Abstract
Background
Survivors of lower extremity (LE) malignancies experience functional deficits.
Purpose
The purpose of this prospective clinical trial was to determine feasibility and functional outcomes of adding pre-habilitation during the 10-12 week period prior to a planned surgery to remove the tumor in children and adolescents with a LE sarcoma.
Design
Pilot study.
Setting
St. Jude Children's Research Hospital (SJCRH).
Patients
Participants included 14 individuals between the ages of 8 and 20 years who were diagnosed with a LE sarcoma. Participant outcomes were compared to a control group of 35 individuals treated for osteosarcoma that obtained the same functional assessments but no pre-habilitation.
Intervention
The intervention group received strengthening exercises and mobility training 3 times per week for 30-60 minutes for 10-12 weeks preoperatively.
Measurements
Participants completed the Functional Mobility Assessment (FMA) and measures of strength and range of motion (ROM) of bilateral lower extremities (BLEs) at baseline, after 10-12 weeks of preoperative PT, and at 20-22 weeks.
Results
Twelve participants completed at least 50% of their schedule pre-habilitative sessions. The intervention group scored significantly better on the FMA than the control group at weeks 20-22 (35.6 vs. 25.7, p .0267). No significant difference was found in ROM or strength.
Limitations
Due to this study being a pilot study, the sample size was small. Therefore, we cannot infer generalizability.
Conclusions
Findings suggest that those diagnosed with a LE malignancy awaiting a limb sparing procedure or amputation participate in at least 50% of scheduled PT sessions and benefit from them.
Keywords: Pre-habilitation, pediatrics, cancer, adolescents, sarcoma, physical therapy, exercise
Introduction and Purpose
Survivors of childhood cancer are at increased risk for physical performance limitations and restricted abilities to participate in activities required to fully function in adult social roles.1 More specifically, functional outcomes for survivors of a lower extremity malignancy whose treatment required a limb sparing procedure or amputation are poor. Bekkering and peers2,3 evaluated survivors of osteosarcoma and Ewing sarcoma who had a limb sparing surgery or an ablative surgery. They determined that adolescents who received a limb-savage or ablative surgery had moderate disability according to the Toronto Extremity Salvage Score (TESS) and Baecke questionnaire as compared to healthy peers. Additionally, when compared to their siblings, long-term bone tumor survivors are 2.9 (95% CI 2.6-3.3) times more likely to report restricted personal skills, 6.3 (95% CI 4.5-9.0) times more likely to report restricted abilities to participate in routine activities, and 6.8 (95% CI 5.0-9.3) times more likely to indicate that poor health prevents school or work attendance.4 Data also indicate that those treated with a LE malignancy are at a 50% increased risk for activity limitations.5
Because exercise training is known to lead to skeletal muscle adaptations that increase both strength and endurance,6 addressing existing impairments preoperatively (pre-habilitation) may improve post-operative outcomes. Pre-habilitation is “a process on the cancer continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment and includes physical and psychological assessments that establish a baseline functional level, identify impairments, and provide interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments.”7 Pre-habilitation has shown to reduce the risk of death from disease in adult breast and colorectal cancer.8,9
Because previous studies highlight long-term problems with function and mobility among survivors of a LE malignancy who received a limb-sparing procedure or amputation, and because no studies have evaluated the feasibility and functional outcomes of pre-habilitation in this patient population, the aims of this study were to determine if children and adolescents were able to participate in strength and mobility training during 10-12 weeks of chemotherapy prior to limb sparing procedure or amputation. We also evaluated the impact of preoperative exercise training on function at 10-12 weeks postoperatively and compared their outcomes to those who did not receive pre-habilitation. We hypothesized that 60% of individuals with a malignancy of the LE would be able to participate in at least 50% of their scheduled physical therapy (PT) sessions before receiving a limb sparing procedure or amputation. In addition, we hypothesized that children who received pre-habilitation would demonstrate better functional outcomes both pre-surgically and at 10-12 weeks postoperatively when compared to a historical control group who received no preoperative PT intervention.
