspheres can myelinate the axons of cultured DRG. However, the limitation of this method is that the number of cells that attach to the surface of the microspheres is low20.
In this study, we developed a method that can efficiently generate collagen microspheres by injecting the mixture of collagen and astrocytes into a cell culture medium with a syringe attached to a syringe pump. The size of the microspheres can be determined by regulating the flow rate of the collagen solution in the syringe, which in turn is controlled by the syringe pump. Astrocytes transfected with plasmids encoding nerve growth factor (NGF)-ires-enhanced green fluorescent protein (EGFP) genes were fabricated in the microspheres. The expression of NGF in microspheres was determined, and the effect of the secreted NGF on DRG axon growth was studied.
MATERIALS AND METHODS
Fabrication of collagen microspheres
Type I collagen (5 mg/ml) isolated from bovine Achilles tendon was used to fabricate the collagen microspheres. Either the collagen solution alone or collagen solution mixed with poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-StarPEG) (0.01 mM and 0.1 mM) was transferred into a syringe with a needle (25 G1, BD Biosciences, Franklin Lakes, NJ). The collagen solution was injected directly into the cell culture medium on a cell culture plate at the rate of either 0.1 ml/min or 0.4 ml/min using a syringe pump (New Era Pump Systems, Inc., Farmingdale, NY). The cell culture plate was then transferred to an incubator (37°C, 5% CO2). Images of the microspheres at various time points (4 h, day 3, day 6, and day 10) after fabrication were taken using a microscope (Olympus IX51 Inverted Microscope, Center Valley, PA), and the diameters of the microspheres were measured and quantified.
Degradation test
Non-crosslinked collagen microspheres and collagen microspheres crosslinked with 4S-StarPEG (0.01 mM, or 0.1 mM) were produced in the cell culture medium. Images of the microspheres were taken 4 h after the microspheres were generated. Then collagenase from clostridium histolyticum (Sigma-Aldrich, St. Louis, MO) prepared in 0.1 mM Tris (pH 7.4) containing 0.05 M calcium chloride (CaCl2) was added to the cell culture medium with a final concentration of 0.25 mM. The samples were then incubated (37°C and 5% CO2). After the collagenase was added into the cell culture medium, images of the microspheres were taken at various time points (20 min, 1 h, 2 h, 4 h, 6 h, and 24 h), and the diameters of the microspheres were measured and quantified.
Astrocyte growth in collagen microspheres
All procedures involving animals in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Wichita State University. The astrocyte culture was prepared from the brains of newborn (postnatal, day 1 to day 3) Sprague Dawley rats as previously described21, 22. In brief, cerebral cortices were isolated from their brains after being sacrificed. Cortex tissues were triturated gently through a 5 ml syringe with a needle. The tissue suspension was passed through a 70-mm nylon cell strainer, and the flow-through was collected with a 50 ml conical tube. The isolated cells were cultured for about 7–14 days. After reaching confluency, the cultures were shaken to remove macrophages and other progenitor cells. The adherent astrocytes were subsequently cultured for growing in microspheres. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Lifetechnology, Grand Island, NY). The medium was changed every 3 days, and the cell culture was placed in a 37°C incubator with 5% CO2. Cells of passages 2, 3, and 4 were used in these studies. To grow astrocytes in collagen microspheres, cells were added to the neutralized collagen solution containing various amounts of 4S-StarPEG (0 mM, 0.01 mM, and 0.1 mM). Then the mixture was injected into the cell culture medium in cell culture dishes at a rate of 0.1 ml/min, and the dishes were transferred to an incubator. After the microspheres were produced, images were taken at various time points (4 h, day 3, day 7, and day 10), and diameters of the microspheres were measured and quantified.
LIVE/DEAD® cell viability assay
Astrocytes (50,000 cells) were mixed in collagen solutions (100 µl) with varying concentrations of 4S-StarPEG (0 mM, 0.01 mM, and 0.1 mM). The LIVE/DEAD® cell viability assay (Lifetechnology, Grand Island, NY) was performed for astrocytes cultured in the microspheres for 4 days. Reagents for the LIVE/DEAD® assay were ethidium homodimer-1 (Ethd-1) and calcein AM. Solutions of the assay were removed from the freezer and allowed to warm to room temperature. EthD-1 stock solution (2 µl of 2 mM) and calcein AM stock solution (0.5 µl of 4 mM) were added to a sterile PBS solution (1 ml) and vortexed. The solution (300 µl) was added directly to each cell culture well and then incubated for 30 min at room temperature. The cells were viewed under a fluorescent microscope (Axiovert 200M; Carl Zeiss, Inc.). At least three independent experiments were performed in this study. In each experiment, four images of cells within the gel were recorded. The live and dead cells in the images were counted, and the ratio of live cells to total cells was calculated.
