Abstract
Esophageal cancer is a malignant neoplasm with poor outcomes. Determination of local disease progression is a major determining factor in treatment modality, radiation dose, radiation field and subsequent surgical therapy. Discrimination of true tumor extent is difficult given the similarity of soft tissues of the malignancy compared to non-malignant tissues using current imaging modalities. A possible method to discriminate between these tissues may be to exploit mechanical properties to diagnostic advantage, as malignant tissues tend to be stiffer relative to normal adjacent tissue. Shear waves propagate faster in stiffer tissues relative to softer tissues. This may be measured by using ultrasound based shear wave vibrometry. In this method, acoustic radiation force is used to create a shear wave in the tissue of interest and ultrafast ultrasound imaging is used to track the propagating wave to measure the wave velocity and estimate the shear moduli. In this study we created simulated malignant lesions (1.5 cm length) using radiofrequency ablation in ex vivo esophageal samples with varied progression (partial thickness n = 4, and full thickness n = 5) and used normal regions of the same esophageal specimen as controls. Shear wave vibrometry was used to measure shear wave group velocity and shear wave phase velocity in the ex vivo specimens. These values were used to estimate shear moduli using an elastic shear wave model and elastic and viscoelastic Lamb wave models. Our results show that the group and phase velocities increase due to both full and mucosal ablation, and that discrimination may be provided by higher order analysis using viscoelastic Lamb wave fitting. This technique may have application for determination of extent of early esophageal malignancy and warrants further investigation using in vivo approaches to determine performance compared to current imaging modalities.
Keywords: Esophagus, ablation, cancer, shear wave, Lamb wave, malignancy
Introduction
Cancers of the gastrointestinal tract (GI), such as esophageal cancer have significant impact on patient quality and duration of life. Esophageal cancer is a common condition, with approximately 500,000 new cases worldwide per year, and is increasing in the United States [1], [2]. Recently, there has been a shift from the historical predominance of esophageal squamous cell carcinoma to increased rates and incidence of adenocarcinoma [1], [2] in the Western world, presumably due to decreased smoking, increased endoscopic surveillance of Barrett’s esophagus, gastroesophageal reflux disease, and obesity [3], [4]. In Asian countries where endoscopic surveillance is common for squamous cell carcinoma of the esophagus there has been an increase in early stage cancers with minimal invasion [5], [6]. Ultimately, the optimal treatment of these cancers depends on resectability which is based on degree of tumor invasion, location, and metastatic spread [7].
The prognosis of esophageal malignancy of both squamous cell and adenocarcinoma depend on the degree of tumor extension outside of the esophagus, the degree of lymph node involvement, as well as metastatic spread [8]. Obtaining accurate information on degree of disease extension throughout the patient’s body is essential to providing optimal clinical therapy. While distant disease may be treated with chemotherapeutics and radiation, local disease may be amenable to surgical or endoscopic approaches [5], [9] with or without a neoadjuvant chemotherapeutic regimen. Current treatment requires resection for definitive determination of malignant extent [10]. Obtaining information about extent of tumor involvement is essential.
Current diagnostic approaches using magnetic resonance imaging, x-ray computed tomography (CT), and positron emission tomography with or without CT fusion, while useful in determining metastatic spread [11], [12], provide poor visualization to determine local tumor extent [11], lymph node disease, and tumor stage. Endoscopic ultrasound (EUS) provides superior determination of local tumor and nodal status [11]. Unfortunately, there are limitations with EUS as it depends solely on the echogenic tissue differences and requires higher frequency to provide optimal spatial resolution, therefore it suffers significant attenuation with tissue depth. Current probe technology for EUS typically utilizes frequencies of 12–20 MHz with soft tissue penetration depths of 29 mm and 18 mm, as probe frequency increases over this range [13]. While for superficial malignancies this may provide complete depth evaluation, for bulkier tumors these penetrations are inadequate for complete depth evaluation and provide suboptimal soft tissue contrast for both early and late stage tumors. Despite drawbacks in fundamental physics associated with EUS it remains the current standard of care when combined with biopsy for determination of diagnosis and depth of invasion [14]. Unfortunately, biopsy sampling combined with EUS may not provide determination of true tumor longitudinal or circumferential or depth of invasion, which would have significant clinical utility to determine radiation field, response to therapy, and definitive surgical or endoscopic resection.
It is well accepted that malignant tissues in a variety of organ systems are stiffer than their nonmalignant counterparts [15]–[17]. On the cellular level, it appears that malignancy is correlated with less stiff and more deformable cells [18]–[21], which may facilitate metastatic spread. However, data suggests that on the macroscopic tissue level malignant disease may be strongly related to increased stiffness [22]–[25]. While the cause of increased stiffness in esophageal malignancy is unclear, in other cancers this increased stiffness may be from both increased collagen content [26], [27] and increased collagen cross linking [28]. While there are biomarkers for detection of malignancy in a variety of organ systems, there is no such chemical biomarker currently for esophageal cancer. A possible avenue for identification of a biomarker for esophageal cancer may be biomechanical properties themselves, and may be related to metastatic disease [22].
An approach to provide this information is leveraging tissue mechanical properties to determine true extent and degree of invasion. One way to investigate material properties is using shear wave elastography [29]. This method typically uses focused ultrasound to “push” on the tissue internally to generate a propagating shear wave. The shear wave velocity is related to the stiffness of the medium, so measurement of the wave velocity can provide quantitative information related to the tissue material properties. Using shear wave velocity variations in different tissue may allow comparison of native unaffected esophageal tissue compared to malignant tissue. This may provide a more optimal approach to determining extent of disease in respiratory and GI malignancy such as esophageal, colonic, and gastric carcinomas. Within this work we apply ultrasound-based shear wave vibrometry to characterize ex vivo swine esophagi and compare material changes in control and radiofrequency ablated esophageal tissue simulating malignant esophageal lesions of varying depth.