Methods
Setting and Participants
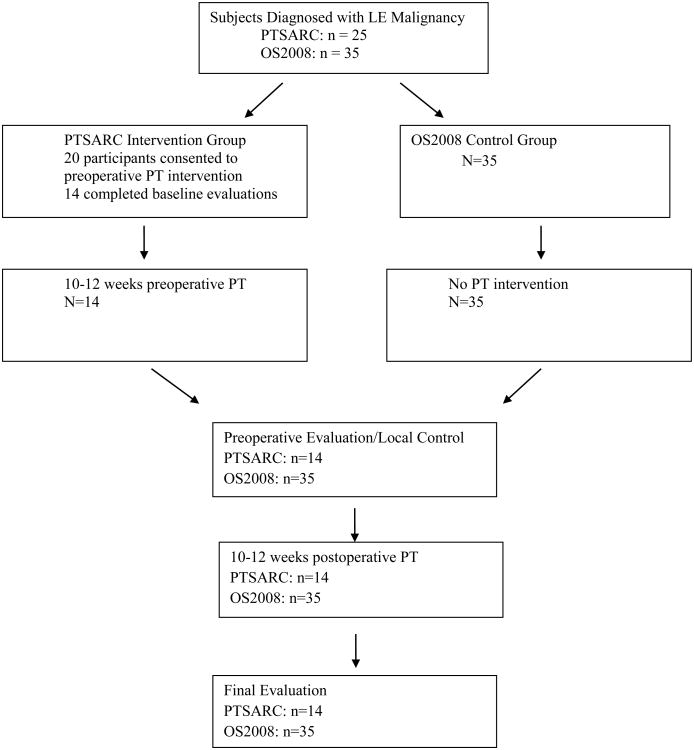
The Institutional Review Boards of SJCRH approved this study. Written, informed consent was obtained by parents or guardians, and assent was obtained per institutional policy. Participants included 14 children and adolescents between the ages of 8 and 20 years with biopsy confirmed LE malignancies between 2012 and 2016 and who were receiving treatment at SJCRH. Diagnoses included osteosarcoma, Ewing's sarcoma, and an undifferentiated sarcoma involving the soleus muscle. Study exclusion criteria for individuals in the intervention group were those with serious, non-healing wounds, ulcer or bone fracture (other than pathologic fracture), a pre-morbid condition that prevented the patient from ambulating, and patients whose pre-operative chemotherapy period was at least 10 weeks before receiving an amputation or limb sparing procedure. The control group included 35 age- and gender-matched individuals treated from 2008 to 2012 for LE osteosarcoma at SJCRH. The control group was evaluated at the same time points with the same outcome measures as the intervention group. They did not receive any preoperative PT or pre-habilitation, but did receive postoperative PT which is standard of care. There were no differences in medical and surgical management between the two groups. Current treatment protocols for LE malignancies typically include 10-12 weeks of chemotherapy to decrease size of tumor, perform local control (e.g., limb sparing procedure or amputation), then continue with chemotherapy for 10-12 weeks.10 Tumor necrosis is the desired outcome of providing chemotherapy preoperatively.11 Participants were seen both inpatient and outpatient at SJCRH by a licensed physical therapist. See Figure 1 for participants diagram through each stage of study. Of twenty-five individuals approached, twenty consented to participate, and fourteen (70%) completed all study assessments (baseline, pre-surgery, and post-surgery). One participant was deemed to be ineligible after consent, one expired prior to completing all assessments and four withdrew after consenting due to patient deciding against participating in study. One of the participants in the intervention group received 20 weeks of preoperative PT rather than 10-12 weeks due to a delay in surgery.
Figure 1.