AlamarBlue® assay
The metabolic activity of astrocytes in the microspheres was studied by AlamarBlue® assay (Pierce Biotechnology, Rockford, lL). The astrocytes (100,000) were mixed with 100 µl collagen solutions with varying concentrations of 4S-StarPEG (0 mM, 0.01 mM, and 0.1 mM). Cells in the collagen microspheres were cultured for 4 days and then incubated with a cell culture medium containing 10% (v/v) AlamarBlue® reagent for 4 h. Absorbance was measured at wavelengths of 570 nm and 600 nm in a microplate reader (Synergy Mx Monochromator-Based Multi-Mode Microplate Reader, Winooski, VT).
Measurement of NGF released from astrocytes encapsulated in collagen microspheres
To investigate the releasing profile of NGF from transfected cells cultured on a cell culture plate, astrocytes (250,000 cells) were transfected with 2 µg plasmids encoding NGF-ires-EGFP (GeneCopoeia, Inc.Rockville, MD) using the 4D-Nucleofector™ System (Lonza, Allendale, NJ), and then they were seeded in the cell culture dishes. The cell culture medium was collected and changed after culturing for 3 days, 6 days, and 9 days. To study the NGF released from the microspheres encapsulating transfected astrocytes, 250,000 astrocytes were transfected with 2 µg plasmids encoding NGF-ires-EGFP and then mixed with 100 µl of collagen solution or a collagen solution containing 4S-StarPEG at a final concentration of 0.1 mM. Microspheres were generated using the mixture of collagen solution and transfected astrocytes. Microspheres produced by non-transfected astrocytes and the collagen solution served as a control group. After the cells were cultured in the microspheres for 3 days, the cell culture medium was collected. The microspheres were digested with collagenase, and the digestion solution was centrifuged. The cell pellets and supernatants were collected separately. The cell pellets were reconstructed with 0.5 ml of cell culture medium and sonicated. In each study, three independent studies were performed.
The amounts of NGF in the cell culture medium, digested collagen microspheres, and astrocytes were measured separately using an enzyme-linked immunosorbent assay (ELISA) kit (Human Beta NGF Duoset, R&D Systems, Minneapolis, MN). The ELISA plates (NUNC, Polylabo, Strasbourg, France) were coated with the captured monoclonal antibodies and blocked with bovine serum albumin (1% w/v) for 1 h. Appropriately diluted samples of supernatant were added to the ELISA plates, and the amount of bound NGF was detected using anti-human NGF monoclonal antibodies. Streptavidine-conjugated horse radish peroxidase and its substrate (tetramethylbenzidine and peroxide) were added, and the plates were incubated for 20 min. The reaction was stopped using a stopping solution of 2N H2SO4 (R&D Systems, Minneapolis, MN). The absorbance of the samples was read at 450 nm using a Victor 3VTM multilabel counter (Perkin–Elmer Precisely, Waltham, MA). The amount of NGF in the supernatants was determined from a calibration curve based on the known concentration of NGF.
Co-culture of DRGs and astrocytes encapsulated in microspheres
DRGs from newborn (postnatal, day 1 to day 3) Sprague Dawley rats were isolated and cultured with Neurobasal-A medium with B-27 and L-Glutamine supplements in cell culture dishes. As shown previously, the crosslinked collagen microsphere (0.1 mM 4S-StarPEG)-encapsulated non-transfected astrocytes or astrocytes transfected with NGF-ires-EGFP plasmids were generated and added to dishes with the DRG culture. In a positive control group, the cultured DRGs were treated with cell culture medium containing NGF (20 ng/ml). A group of cultured DRGs that was not treated with NGF or microspheres was used as the negative control. After 6 days, images of DRGs were taken. Then the cells were fixed with 4% paraformaldehyde. The DRGs were labeled with anti-Tuj1 primary antibody and Alexa Fluor® 488 AffiniPure Donkey Anti-Mouse secondary antibody. Measurements of radial neurite outgrowth were taken using NIH Image J software. Neurite length was measured from the center of the ganglion to the edge of the longest neuronal process. The average neurite length (in mm) was calculated from explants from three independent experiments.
Statistics
Statistical analysis was conducted using a two-tailed Student’s t-test. A p-value of 0.05 was considered to be statistically significant. Data are expressed as means ± standard deviation.