Methods
Specimen Preparation
Nine esophagi were harvested from 60–70 kg cross bred domestic swine consistent with Institutional Animal Care Use Committee guidelines. The complete esophagus was extirpated after animal sacrifice. Esophageal tissue was placed in PBS buffered crystalloid solution (NaCl 137 mmol/L KCL 2.7 mmol/L Na2HPO4 10mmol/L KH2PO4 1.8 mmol/L) and stored at −80 °C. Esophageal specimens were gradually rewarmed in PBS to atmospheric temperature (assumed 25 °C). Specimens were then inverted so the mucosa was extra-luminal and the muscular surface was luminal. This was performed to allow surface ultrasound probe use. The specimens were then ablated in either full thickness (entire esophageal wall, mucosa through muscular layer) (n = 5) or partial thickness (mucosa and submucosa only) (n = 4) using radiofrequency (RF) energy (Force 2 CEM, Valleylab, Boulder, CO) [30]–[33] by inserting the RF probe into an incision in the mucosal-submucosa complex with a non-conductive surface deeper to isolate the thermal energy from the muscular layer. Further thermal protection using a 60 French bougie dilator inserted luminally was used to prevent ablation of the radial opposite wall. Full thickness ablation was performed similarly without the non-conductive probe between the mucosal-submucosa complex and muscular layers. Ablations were generated to be approximately 1.5 cm in length.
The specimen was then trimmed so 12 cm of mid-thoracic esophagus was isolated which was then mounted on cannulae fixed with plastic securement ties so that approximately 10 cm of esophageal tissue was exposed. The mounting apparatus was then immersed in a bath of degassed water. The lumen was then infused with degassed water and allowed to equilibrate with atmospheric pressure. A Verasonics V-1 programmable ultrasound instrument (Verasonics, Inc., Kirkland, WA) was used for the experiments. The ultrasound shear wave elastography was performed with a linear array transducer (L7–4, Philips Healthcare, Andover, MA). The speed of sound in the water media is assumed 1480 m/s.
Mechanical Property Characterization
The characterization of the mechanical properties of the esophagi after generation of simulated malignant lesions was performed using ultrasound-based shear wave vibrometry. Shear wave vibrometry constitutes the principle behind a number of methods that have been used for more than two decades to assess non-invasively the status of different body tissues under healthy and pathological conditions. Shear wave vibrometry was used to directly measure shear wave group velocity and shear wave phase velocity in excised porcine esophagi. Shear wave vibrometry measurements were then used to detect changes in esophageal stiffness due to ablation. The ablated zone (1.5 cm in length) was assessed for shear wave velocity and the non-ablated wall of the esophagus was used as a control.
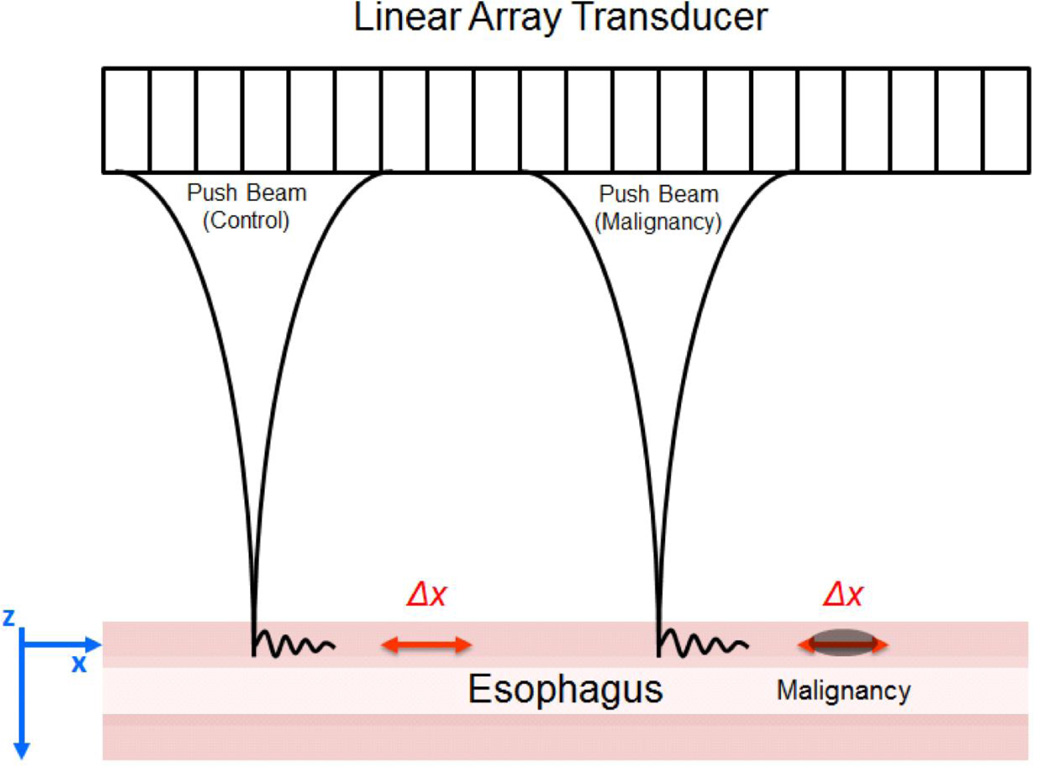
Shear wave vibrometry uses acoustic radiation force (ARF) to excite propagating waves in the esophagus and plane wave imaging to track the wave motion. The excitation force is in the form of a short impulse that is 400 µs in duration and is focused at a user-defined position (push beam) with the ultrasound transducer as shown in Fig. 1. This impulsive ARF perturbs the tissue and creates waves that propagate perpendicular to the z-axis (along x-axis in Fig. 1).