Intervention
The intervention group received PT 3 times per week for a maximum of 60 minutes each session for 10-12 weeks. The therapy sessions included endurance, strengthening, and stretching exercises. Endurance exercise consisted of ambulating with assistive devices as needed, upper extremity (UE) ergometry, and/or playing Wii Sports, which is a video platform that promotes simulation of boxing, bowling, golf, tennis, and baseball. The therapist identified appropriate intensity and duration of the endurance exercise by monitoring heart rate and/or RPE.12 The desired heart rate range during exercise was 50-70% of their maximum heart rate. Maximum heart rate is defined as the difference between 220 and the patient's age.13 Fifty-70% of age predicted heart rate was the goal, but we also used symptom monitoring and RPE to manage PT sessions because individuals diagnosed with malignancies and who are receiving chemotherapy may have poor cardiorespiratory fitness.4 Strengthening exercises involved both UE and LE's. Participants were first instructed to perform 3 sets of 10 without any resistance and resistance was as added as appropriate. For UE's, bicep curls, triceps curls, shoulder flexion, and/or press-ups from the therapy mat or wheelchair were included. For the LE's, bridging, long arc quads, hamstring curls, calf raises in standing, single LE squats, and/or dorsiflexion (DF) in supine were performed on the uninvolved extremity. Stretching consisted of ankle stretch into DF in supine or long sitting and hamstring stretch in supine with hip flexed to 90 degrees. Stretching was held for 30-60 seconds for 2-3 sets.12 Exercise time and resistance were progressed per individual's medical status and per participant's tolerance. Progression was determined by ensuring participants were reporting 12-16 or the “somewhat hard to hard” area on the RPE scale. Participants participated in PT for at least 10 weeks prior to surgery and then 10-12 weeks after surgery, which was the usual standard of care. Usual standard of care begins with aggressive PT post-operative day 1. Patients are seen daily while inpatient and once discharged are seen 3 days per week outpatient. Depending on individual progress, frequency of sessions per week may continue at 3 days or may decrease to 1-2 days for 10-12 weeks. All patients are seen for 30 minutes each session. Treatment sessions included standing, transfer training, gait training, and strengthening and ROM exercises for bilateral LEs. PT sessions were modified per patient's precautions (i.e. non-weightbearing, no knee ROM). Subjects were seen by a licensed physical therapist both when inpatient and outpatient. Patients were not seen for PT if platelet count was less than 20,000mm3 and/or hemoglobin was less than 8g/dL.14
The historical control group did not receive any pre-habilitation except for gait training with an assistive device and/or to be fit with a knee brace. They received PT postoperatively which is usual standard of care.
Outcomes
Feasibility was defined as successful if at least 60% of patients were able to complete 50% or more of their scheduled PT sessions, as well as completion of baseline, pre-surgical, and post-surgical assessment (Table 1). We used 50% of sessions to indicate success because our previous experience with OS2008 was that children and adolescents did not participate in pre-habiliation at all due to the intensity of chemotherapy and the frequency of inpatient hospitalization for chemotherapy-related side effects. With this study, we wanted to keep the threshold low due to the uncertainty of participants being able to attend due to chemotherapy-related side effects.
Table 1.
| Test | Pre-Intervention | 10-12 Weeks (Pre-surgery) | 20-22 Weeks (Post-surgery) |
|---|---|---|---|
| Functional Mobility Assessment | X | X | X |
| Range of Motion using a goniometer | X | X | X |
| Strength using a myometer | X | X | X |
The Functional Mobility Assessment (FMA)15 was used to evaluate physical function. The FMA was validated specifically on children and adolescents with a LE sarcoma after surgical intervention to determine functional mobility. It includes six categories: (1) pain, (2) function using timed up and down stairs (TUDS) time and timed up and go (TUG) time, heart rate (HR) and rate of perceived of exertion (RPE) are also assessed during the TUDS and TUG, (3) use of assistive devices, (4) satisfaction with walking quality, (5) participation in work, school, sports, and (6) endurance measured by the 9-Minute Walk-Run (9MWR). Physiological cost index (PCI), heart rate (HR), and rate of perceived exertion (RPE) were also measured during the 9MWR. The maximum score that can be obtained on the FMA is 70 indicating the best functional outcome. (See FMA in Appendix)
Range of Motion (ROM) was measured both actively and passively using a goniometer16,17. Measurements included hip flexion and extension, hip abduction and adduction, hip internal and external rotation, knee flexion and extension, and ankle dorsiflexion and plantarflexion. ROM was attempted on bilateral lower extremities, however, due to precautions and to prevent injury, the involved extremity may not have been measured.
Strength was measured using a Chatillon MSC-500 hand held myometer. The strength was measured in Newtons. Strength assessment was measured using the break test18. The break test required the participants to hold the extremity after placed in the position below and not allow the examiner to “break” the hold. Hip flexion, knee extension, and ankle dorsiflexion was tested but only on the unaffected lower extremity. Strength was only obtained in the intervention group.