RESULTS
Influence of crosslinking and solution flow rate on microsphere diameter
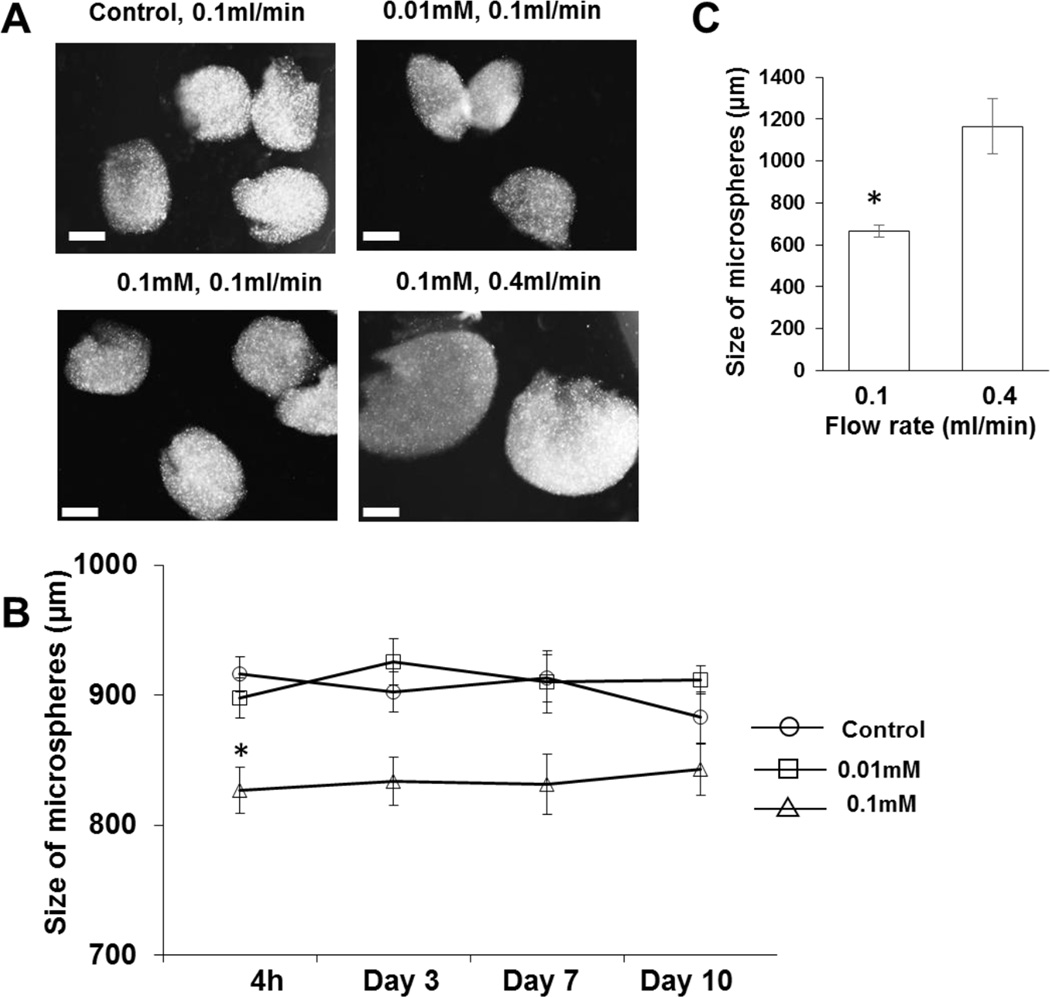
Collagen microspheres with or without crosslinking were fabricated in a cell culture medium (Figure 1A). The size of the non-crosslinked collagen microspheres was 916.2 ± 74.4 µm, which is higher than that of microspheres crosslinked with 0.01 mM 4S-StarPEG (897.7 ± 82.6 µm) or 0.1 mM 4S-StarPEG (826.8 ± 100.6 µm) (p < 0.01). The size of these microspheres did not change significantly after they were incubated in the cell culture medium for 10 days. After that time, the size of the non-crosslinked collagen microspheres and microspheres crosslinked with 0.01 mM or 0.1 mM 4S-StarPEG was 882.9 ± 104.7 µm, 911.7 ± 56.5 µm, and 842.7 ± 118.2 µm, respectively (Figure 1B). The size of the microspheres crosslinked with 0.1 mM 4S-StarPEG increased from 663.3 ± 28.5 µm to 1165 ± 131.9 µm (p < 0.01) when the flow rate of the collagen in the syringe increased from 0.1 ml/min to 0.4 ml/min (Figure 1C).
Figure 1.
Fabrication of crosslinked collagen microspheres: (A) Images of non-crosslinked and crosslinked collagen microspheres. (B) Slight change in size of collagen after incubation in cell culture medium for 10 days. (C) Increased size of produced microspheres with increase in collagen solution flow rate. *, p < 0.05, compared with control and 0.01 mM groups at time point of 4 h. Scale bar: 400 µm.
Reduction of degradation rate of microspheres by crosslinking
After the microspheres were digested with collagenase, their size decreased with time (Figure 2A). Measurement and quantification of the microspheres showed that the size reduction of non-crosslinked microspheres was faster than that of microspheres crosslinked with 4S-StarPEG. The degradation rate of microspheres crosslinked with 0.1 mM 4S-StarPEG was slower than that crosslinked with 0.01 mM 4S-StarPEG (Figure 2B).
Figure 2.
Decrease in microsphere degradation rate by crosslinking collagen microspheres with 4S-StarPEG: (A) Degradation of collagen microspheres crosslinked with different amounts of 4S-StarPEG. Arrows indicate typical microspheres digested with collagenase. (A’) Magnified images of microspheres. (B) Quantification of size of microspheres digested with collagenase. *p < 0.05, compared with control at same time point; #, p < 0.05, compared with 0.01 mM group at same time point; ^, p < 0.05, compared with 0.01 mM group at same time point. Scale bar: 400 µm.