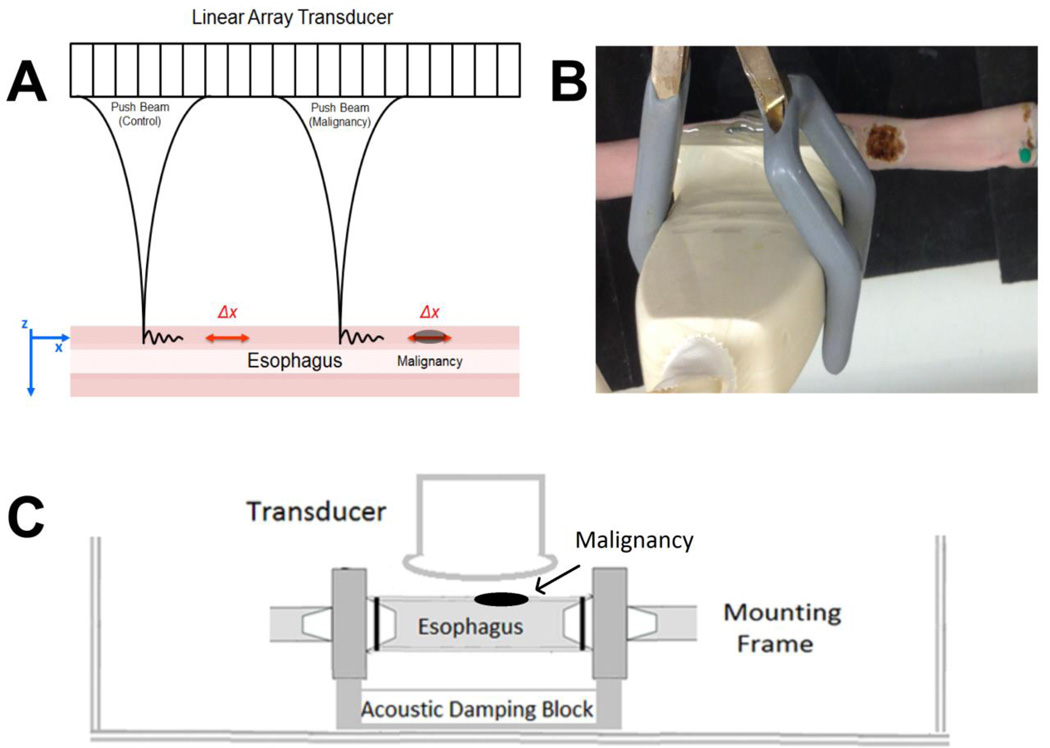
Figure 1.
Experimental setup with linear array transducer performing shear wave vibrometry on an esophageal specimen. A. The experiments were performed with the push beams focused in normal esophagus (control) positioned on the left, and near the simulated malignancy on the right. B. A top view of the esophagus and transducer immersed in a water tank with degassed water. C. Diagrammatic scheme of the experimental conditions.
Following the wave excitation, the probe is switched to a plane wave imaging mode to track wave propagation at a frame rate of 12 kHz (detection beam). The acquired in-phase/quadrature (IQ) data from the Verasonics system (V1, Verasonics, Inc., Kirkland, WA) using the linear array transducer (L7–4, Philips Healthcare, Andover, MA) was analyzed using autocorrelation analysis to obtain the particle velocity [34]. This allows us to track wave propagation as a function of time (t) and distance (x). Under the assumptions that the tissue is locally homogeneous, linear, elastic and isotropic the group velocity can be related to the mechanical properties of the tissue. Group velocity can be estimated by calculating the slope of the impulse propagation as a function of time and distance [35]. The group velocity (cg) can be used to calculate the elastic shear modulus using μ = ρmcg2 where tissue density ρm is assumed to be 1000 kg/m3.
In reality, most biological tissues are not purely elastic but inherently viscoelastic, and therefore in addition to group velocity measures it is necessary to obtain estimators that account for the viscous nature of the tissue such as phase velocity in order to uniquely characterize the tissue mechanical properties. Additionally, the esophagus is not a large organ like the liver or breast, so the propagating waves are affected by the boundaries of the esophagus.
Lamb wave Dispersion Ultrasound Vibrometry (LDUV) is a technique developed and validated by our laboratory described in the original paper by Nenadic, et al. [36] for measuring mechanical properties of boundary sensitive soft tissues such as free-wall myocardium, arterial wall, bladder, and tendons [37]–[39], and in this case the esophagus.