Statistical Analysis
For demographic and clinical variables, means and standard deviations were calculated for continuous variables and two-sample t-tests were conducted to compare the characteristics between participants and controls. Frequencies were calculated for categorical variables and Chi-Square tests were used to assess significance. Multivariable linear regression analysis was used to test the pre-surgery PT intervention effect on the post-surgery FMA, adjusted for pre-intervention FMA, sex, age at diagnosis, and surgical procedure. One-sample t-tests were applied to compare mean change from zero (0) in strength in the uninvolved extremity from pre-intervention to pre-surgery, pre-surgery to post-surgery, and pre-intervention to post surgery, respectively. Statistical significance was pre-defined at a p-value < 0.05, without adjustment for multiple testing. All analyses were conducted in SAS 9.3 (SAS Institute, Cary NC) or StatXact v11.1 (Cytel Inc, Cambridge MA).
Results
The characteristics of the study population for each group are depicted in Table 2. There were no significant differences in demographic variables between the two groups except for diagnosis. All participants in the control group had osteosarcoma and most of the children and/or adolescents in the intervention group had osteosarcoma as well at 78.6%. Mean (SD) ages at time of baseline evaluation were 13.5 (3.5) for intervention group and 13.1 (3.5) for the control group. More females were in the control group (45.7%) than in the intervention group (28.6%). Most individuals in the intervention and control group had a limb sparing procedure of their femur (30.0% and 54.3%, respectively). The involved extremity was nearly equal in terms of laterality in the control group at 51.4% of the left lower extremity (LLE) and 48.6% of the right lower extremity (RLE). The involved extremity in the intervention group was 64.3% affecting the LLE and 35.7% affecting the RLE.
Table 2.
| Patient Demographics | Intervention N=14 (%) | Control N=35 (%) |
|---|---|---|
| Gender | ||
| F | 4 (28.6) | 16 (45.7) |
| M | 10 (71.4) | 19 (54.3) |
| Race | ||
| Black | 1 (7.1) | 10 (28.6) |
| Other | 4 (28.6) | 5 (14.3) |
| White | 9 (64.3) | 20 (57.1) |
| Age at diagnosis | ||
| Mean (SD) | 13.5 (3.5) | 13.1 (3.5) |
| Laterality | ||
| Left | 9 (64.3) | 18 (51.4) |
| Right | 5 (35.7) | 17 (48.6) |
| Local control | ||
| AKA | 2 (14.2) | 2 (5.7) |
| BKA | 3 (8.6) | |
| Hemipelvectomy | 1 (2.9) | |
| Hip disarticulation | 1 (2.9) | |
| Limb sparing – femur | 7 (30.0) | 19 (54.3) |
| Limb sparing Tibia or Fibula | 5 (35.7) | 9 (25.7) |
| Diagnosis | ||
| Osteosarcoma | 11 (78.6) | 35 (100.0) |
| Ewing sarcoma | 2 (14.3) | |
| Chondroblastoma | 1 (7.1) |
Feasibility
Twelve of the 14 participants (85%) in the intervention group completed at least 50% of their scheduled PT sessions. The other two participants attended 43% and 47% of their scheduled sessions. Reasons for not attending sessions included: illness, previous appointment ran late, and unknown reasons.
Physical Performance
After adjusting for age at diagnosis, sex, and surgical procedure, those in the intervention group had a significantly higher score (10 points) on the FMA than those in the control group at 20-22 weeks (Table 3), and the significant difference did not change after adjusting for pre-intervention FMA total score, sex, age at diagnosis, and surgical procedures (p=0.03, data not shown). This was primarily due to their better performance on the 9MWR. When compared to controls, whose FMA scale score on the 9MWR test decreased by 0.7 points, intervention group participants improved by 0.6 points from baseline to 20-22 weeks (p=0.03, data not shown).