Cell viability of astrocytes encapsulated in microspheres
The cell viability of astrocytes in the collagen microspheres was determined using a LIVE/DEAD® cell vitality assay kit and viewed under a fluorescent microscope. After the cells were cultured for 4 days, most of them survived in the non-crosslinked microspheres and microspheres crosslinked with 4S-StarPEG (Figures 3A). The astrocytes showed a flat star shape with multiple processes (Figure 3B). They also showed extended multiple processes in the non-crosslinked and crosslinked microspheres (Figure 3C and D). Quantification of live and dead cells in the microspheres showed that the ratio of live astrocytes to total cells was 93.6 ± 0.4% in the non-crosslinked microspheres, 91.3 ± 4.0 in microspheres crosslinked with 0.01 mM 4S-StarPEG, and 89.6 ± 3.0% in microspheres crosslinked with 0.1 mM 4S-StarPEG (Figure 3C).
Figure 3.
LIVE/DEAD® cell viability assay for astrocytes grown in microspheres: (A) Most astrocytes grown in non-crosslinked microspheres and microspheres crosslinked with 0.1 mM 4S-StarPEG exhibiting high cell viability. Scale bar: 200 µm. (B) Astrocytes cultured on cell culture plate labeled with astrocyte marker (GFAP). Scale bar: 50 µm. (C) Astrocytes showing multiple processes in non-crosslinked microspheres. (D) Astrocytes showing multiple processes in crosslinked microspheres. Scale bar: 50 µm. (E) Percentage of live cells in collagen microspheres as determined by LIVE/DEAD® cell assay.
The AlamarBlue® assay showed the metabolic activity of astrocytes in collagen microspheres (Figure 4). The reduction of AlamarBlue® reagent for astrocytes in non-crosslinked collagen microspheres (102.5 ± 6.7%), microspheres crosslinked with 0.01 mM 4S-StarPEG (101.1 ± 6.3%), and microspheres crosslinked with 0.1 mM 4S-StarPEG (104.0 ± 11.8%) was not statistically different.
Figure 4.
AlamarBlue® assay for astrocytes grown in non-crosslinked collagen microspheres and collagen microspheres crosslinked with 4S-StarPEG.
Astrocyte growth in collagen microspheres remodeled microspheres
The size of the non-crosslinked and crosslinked microspheres encapsulating astrocytes decreased significantly after the cells were cultured in microspheres for 3 days. The size of microspheres continued to decrease significantly from day 3 to day 7. The size of the microspheres was stable from day 7 to day 10 (Figure 5A). The size of non-crosslinked and crosslinked microspheres encapsulating astrocytes was not significantly different at the same time point. Cells showed extended multiple processes in the microspheres at day 10 (Figure 5B). With astrocytes grown in microspheres, the size of non-crosslinked microspheres, microspheres crosslinked with 0.01 mM 4S-StarPEG, and microspheres crosslinked with 0.1 mM were 696.0 ± 16.6 µm, 694.9 ± 12.4 µm, and 659.3 ± 15.5 µm, respectively. The size of these microspheres decreased more than 50% after the astrocytes in microspheres were cultured for 10 days (Figure 5C).
Figure 5.
Size of microspheres modulated by astrocytes grown in collagen microspheres: (A) Significant decrease in size of microspheres encapsulating astrocytes after incubation in cell culture medium for 10 days. Scale bar: 400 µm. (B) Astrocyte growth in microspheres for 10 days. Scale bar: 100 µm. (C) Quantification of size of collagen microspheres encapsulating astrocytes in cell culture medium. *, p < 0.05, compared with size at time point of 4 h. **, p < 0.05, compared with size at time points of 4 h and day 3.
Release of NGF secreted by transfected astrocytes encapsulated in collagen microspheres
The expression of EGFP by transfected astrocytes in both non-crosslinked and crosslinked microspheres was observed by fluorescent microscope after the cells were cultured for 1 day and 3 days (Figure 6 A). The ELISA assay showed that the level of released NGF from astrocytes cultured on the cell culture plate decreased significantly from day 3 (947.1 ± 286.9 pg) to day 6 (101.4 ± 70.4 pg) (p < 0.01).
Figure 6.
Expression of EGFP and NGF by astrocytes transfected with NGF-ires-EGFP plasmids: (A) EGFP expression by astrocytes in microspheres. (B) Measurement of level of NGF secreted by astrocytes cultured on cell culture plate by ELISA assay. *, p < 0.05, compared with NGF level at day 6 and day 9. **, p < 0.05, compared with level at day 9. (C) Non-transfected astrocytes and astrocytes transfected with NGF-ires-EGFP plasmids grown in collagen microspheres. NGF level in cell culture medium, digested microspheres, and astrocyte lysate measured by ELISA assay. *, p < 0.05, compared with NGF level of transfected groups. #, p = 0.059, compared with NGF level in the microspheres of non-crosslinked group. ^, p < 0.05, compared with NGF level in the microspheres of 0.1 mM 4S-StarPEG group. Scale bar: 100 µm.