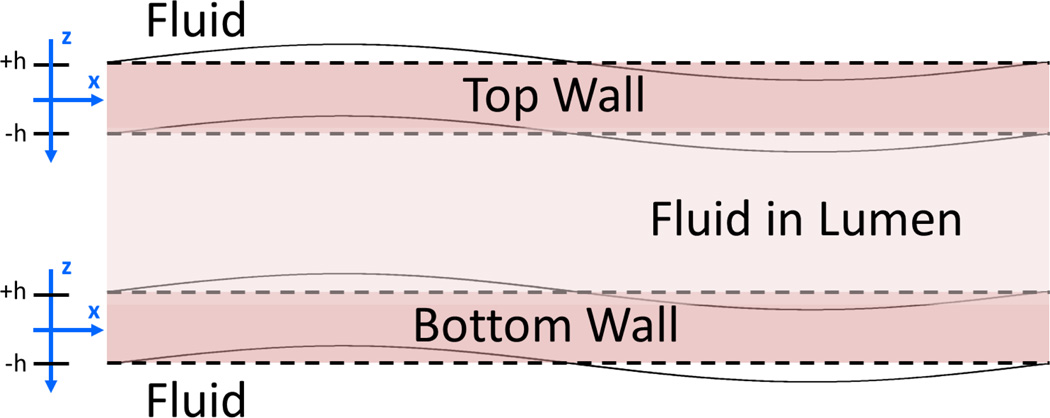
For the purpose of this application, we assumed that wave propagation in the top and bottom wall can be approximated by wave propagation in a plate of the same thickness as the wall of the esophagus Fig 2. This approximation has been validated in a variety of settings [37]–[39]. The wall of the esophagus is approximated as an incompressible, homogenous, isotropic solid submerged in an incompressible nonviscous fluid. The equation governing the Lamb wave dispersion in the esophageal wall is
| (1) |
where kL = ω/cL is the Lamb wave number, ω is the angular frequency, cL is the frequency dependent Lamb wave velocity, is the shear wave number, μ is the shear modulus written in terms of the elastic (μ1) and viscous (μ2) components so that μ = μ1 + iωμ2, ρm is the density of the sample (assumed) and h is the half-thickness of the sample. Equation (1) is fit to the experimentally measured Lamb wave dispersion curves (velocity versus frequency) to obtain elasticity and viscosity coefficients μ1 and μ2.
Figure 2.
Coordinate system used for Lamb wave modeling for esophagus walls surrounded by fluid (water).
The phase velocities of the propagating waves were extracted using a method described by Bernal, et al. [40]. To understand the value of modeling the esophagus as an elastic or viscoelastic organ, we can fit the same phase velocity data to the Lamb wave model in Eq. (1) with the real or complex shear modulus.
In our analyses of comparing the parameters we used a paired Student’s t-test implemented in MATLAB (Mathworks, Natick, MA). We regarded a p value of 0.05 to be statistically significant.
Results
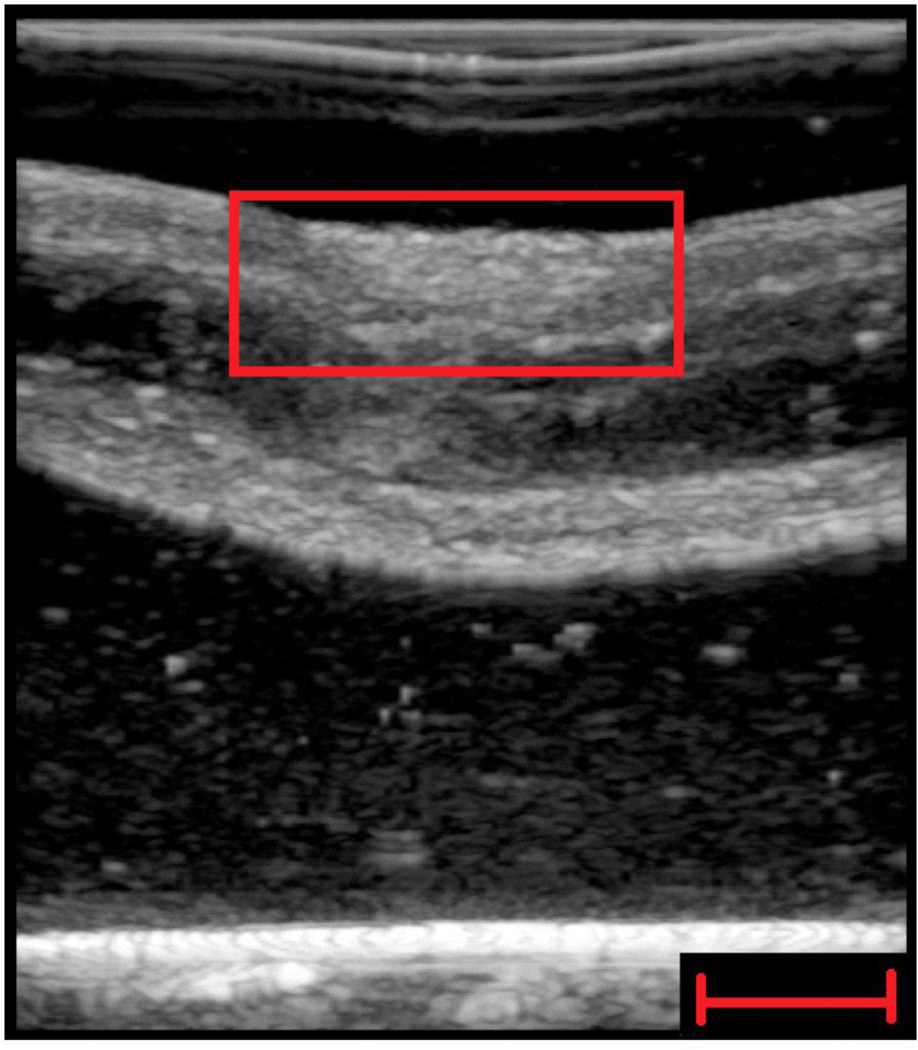
An example of a B-mode image taken with a General Electric Logiq E9 scanner (General Electric Healthcare, Wauwatosa, WI) of a partial thickness ablation sample is shown in Fig. 3. The B-mode contrast is low.