Table 3. Mean and SD of FMA for Control and Intervention Groups.
| Baseline | 10-12 Weeks | 20-22 Weeks | ||||
|---|---|---|---|---|---|---|
| FMA (0-5) | Control | Intervention | Control | Intervention | Control | Intervention |
| Pain | 4.0 (1.3) | 4.3 (1.2) | 4.5 (1.2) | 4.6 (1.3) | 4.2 (1.2) | 4.7 (0.6) |
| TUG time | 0.5 (1.1) | 0.2 (0.6) | 0.7 (1.5) | 0.4 (1.1) | 0.5 (1.1) | 0.6 (1.3) |
| TUG HR | 4.1 (1.5) | 4.7 (0.6) | 4.1 (1.6) | 4.1 (0.9) | 4.3 (1.1) | 4.0 (1.6) |
| TUG RPE | 4.1 (1.6) | 4.9 (0.5) | 4.4 (1.4) | 4.6 (0.7) | 4.6 (0.9) | 4.6 (0.7) |
| TUDS time | 0.6 (1.4) | 0.0 (0.0) | 0.7 (1.5) | 0.0 (0.0) | 0.5 (1.1) | 0.5 (1.2) |
| TUDS HR | 2.4 (1.9) | 2.8 (1.6) | 2.4 (1.9) | 2.3 (2.1) | 2.8 (1.8) | 2.7 (1.8) |
| TUDS RPE | 3.1 (1.8) | 3.9 (0.9) | 3.0 (1.7) | 3.3 (1.5) | 3.0 (1.2) | 3.4 (1.2) |
| Supports | 1.4 (1.7) | 0.8 (0.7) | 1.5 (1.5) | 0.8 (0.7) | 2.0 (1.9) | 2.1 (1.8) |
| Satisfaction | 2.9 (1.2) | 3.4 (0.9) | 3.2 (1.2) | 3.5 (0.5) | 3.2 (0.9) | 3.6 (0.9) |
| 9MWR Distance | 1.5 (1.7) | 0.9 (0.9) | 1.2 (1.0) | 1.1 (0.9) | 0.9 (1.0) | 1.3 (1.1) |
| 9MWR HR | 2.5 (1.4) | 2.3 (1.0) | 1.8 (1.3) | 2.0 (0.9) | 2.3 (0.9) | 2.6 (1.2) |
| 9MWR RPE | 2.4 (1.7) | 2.3 (1.4) | 1.9 (1.8) | 2.1 (1.4) | 1.8 (1.5) | 2.2 (1.4) |
| 9MWR PCI | 0.9 (1.5) | 0.3 (0.6) | 0.7 (1.4) | 0.5 (0.7) | 0.3 (0.6) | 0.5 (0.8) |
| Participation | 1.3 (1.3) | 2.0 (1.6) | 1.6 (1.3) | 2.9 (1.3) | 2.3 (1.5) | 3.1 (1.7) |
| FMA Total Score (0-70) | 25.9 (16.0) | 31.9 (5.0) | 28.9 (14.6) | 31.1 (6.8) | 25.7 (13.7) | 35.6 (10.3) |
ROM
Table 4 includes the mean and standard deviation of range of motion measurements for both involved and uninvolved extremity extensions, among both the intervention and control groups at each time point. There were no detectable differences between groups or between extremities within groups at any time point.
Table 4.
| Baseline | 10-12 Weeks | 20-22 Weeks | ||||
|---|---|---|---|---|---|---|
| AROM | Control | Intervention | Control | Intervention | Control | Intervention |
| Involved Extension | -13 (16.1) | -3.6 (4.2) | -8.3 (10.6) | -0.5 (1.2) | -3.8 (7.3) | -3.7 (5.1) |
| Uninvolved Extension | -1.3 (2.8) | -1.9 (3.4) | -0.8(2.2) | -0.3 (1.1) | -1.6 (4.6) | 0.0 (0.0) |
| Extension Difference (Uninvolved-Involved) | 11.5 (16.1) | 1.6 (4.5) | 7.6 (10.9) | 0.2 (1.0) | 2.9 (7.9) | 3.7 (5.1) |
| Involved Flexion | 102 (42.3) | 128 (45.6) | 123 (33.8) | 146 (8.1) | 86.4 (44.9) | 96.8 (48.7) |
| Uninvolved Flexion | 143 (11.0) | 149 (7.5) | 145 (9.5) | 149 (7.0) | 144 (9.1) | 146 (9.0) |
| Flexion Difference (Uninvolved-Involved) | 40.8 (43.1) | 21.0 (44.2) | 22.7 (31.8) | 3.4 (8.1) | 56.9 (42.9) | 48.7 (54.1) |
Strength
Strength measures for the intervention group are shown in Table 5. Strength declined from baseline to post surgery and did not recover by 20-22 weeks in the intervention group.