After the astrocytes were cultured in non-crosslinked microspheres or microspheres crosslinked with 0.1 mM 4S-StarPEG for 3 days, the NGF level in the cell culture medium, digested microspheres, and astrocyte lysate were measured. The total NGF in the cell culture medium released from the non-crosslinked microspheres and crosslinked microspheres encapsulating the transfected cells was 197.0 ± 87.6 pg and 473.9 ± 167.6 pg, respectively. The NGF level in the medium was higher than that in digested microspheres or in cell lysate. This study showed that most of the NGF secreted by the astrocytes was released into the cell culture medium.
Microsphere-encapsulated astrocytes transfected with NGF-ires-EGFP enhanced neurite growth of cultured DRGs
In this study, we observed the neurite growth from cultured DRGs (Figure 7). The neurite length of cultured DRGs supplemented with microsphere-encapsulated astrocytes transfected with NGF-ires-EGFP was 551.0 ± 186.7 µm (DRGs, n = 26), which was significantly longer than that supplemented with microsphere-encapsulated non-transfected astrocytes (336.4 ± 113.3 µm; DRGs, n = 15; p < 0.01) or without any supplement (157.9 ± 185.1 µm; DRGs, n = 23; p < 0.01). The neurite length of DRGs of the transfected group was significantly lower than the group treated with NGF (701.9 µm ± 238.1 µm; n = 22; p < 0.01).
Figure 7.
Effect of microsphere-encapsulated astrocytes transfected with NGF-ires-EGFP plasmids on neurite growth from cultured DRGs: (A) Cultured DRGs treated with medium supplemented with NGF growth factor (20 ng/ml). (B) Cultured DRGs incubated with crosslinked (0.1 mM 4S-StarPEG) collagen microsphere-encapsulated transfected astrocytes. (C) Cultured DRGs incubated with crosslinked (0.1 mM 4S-StarPEG) collagen microsphere-encapsulated non-transfected astrocytes. (D) Cultured DRGs without any treatment. NGF growth factor supplement in cell culture medium (NGF). Microsphere-encapsulated transfected cells (MTC). Microsphere-encapsulated non-transfected cells (MC). No treatment (NT). *, p < 0.05, compared with NT. Scale bar: 400 µm.
DISCUSSION
Although cell transplantation is an effective approach in the tissue-regeneration process, one of the major concerns with introducing new cells to the body is regarding the environment the cells encounter. Grafted cells are likely to be attacked by the immune system, followed by inflammatory responses. Therefore, a cell-delivery vehicle may help to overcome this problem. A cell-delivery vehicle is a matrix that can encapsulate cells for transplantation into a host. The concept of bioencapsulation was proposed almost half a century ago 23. In the later studies, the implantation of microencapsulated islets was investigated to control diabetes in animal models. The microencapsulated cells remained viable and controlled glucose levels for several weeks in the hosts 24, 25. The transplantation of encapsulated islets for diabetes therapy has been attempted in clinical trials 26, 27. This vehicle must be semipermeable to allow the release of active molecules generated by the cells. To optimize the process of cell encapsulation, the carrier must also have the appropriate mechanical property, low toxicity, biodegradability and biocompatibility, and low immunogenicity28. Natural polymers are strongly favored as cell-delivery vehicles because they usually possess these properties. In a previous study, mesenchymal stem cells (MSCs) were encapsulated in collagen microspheres, and the in vitro osteoconductivity, osteogenicity, and osteoinductivity of the MSCs in the microspheres were evaluated19. The MSCs encapsulated in collagen spheres could be induced for osteogenic differentiation. The study indicated that the microsphere-encapsulated MSCs could provide an alternative method to traditional bone grafts. Injectable microcarriers that encapsulate stem cells at an injured site and the differentiation of those stem cells to the desired lineage may generate a significant clinical impact.
In a previous study, we fabricated collagen microspheres using a water-in-oil emulsion method and crosslinked the microspheres with EDC20. The viability of oligodendrocyte progenitor cells grown on collagen microspheres was not reduced, compared with that on the cell culture plates. We also showed that the OPCs grown on collagen microspheres can differentiate into oligodendrocytes and myelinate the axons of cultured DRGs in vitro. In the present study, 4S-StarPEG was used to crosslink the type I collagen microspheres. The active ester groups in 4S-StarPEG can react with amino groups of collagen and form crosslinks, which create a more stable network for supporting cell growth and proliferation 29. In a previous study, type II collagen hydrogel was crosslinked with 4S-StarPEG, and the effect of crosslinking on the mechanical property of the hydrogels was investigated. The study showed that the increase of 4S-StarPEG level in type II collagen hydrogel increased the stiffness of the hydrogels 30. It was also reported that crosslinking of collagen fibers with 4S-StarPEG significantly increased the stress of fibers at break 31. In this study, astrocytes were mixed with collagen, and microsphere-encapsulated cells were generated when the mixture of collagen solution and cells was directly injected into the cell culture medium. This method can efficiently encapsulate a large number of cells in a relatively small amount of solution. Because the microspheres can be injected into the cell culture medium directly, they can maintain high viability in the microspheres. We showed that the viability and metabolic activity of astrocyte cells in both crosslinked and non-crosslinked collagen microspheres was not significantly different. Additionally, the size of the microspheres was uniform because the flow rate of the collagen solution in the syringe can be controlled by a syringe pump. The size of the microspheres can be regulated by the flow rate of the collagen solution.