Figure 3.
Representative B-mode ultrasound image of partial thickness ablated region of esophagus (red square). Moderately poor contrast discrimination on B-mode ultrasound imaging was observed. Scale bar indicates 1 cm.
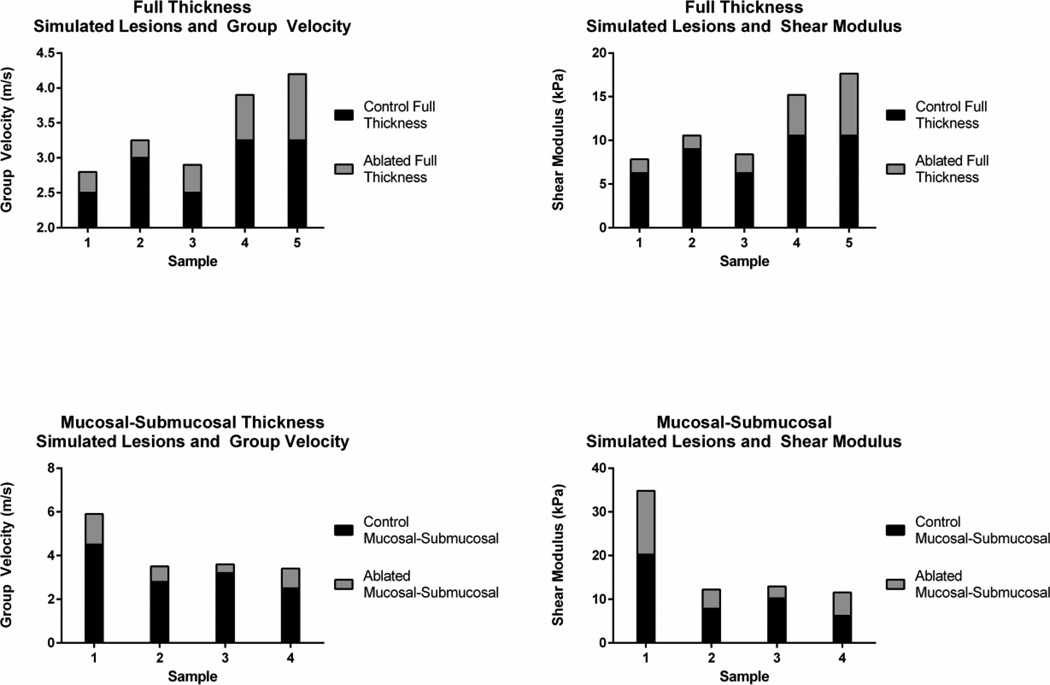
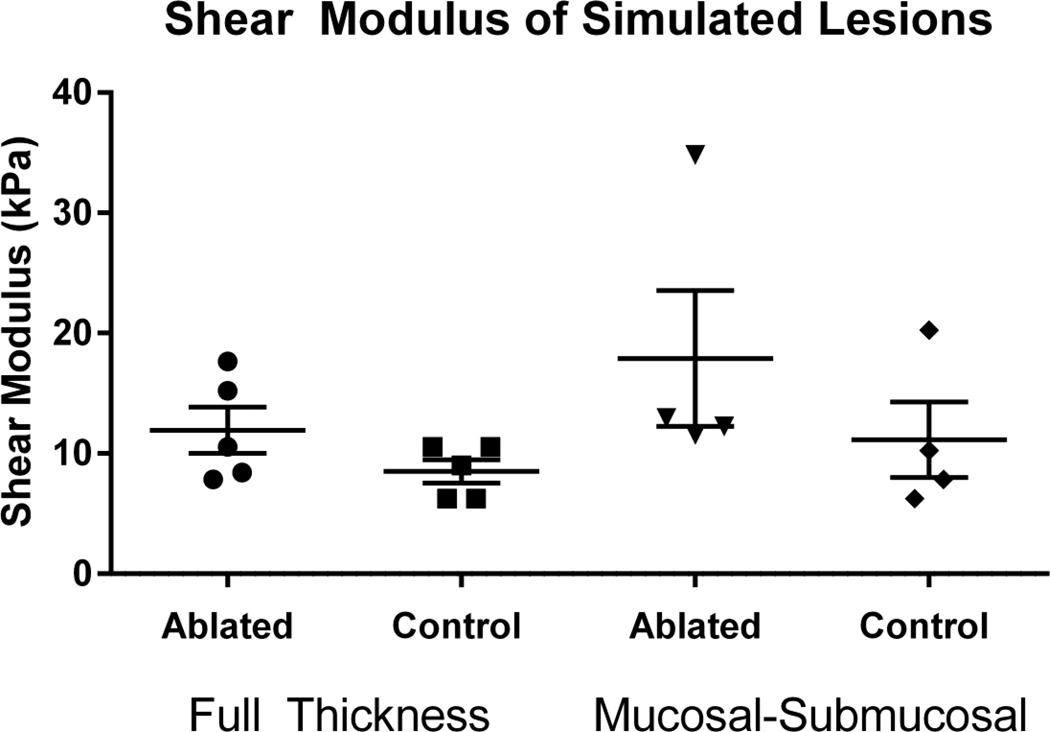
Figure 4 shows the group velocity values and elastic shear moduli values derived from the group velocity measurements for all samples with full thickness and mucosal-submucosal ablations. In all samples, the ablated region had higher values of group velocity and shear moduli compared to control regions in the same esophagus sample. The shear moduli values are further summarized in Fig. 5 with the data points and their associated means and standard deviations represented by the center line and error bars, respectively. The means of the ablated regions were larger than the control regions.