Table 5.
| Strength of Uninvolved Extremity | Time1 (n=13) | Time2 (n=14) | Time3 (n=13) | Pre-Intervention | 10-12 Weeks (Pre-surgery) | 20-22 Weeks (Post-surgery) | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ttest-p* | Mean (SD) | ttest-p* | Mean (SD) | ttest-p* | |
| Dorsiflexion | 273 (112) | 234 (106) | 215 (119) | -38.9 (73.1) | 0.0793 | -15.9 (88.1) | 0.5265 | -71.4 (105.0) | 0.0382 |
| Hip Flexion | 189 (71.9) | 183 (71.6) | 153 (84.6) | -17.1 (27.2) | 0.0426 | -28.8 (40.5) | 0.0249 | -50.0 (51.5) | 0.0063 |
| Knee Extension | 269 (112) | 229 (97.2) | 208 (128) | -48.6 (62.9) | 0.0215 | -21.5 (76.9) | 0.3341 | -76.0 (85.6) | 0.0106 |
Discussion
To our knowledge, this is the first study to evaluate the feasibility and initial efficacy of pre-habilitation in children, adolescents and young adults receiving chemotherapy for a lower extremity malignancy and awaiting a limb sparing procedure or amputation. This pilot study indicates that 85% of children who are awaiting local control while undergoing chemotherapy for a lower extremity malignancy can successfully complete 10-12 weeks of pre-habilitation, and that such a program has potential to significantly improve their physical fitness and functional mobility.
We predicted that 60% of individuals would attend at least 50% of their scheduled PT session preoperatively. Eighty-five percent of the intervention group participants completed at least 50% of their preoperative PT. This finding is consistent with another study evaluating functional outcomes after spinal surgery. Nielson found that 85% of the 28 adults in the intervention group were able to complete an 8-week exercise preoperative PT program.19 Our feasibility outcome was slightly better than one study that recruited 10 adult men diagnosed with prostate cancer to participate in a 6-week, twice-weekly preoperative PT program before undergoing a prostatectomy.20 They found that 50% of participants completed more than 80% of the 12 training sessions and only 1 missing more than 6 sessions. Of note, the individuals in the aforementioned studies were not receiving chemotherapy during pre-habilitation. However, in all studies discussed, there is demonstration of moderate to high probability that individuals can participate in a preoperative PT program.
Predicted maximum heart rate at 50-70% was used for this study along with RPE without any adverse effects in our patient population. This calculation was an easy, safe way to guide treatment and ensure effective cardiovascular training that lead to better functional outcomes in this pilot study. Future studies could compare predicted maximum heart rate calculation and exercise testing to determine heart rate range for pre-habilitation in this patient population.
Our study found an increase in walking distance on the 9MWR and a higher score on the FMA at week 20-22 in the intervention group when compared to the control group. Most research on functional outcomes of pre-habilitation has been performed on individuals awaiting a total hip or knee arthroscopy in the adult population. A literature review performed by Gill and McBurney21 found that adults who participated in preoperative PT before a total hip replacement surgery had better self-reported function than controls. In one study, twenty-six individuals 50 years of age and older participated in pre-habilitation before receiving a total knee arthroscopy (TKA). They were compared to 28 individuals who received usual standard of care of post-operative PT. The intervention group on average received 13 sessions preoperatively and exercises consisted of flexibility, resistance, and step training. All individuals were evaluated at baseline, preoperatively, 1 month postoperatively, and 3 months postoperatively. At 1 and 3 months post-operatively, the intervention group demonstrated an improvement in function; whereas, the control group had a significant decrease in their 6-minute walk distance at 1 month post-operatively.22
Strength declined in the intervention group overtime and no change was seen in ROM between groups. A literature review performed by Baker and McKeon23 had similar findings in individuals awaiting a total knee arthroscopy. They looked at the geriatric population and determined that individuals who received preoperative PT did not differ from the control groups in terms of strength and ROM.