Biomaterial microspheres provide a permissive environment for cell growth. Cells growing on microspheres can modulate their structure. When adipose-derived stem cells (ADSCs) were grown on porous chitosan microspheres, the cells proliferated on the surface of the spheres and infiltrated the pores to grow within the spheres18. In a previous study, we found that after OPCs were cultured on the surface of collagen microspheres for 8 days, round collagen microspheres changed to an irregular morphology20. In this study, we observed the structural modification of astrocytes to the collagen microspheres. Without cell growth, the size of the collagen microspheres changed slightly after they were incubated in the cell culture medium for 10 days. However, the size of the collagen microspheres encapsulating the astrocytes decreased 50% after the microspheres were incubated in the cell culture medium for 10 days. Degradation of collagen scaffolds can be regulated by altering the crosslinking reagent concentration. We have shown that the degradation rate of the collagen microspheres can be regulated by modifying the level of 4S-StarPEG and that this was actually reduced by increasing the amount of crosslinker.
Micro- and nanoparticle technology hold promise in tissue regeneration with the controlled release of therapeutic molecules. Drug delivery by biodegradable microspheres is an efficient approach for the controlled release of these molecules32. The fabrication and use of collagen microspheres in the sustained release of vascular endothelial growth factor (VEGF) has been reported33. VEGF releasing was sustained over the course of 4 weeks, and the VEGF retained its bioactive properties. Also, an in vivo study showed that nerve regeneration could be enhanced when the repair site of a rat nerve was treated with fibrin containing poly(lactic-c-gly-colic acid) microsphere-encapsulated glial-derived neurotrophic factor (GDNF)34. In a similar neural regenerative study, the continuous release of NGF for an extended course of time from microspheres loaded into nerve guidance conduits (NGCs) enhanced peripheral nerve generation35. Cell transplantation is an alternative to controlled exogenous drug release. Because of the elastic property, the local delivery of collagen-based microspheres to neural tissue by injection causes little damage to healthy tissue. Cells delivered by collagen-based microspheres may constantly generate therapeutic molecules into the injury site. Cells encapsulated in microspheres can release growth factors in a specific location and maintain isolation from attack by the immune system. In a previous study, ADSCs and hollow collagen microspheres containing plasmid RNA polyplexes were loaded into a type II collagen/hyaluronan microgel. Because the ADSCs were constantly transfected in the microgel, increasing levels of luciferase were secreted from the microgels over 7 days of culturing36.
NGF is important in both the central nervous system and peripheral nervous system 37–40. It has demonstrated a neuroprotective effect and can improve the recovery of wounded spinal cord 41–43. In this study, astrocytes were transfected with NGS-ires-EGFP plasmids, and the releasing profile of the NGF from collagen microspheres was studied. Transfected with plasmids encoding NGS-ires-EGFP, the astrocytes can express both NGF and EGFP. NGF can promote neural repair, and EGFP is an indicator of plasmid transfection. The EGFP can also serve as a marker of transplanted astrocytes in vivo. Additionally, this study is a proof of concept to study growth-factor release from microspheres. In the future in vivo study, the NGF can be replaced by other growth factor genes. We found that most NGF generated by astrocytes was released into the cell culture medium; only about 10% of the total secreted NGF remained in the collagen microspheres. Crosslinking with 4S-StarPEG did not significantly increase the retention of NGF in the microspheres. To demonstrate the function of released NGF on neurite growth, we incubated cultured DRGs with microsphere-encapsulated astrocytes transfected with NGF plasmids and found that NGF secreted by the transfected astrocytes significantly enhanced neurite growth compared to the non-transfected control group. This study suggests that astrocytes encapsulated in crosslinked collagen microspheres can be potentially delivered to wounded nerve tissue to reestablish physiological function. Astrocytes transfected with nerve growth factor gene vectors may significantly enhance neural regeneration and functional recovery.
In our study, we found that the level of NGF in the medium of cultured transfected astrocytes on a cell culture plate was higher than that released from transfected astrocytes in microspheres. This observation indicates that cells have different abilities to produce NGF under different culture conditions. We also showed that the level of NGF in the cell culture medium of the crosslinked microsphere group was higher than that in the non-crosslinked group, although the difference between these two groups was not statistically significant. The 4S-StarPEG in the microspheres might stimulate NGF generation. However, further experiments are needed to confirm this finding and explore the mechanism by which it occurs.
CONCLUSION
In this study, we fabricated collagen microsphere-encapsulated astrocytes by injecting a mixture of collagen and astrocytes into a cell culture medium. The size of microspheres can be controlled by regulating the flow rate of the collagen solution in a syringe attached to a syringe pump. The collagen microspheres crosslinked with 4S-StarPEG reduced the degradation rate of the microspheres subjected to collagenase digestion. The size of the microspheres encapsulating astrocytes decreased after the cells were cultured in the microspheres for 10 days. The viability of the cells in the crosslinked microspheres was higher than 90%. Astrocytes were transfected with plasmids encoding NGF-ires-EGFP genes by electroporation and were encapsulated in crosslinked microspheres. Then the amount of NGF secreted by the astrocytes in the microspheres was determined by ELISA assay. The level of NGF released into the cell culture medium was higher than that remaining in the microsphere or in astrocytes. When microsphere- encapsulated astrocytes transfected with plasmids encoding NGF-ires-EGFP genes were incubated with the cultured rat dorsal root ganglion, the axonal growth was significantly enhanced by NGF secreted by transfected astrocytes. This study demonstrated a method that can efficiently encapsulate astrocytes in collagen microspheres, and the astrocytes delivered by the microspheres can potentially be used to promote neural regeneration.