Figure 4.
Group velocity cg and shear moduli (μ) results for each sample with full thickness and mucosal-submucosal ablation. Statistical analysis is found in Table 1.
Figure 5.
Summary of shear modulus in simulated full thickness and partial thickness (mucosal-submucosal) lesions. The center line and error bars represent the mean and standard error of the mean of the values.
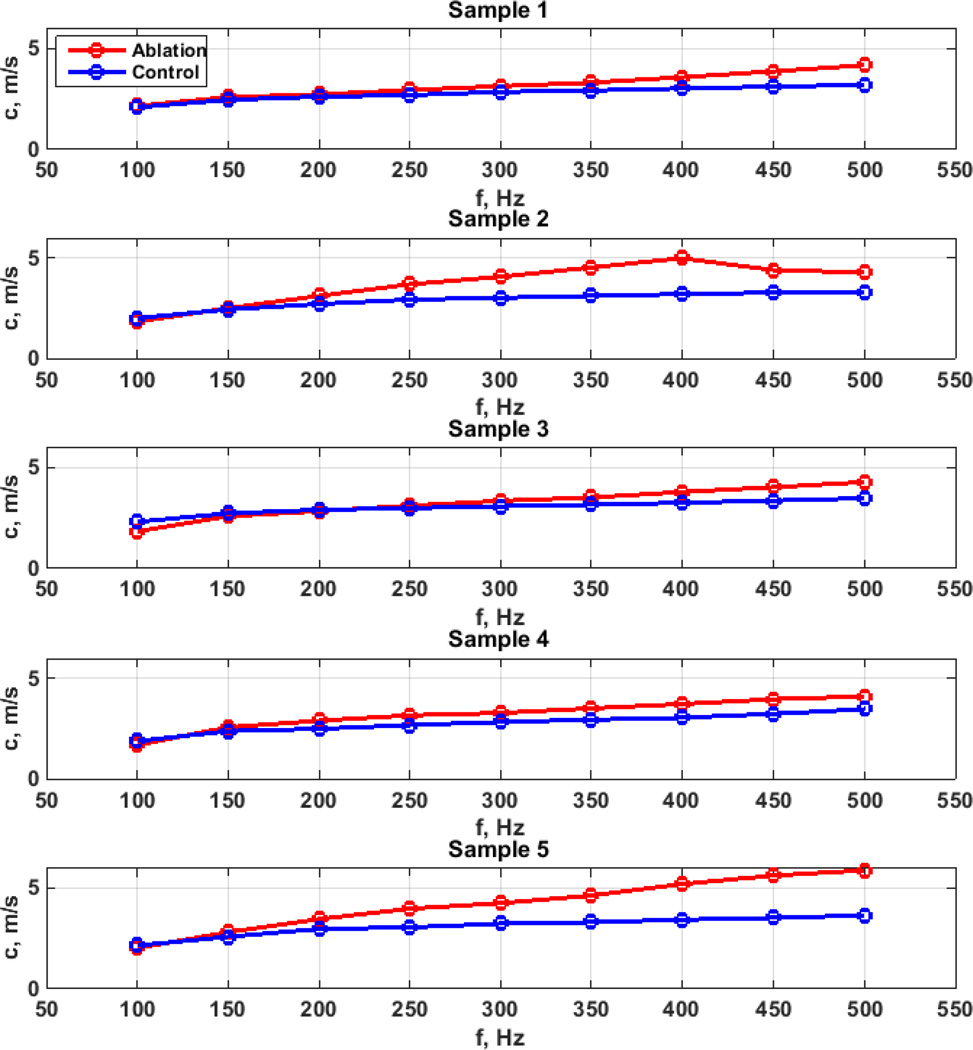
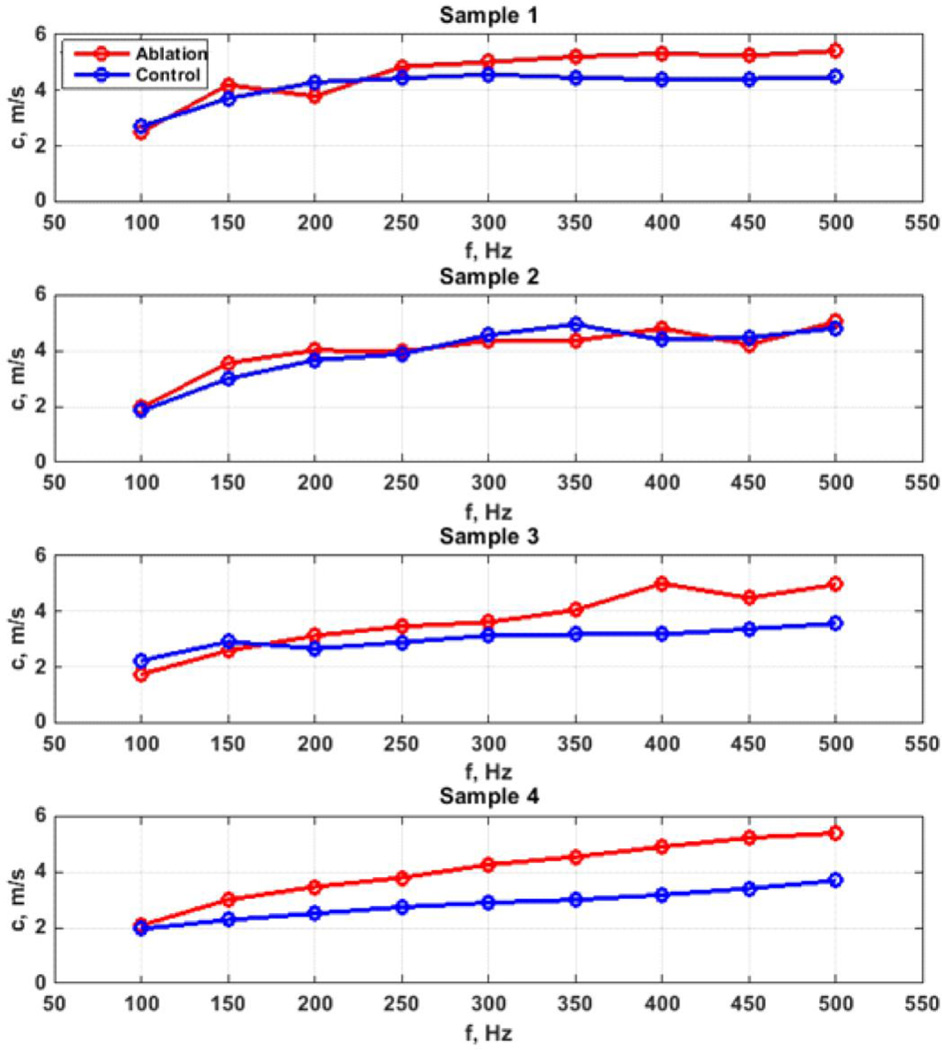
Figures 6 and 7 show the phase velocity dispersion measured in control and ablated regions for the full thickness and partial thickness samples, respectively. The phase velocities are generally higher in the ablated regions compared to the control regions in almost all samples. The differences in phase velocities increased at higher frequencies.