Future research should examine if there is a difference in strength of the uninvolved extremity between individuals who received preoperative PT and those that did not. Our study did not have strength measurements for the control group; therefore, we were unable to compare the two groups. While our study showed a decline in strength from baseline to weeks 20-22, it is possible the decline was not as severe as it could have been if PT was not administered before surgery. Another limitation of the study was that the two groups were treated at different time periods, PTSARC from 2012-2016 and OS2008 from 2008-2012. Since previous studies have determined long term functional deficits and decreased physical fitness, it would be of importance if future research would perform an evaluation several years postoperatively to determine long term effects when a preoperative PT program was administered. Lastly, a randomized controlled trial with larger sample sizes should be performed to strengthen the argument to administer PT before local control while undergoing chemotherapy.
Conclusion
It is well documented that lasting functional deficits occur as a result of a local control and chemotherapy1-4,24. By adding a pre-habilitation regimen to those awaiting a limb sparing procedure or amputation while undergoing chemotherapy, it is possible to negate or lessen the functional deficits found in these individuals. Ongoing investigations into comparing strength between groups and longer follow-up times to determine if function and physical fitness are maintained overtime are warranted.
Acknowledgments
This study was financially supported by a grant from the Tennessee Physical Therapy Association. I would like to thank Dr. Kiri Ness for her support in creating and administering this study. I would also like to thank my fellow physical therapists at SJCRH. Without their support, this study would not have been possible.
Appendix
Table 2.
Functional mobility assessment.
| Score | Pain 0–10 scale (0 = none, 10 = worst) | Function TUDS | TUG | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Time (seconds) | HR (beats per minute) | RPE (6–20 scale) | Time (seconds) | HR (beats per minute) | RPL (6–20 scale) | ||
| 5 | 0 | ≤8 | ≤127 | ≤7 | ≤4 | ≤127 | ≤7 |
| 4 | 1–2 | 9–12 | 128–137 | 8–9 | 5 | 128–137 | 8–9 |
| 3 | 3–1 | 13–16 | 138–147 | 10–11 | 6 | 138–147 | 10–11 |
| 2 | 5–6 | 17–20 | 148–157 | 12–13 | 7 | 148–157 | 12–13 |
| 1 | 7–8 | 21–24 | 158–167 | 14–15 | 8 | 158–167 | 14–15 |
| 0 | 9–10 | >24 | >167 | >15 | >8 | >167 | >15 |
| Subtotal score | |||||||
| Score | Supports | Satisfaction with my walking quality | Participation | Endurance (9-min walk run) | |||
|
|
|||||||
| Distance (feet | PCI | HR | RPE | ||||
|
|
|||||||
| 5 | None | Very satisfied | Participate in work or school and sports | ≥4000 | ≤0.20 | <115 | ≤7 |
| 4 | 1 crutch or cane <5 h a day | Happy with my walking quality | Participate in all activities including work/school, but limited sports | 3000–3999 | 0.21–0.41 | 115–134 | 8–9 |
| 3 | 1 crutch or cane all day | Happy but hope it improves | Limited in work or school and sports | 2250–2999 | 0.42–0.62 | 135–154 | 10–11 |
| 2 | 2 crutches or canes <5 h a day | Disappointed and want to improve | Limited in work or school and do not participate in sports | 1500–2249 | 0.63–483 | 155–174 | 12–13 |
| 1 | 2 crutches or canes >5 h a day | Disappointed and afraid it will not improve | Limited in work or school and activities of daily living | 1000–1499 | 0.84–1.09 | 175–194 | 14–15 |
| 0 | Wheelchair | Very unhappy and rarely leave the house | Unable to go to work or school | <1000 | ≥1.10 | ≥195 | >15 |
| Subtotal | |||||||
| Total (add all 14 boxes (range 0–70) | |||||||
Timed up and down stairs (TUDS), timed up and go (TUG), heart rate (HR), rating of perceived exertion (RPL), physiological cost index (PCI)PCl = HR end of walk -HR beginning of walk (/metres walkcd/9 min.