Acknowledgments
We acknowledge Li Yao’ s start-up funding, Wichita State University, and the National Institute of General Medical Sciences (P20 GM103418) of the National Institutes of Health.
Abbreviation
- IACUC
Institutional Animal Care and Use Committee
- 4S-StarPEG
poly(ethylene glycol) ether tetrasuccinimidyl glutarate
- NGF
nerve growth factor
- EGFP
enhanced green fluorescent protein
- OPC
oligodendrocyte progenitor cell
- EDC
1-ethyl-3-(3-dimethylaminopropryl) carbodiimide
- DRG
dorsal root ganglion
- FBS
fetal bovine serum
- DMEM
Dulbecco’s modified Eagle’s medium
- MSC
mesenchymal stem cell
- ADSC
adipose-derived stem cell
- GDNF
glial-derived neurotrophic factor
- NGC
nerve guidance conduit
- VEGF
vascular endothelial growth factor
Footnotes
The authors declare no competing financial interest.
Author Contributions
This manuscript was written with contributions from all authors who have approved the final version of the manuscript.
REFERENCES
- 1.Jin S, Ye K. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol Prog. 2007;23:32–41. doi: 10.1021/bp060348j. [DOI] [PubMed] [Google Scholar]
- 2.Sultana S, Khan MR, Kumar M, Kumar S, Ali M. Nanoparticles-mediated drug delivery approaches for cancer targeting: a review. J Drug Target. 2013;21:107–125. doi: 10.3109/1061186X.2012.712130. [DOI] [PubMed] [Google Scholar]
- 3.Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- 4.Kim YT, Caldwell JM, Bellamkonda RV. Nanoparticle-mediated local delivery of methylprednisolone after spinal cord injury. Biomaterials. 2009;30:2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranjan OP, Shavi GV, Nayak UY, Arumugam K, Averineni RK, Meka SR, Sureshwar P. Controlled release chitosan microspheres of mirtazapine: in vitro and in vivo evaluation. Arch Pharm Res. 2011;34:1919–1929. doi: 10.1007/s12272-011-1112-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q, Han B, Wang Z, Gao C, Peng C, Shen J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle:doxorubicin loading and in vitro and in vivo studies. Nanomedicine. 2007;3:63–74. doi: 10.1016/j.nano.2006.11.007. DOI: http://dx.doi.org/10.1016/j.nano.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Kettenmann H, Verkhratsky A. Neuroglia--living nerve glue. Fortschritte der Neurologie· Psychiatrie. 2011;79:588–597. doi: 10.1055/s-0031-1281704. [DOI] [PubMed] [Google Scholar]
- 8.Wang JJ, Chuan ML, Yew DT, Leung PC, Tsang DC. Effects of astrocyte implantation into the hemisected adult rat spinal cord. Neuroscience. 1995;65:973–981. doi: 10.1016/0306-4522(94)00519-b. [DOI] [PubMed] [Google Scholar]
- 9.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJA. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJA. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J. Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies SJA, Shih CH, Noble M, Mayer-Proschel M, Davies JE, Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PloS. one. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan C, Zheng Y, Cheng X, Qi X, Bu P, Luo X, Kim DH, Cao Q. Transplantation of D15A–expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int. J. Biol. Sci. 2013;9:78. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J. Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliot M, Smith GM, Siegal JD, Silver J. Astrocyte-polymer implants promote regeneration of dorsal root fibers into the adult mammalian spinal cord. Exp. Neurol. 1990;109:57–69. doi: 10.1016/s0014-4886(05)80008-1. [DOI] [PubMed] [Google Scholar]
- 15.Daly WT, Yao L, Abu-rub MT, O’Connell C, Zeugolis DI, Windebank AJ, Pandit AS. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials. 2012;33:6660–6671. doi: 10.1016/j.biomaterials.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Yao L, Daly W, Newland B, Yao S, Wang W, Chen BK, Madigan N, Windebank A, Pandit A. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Ther. 2013;20:1149–1157. doi: 10.1038/gt.2013.42. [DOI] [PubMed] [Google Scholar]
- 17.Yao R, Zhang R, Luan J, Lin F. Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication. 2012;4:025007. doi: 10.1088/1758-5082/4/2/025007. [DOI] [PubMed] [Google Scholar]
- 18.Natesan S, Baer DG, Walters TJ, Babu M, Christy RJ. Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng Part A. 2010;16:1369–1384. doi: 10.1089/ten.tea.2009.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan BP, Hui TY, Wong MY, Yip KH, Chan GC. Mesenchymal stem cellencapsulated collagen microspheres for bone tissue engineering. Tissue Eng Part C Methods. 2010;16:225–235. doi: 10.1089/ten.tec.2008.0709. [DOI] [PubMed] [Google Scholar]