Figure 6.
Phase velocity comparison for full thickness ablation studies.
Figure 7.
Phase velocity comparison for mucosal-submucosal ablation studies.
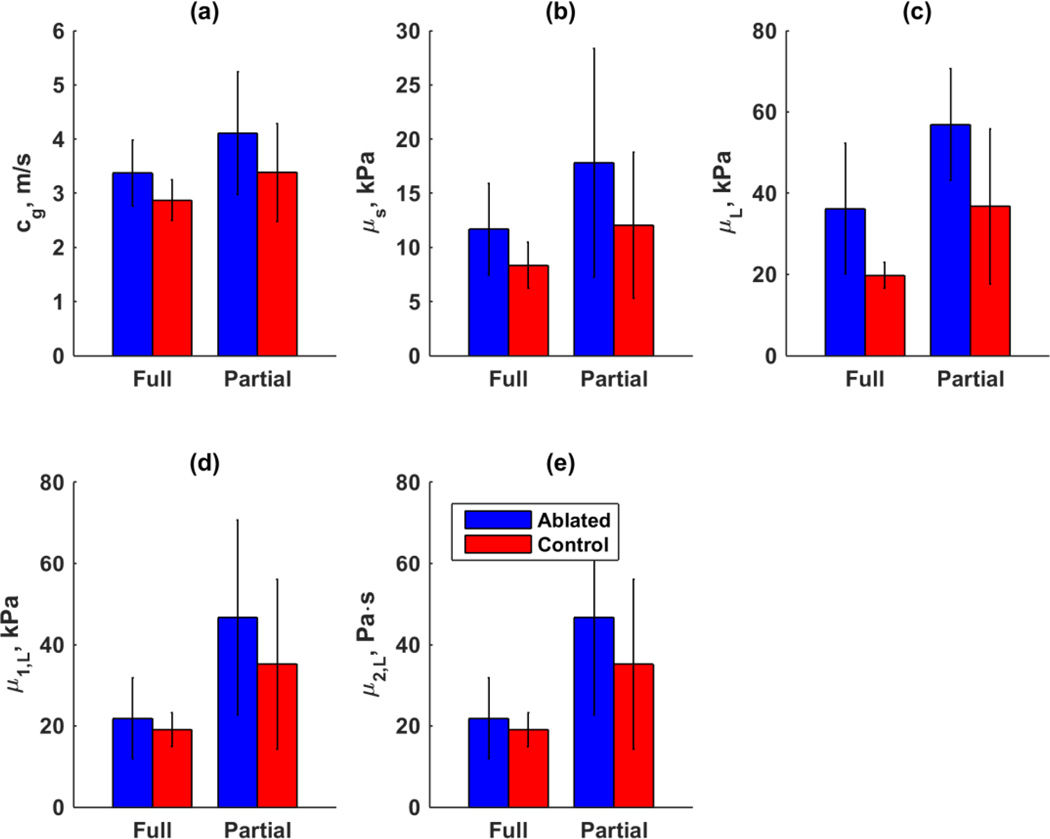
Figure 8 shows a summary of the different parameters that were derived from the group velocity and phase velocity measurements. The error bars represent the standard deviations of the parameter for all samples. Figure 8(a) shows the group velocity and Fig. 8(b) shows the elastic shear moduli derived from the group velocity. Table 1 summarizes the p values of the paired Student’s t-test between the values for the control and ablated regions. Using group velocity for these comparisons demonstrated statistically significant differences and the elastic shear modulus also demonstrated a statistically significant difference for the full thickness ablation (p = 0.038).
Figure 8.