References
- 1.Fernandez-Pineda I, Hudson MM, Pappo AS, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St. Jude Lifetime Cohort Study. Journal of cancer survivorship : research and practice. 2016 doi: 10.1007/s11764-016-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekkering WP, Vliet Vlieland TP, Koopman HM, et al. Functional ability and physical activity in children and young adults after limb-salvage or ablative surgery for lower extremity bone tumors. Journal of surgical oncology. 2011;103(3):276–282. doi: 10.1002/jso.21828. [DOI] [PubMed] [Google Scholar]
- 3.Bekkering WP, Vliet Vlieland TP, Koopman HM, et al. Quality of life in young patients after bone tumor surgery around the knee joint and comparison with healthy controls. Pediatric blood & cancer. 2010;54(5):738–745. doi: 10.1002/pbc.22439. [DOI] [PubMed] [Google Scholar]
- 4.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Annals of internal medicine. 2005;143(9):639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Marina N, Hudson MM, Jones KE, et al. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: a report from the childhood cancer survivor study. Archives of physical medicine and rehabilitation. 2013;94(6):1062–1073. doi: 10.1016/j.apmr.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair SN, Morris JN. Healthy hearts--and the universal benefits of being physically active: physical activity and health. Annals of epidemiology. 2009;19(4):253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA: a cancer journal for clinicians. 2013;63(5):295–317. doi: 10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 8.Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast cancer research : BCR. 2009;11(5):106. doi: 10.1186/bcr2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 10.Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. The Lancet Oncology. 2016;17(10):1396–1408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(1):76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 12.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatric blood & cancer. 2004;42(2):127–133. doi: 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- 13.Target Heart Rate and Estimated Maximum Heart Rate. 2015 [Google Scholar]
- 14.Ghasemi Z, M T. Laboratory values in the intensive care unit. Newsletter of the acute care/hospital clinical practice section. 1995 [Google Scholar]
- 15.Marchese VG, Rai SN, Carlson CA, et al. Assessing functional mobility in survivors of lower-extremity sarcoma: reliability and validity of a new assessment tool. Pediatric blood & cancer. 2007;49(2):183–189. doi: 10.1002/pbc.20932. [DOI] [PubMed] [Google Scholar]
- 16.Piriyaprasarth P, Morris ME. Psychometric properties of measurement tools for quantifying knee joint position and movement: a systematic review. The Knee. 2007;14(1):2–8. doi: 10.1016/j.knee.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Pua YH, Wrigley TV, Cowan SM, Bennell KL. Intrarater test-retest reliability of hip range of motion and hip muscle strength measurements in persons with hip osteoarthritis. Archives of physical medicine and rehabilitation. 2008;89(6):1146–1154. doi: 10.1016/j.apmr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Kendall FP, M E, Provance PG, Rodgers MM, Romani WA. Muscles: Testing and Function With Posture and Pain. 5th. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 19.Nielsen PR, Jorgensen LD, Dahl B, Pedersen T, Tonnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clinical rehabilitation. 2010;24(2):137–148. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 20.Singh F, Newton RU, Baker MK, et al. Feasibility of Presurgical Exercise in Men With Prostate Cancer Undergoing Prostatectomy. Integrative cancer therapies. 2016 doi: 10.1177/1534735416666373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill SD, McBurney H. Does exercise reduce pain and improve physical function before hip or knee replacement surgery? A systematic review and meta-analysis of randomized controlled trials. Archives of physical medicine and rehabilitation. 2013;94(1):164–176. doi: 10.1016/j.apmr.2012.08.211. [DOI] [PubMed] [Google Scholar]
- 22.Topp R, Swank AM, Quesada PM, Nyland J, Malkani A. The effect of prehabilitation exercise on strength and functioning after total knee arthroplasty. PM & R : the journal of injury, function, and rehabilitation. 2009;1(8):729–735. doi: 10.1016/j.pmrj.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Silkman Baker C, McKeon JM. Does preoperative rehabilitation improve patient-based outcomes in persons who have undergone total knee arthroplasty? A systematic review. PM & R : the journal of injury, function, and rehabilitation. 2012;4(10):756–767. doi: 10.1016/j.pmrj.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Gerber LH, Hoffman K, Chaudhry U, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Archives of physical medicine and rehabilitation. 2006;87(12):1611–1617. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]