- 20.Yao L, Phan F, Li Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res Ther. 2013;4:109. doi: 10.1186/scrt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houweling DA, Lankhorst AJ, Gispen WH, Bär PR, Joosten EA. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp Neurol. 1998;153:49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 22.Seyedhassantehrani N, Li Y, Yao L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr Biol (Camb) 2016;16:624–634. doi: 10.1039/c6ib00003g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang TMS. Semipermeable Microcapsules. Science. 1964;146:524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 24.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 25.Ricci M, Blasi P, Giovagnoli S, Rossi C, Macchiarulo G, Luca G, Basta G, Calafiore R. Ketoprofen controlled release from composite microcapsules for cell encapsulation: effect on post-transplant acute inflammation. J Control Release. 2005;107:395–407. doi: 10.1016/j.jconrel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32:1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott RB, Escobar L, Tan PL, Muzina M, Zwain S, Buchanan C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Prakash S, Soe-Lin H. Strategy for cell therapy: polymers for live cell encapsulation and delivery. Trends Biomater. Artif. Organs. 2004;18:24–35. [Google Scholar]
- 29.Taguchi T, Xu L, Kobayashi H, Taniguchi A, Kataoka K, Tanaka J. Encapsulation of chondrocytes in injectable alkali-treated collagen gels prepared using poly(ethylene glycol)-based 4-armed star polymer. Biomaterials. 2005;26:1247–1252. doi: 10.1016/j.biomaterials.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 30.Collin EC, Grad S, Zeugolis DI, Vinatier CS, Clouet JR, Guicheux JJ, Weiss P, Alini M, Pandit AS. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–2870. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Sanami M, Sweeney I, Shtein Z, Meirovich S, Sorushanova A, Mullen AM, Miraftab M, Shoseyov O, O’Dowd C, Pandit A, Zeugolis DI. The influence of poly(ethylene glycol) ether tetrasuccinimidyl glutarate on the structural, physical, and biological properties of collagen fibers. J Biomed Mater Res B Appl Biomater. 2016;104:914–922. doi: 10.1002/jbm.b.33445. [DOI] [PubMed] [Google Scholar]
- 32.Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339. doi: 10.1016/s0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 33.Nagai N, Kumasaka N, Kawashima T, Kaji H, Nishizawa M, Abe T. Preparation and characterization of collagen microspheres for sustained release of VEGF. J Mater Sci Mater Med. 2010;21:1891–1898. doi: 10.1007/s10856-010-4054-0. [DOI] [PubMed] [Google Scholar]
- 34.Wood MD, Kim H, Bilbily A, Kemp SW, Lafontaine C, Gordon T, Shoichet MS, Borschel GH. GDNF released from microspheres enhances nerve regeneration after delayed repair. Muscle Nerve. 2012;46:122–124. doi: 10.1002/mus.23295. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Yee WC, Hwang PY, Yu H, Wan AC, Gao S, Boon KL, Mao HQ, Leong KW, Wang S. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24:2405–2412. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 36.Fontana G, Srivastava A, Thomas D, Lalor P, Dockery P, Pandit A. Three-Dimensional Microgel Platform for the Production of Cell Factories Tailored for the Nucleus Pulposus. Bioconjug Chem. 2015;26:1297–1306. doi: 10.1021/bc5004247. [DOI] [PubMed] [Google Scholar]
- 37.Springer JE. Nerve growth factor receptors in the central nervous system. Exp Neurol. 1988;102:354–365. doi: 10.1016/0014-4886(88)90231-2. [DOI] [PubMed] [Google Scholar]
- 38.Gage FH, Björklund A. Trophic and growth-regulating mechanisms in the central nervous system monitored by intracerebral neural transplants. Ciba Found Symp. 1987;126:143–159. doi: 10.1002/9780470513422.ch9. [DOI] [PubMed] [Google Scholar]
- 39.Varon S, Conner JM. Nerve growth factor in CNS repair. J Neurotrauma. 1994;11:473–486. doi: 10.1089/neu.1994.11.473. [DOI] [PubMed] [Google Scholar]
- 40.Freed WJ. The role of nerve-growth factor (NGF) in the central nervous system. Brain Res Bull. 1976;1:393–412. doi: 10.1016/0361-9230(76)90033-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhao YZ, Jiang X, Xiao J, Lin Q, Yu WZ, Tian FR, Mao KL, Yang W, Wong HL, Lu CT. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater. 2016;29:71–80. doi: 10.1016/j.actbio.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin CL, Heron P, Hamann SR, Smith GM. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neuroscience. 2014;272:76–87. doi: 10.1016/j.neuroscience.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Zhang Z, Wang S, Lv D. Combined treatment with FK506 and nerve growth factor for spinal cord injury in rats. Exp Ther Med. 2013;6:868–872. doi: 10.3892/etm.2013.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]