Summary of wave propagation results. (a) Group velocity, (b) μs from group velocity, (c) μL from elastic Lamb wave fitting, (d) μ1 from viscoelastic Lamb wave fitting, (e) μ2 from viscoelastic Lamb wave fitting
Table 1.
p-values for t-tests between Ablated Regions and Controls for Different Parameters Obtained with Shear Wave Vibrometry Measurements.
| Parameter | Full Thickness Ablation |
Partial Thickness Ablation |
|---|---|---|
| Group Velocity, cg | 0.021 | 0.025 |
| Elastic Shear Modulus, μ | 0.038 | 0.072 |
| Elastic Shear Modulus from Lamb Wave, μL | 0.053 | 0.061 |
| Viscoelastic Shear Modulus from Lamb Wave, μ1,L |
0.609 | 0.172 |
| Viscoelastic Shear Viscosity from Lamb Wave, μ2,L |
0.031 | 0.152 |
Figure 8(c) shows the results for the Lamb wave fitting of the data in Figs. 6 and 7 with an elastic shear modulus, and Figs. 8(d)–(e) show the results of the Lamb wave fitting of the same data with a complex shear modulus. When fitting wave propagation to the elastic Lamb wave model no statistical significant results was observed. However, when viscoelastic Lamb wave fitting was used, μ2 for full thickness ablations was significantly different than partial, while in partial thickness ablation this comparison did not yield a statistically different result. Together these results show the discriminatory potential of shear wave vibrometry using the viscosity to distinguish between partial and full thickness ablations (Table 1).
Discussion
We have shown within this work that group velocity in full thickness and partial thickness lesions are able to be discriminated from normal regions of esophageal tissue using shear wave vibrometry. Elevations in group velocity cg suggest there is some degree of ablation present. Furthermore, we have shown that by using higher order analysis of the shear moduli analyzed in terms of the elastic (μ1) and viscous (μ2) components, full thickness lesions can be discriminated from partial thickness lesions based on elevations in those parameters. This methodology may have substantial clinical utility for characterization of benign and malignant esophageal tumors. In practice benign lesions may be more easily characterized by distinct boundries or relative homogeneity and distinct tissue type compared to surrounding esophageal tissue. Currently the standard of care for diagnosis of malignant esophageal neoplasms is endoscopic ultrasound combined with biopsy, which has suboptimal soft tissue contrast. The ability to guide tissue biopsies for definitive diagnosis to the area most suspicious for metaplastic and most severe malignant degeneration would have potentially widespread utility in clinical practice for both benign and malignant tumors. Furthermore, using analysis of group velocity for a screening application and then higher order analysis based on the elastic and viscious components of the shear modulus may allow discrimination between extent of tumor invasion through the esophageal wall. Use of ultrasound-based vibrometry in this setting would allow for realtively simple study and translation of clinical practice, as the same devices are already used in this setting, and operators have familiarity with the equipment. Incorporating another additional method to generate contrast for determining malignant tissue from normal based on biomechanical properties with the same probe would have benefit as an additional diagnostic modality.
This proof-of-concept study to investigate if shear wave-based metrics could be used to distinguish between normal esophagus tissue and simulated lesions of varied stage created using RF ablation suggest this method is feasible for clinical practice and has promise for determining degree of tumor progression. The equipment configuration used in this study could be used in an intraoperative setting currently during tumor resection as there is no current barrier to instilling fluid intraoperatively into the thoracic cavity and is often performed in other settings for intraoperative ultrasound. In the future, further enhancements of our appraoch could be considered, as ideally information would be provided before an incision is made on the patient. To make the method minimally invasive and provide preoperative information and use the standard EUS equipment, the shear wave elastography method would have to be implemented on an endoscopic ultrasound probe or a transesophageal probe. The radial geometry of the probe would have to be taken into account from both a theoretical and practical perspective before the implementation of this method. A possible method may be a shallow application of the ARF push which could be done with an unfocused push [41]. Additionally, measurements in an ex vivo porcine heart have been done with a transesophageal probe in contact with the myocardium [42] and should be surmountable from an engineering basis.
It has been shown previously that in various organs that malignant tissues are stiffer than benign tissues, and this correlates in breast tissue with risk of metastatic disease [15]–[17]. It is unclear to our knowledge if this extends to esophageal cancers in vivo. In our study partial and full ablations increase stiffness relative to control esophagi. Interestingly in our study parameter estimates for partial ablation were increased relative to full thickness ablation. We propose there may be several reasons for this. One of the reasons is that at baseline in the normal esophagus there are marked mechanical differences between the muscular and submucosal layers [43]–[45] and ablation of the mucosal-submucosal layer may alter the mechanical coupling of the submucosal layer to the the outer muscular layer and stiffen them both because the ablated mucosal-submucosal layer may produce increased strain between the layers. In the full thickness ablation both of these layers are denatured to the same degree and behave more mechanically similar. While this may be the case for ablation of the esophagus it is unclear if this is also the case in the setting of malignancy.
We propose that if this increased stiffness could be proven in vivo that it be considered a biomarker for malignancy and that further study could be aimed at determining either failure of therapy or risk of metastatic spread based on tissue stiffness. While historically mechanical assessment has relied on methods of applying either stresses or deformations using variable methods to quantitate in order to elucidate biomechanical tissue properties, in vivo use of these techniques is often not feasible, in particular if the tissue needs to be tested intact. Using ultrasound to non-destructively determine complex elastic moduli and in particular to this work, stiffness of tissue which has undergone malignant changes has promise as incorporating biomechanical measurements into clinical practice. Using short impulses of ARF to apply deformations and generate shear waves which propagate through the tissues which are rapidly measured at high framerate allows high resolution micrometer scale and temporal displacements to be aquired, velocity, and consequently mechanical properties to be determined.
The applications of this method using currently available ultrasound transducer arrays for an additional modality which provides information of biomechanical changes in tissue has broad applications. Applications in addition to detection of malignancy may include other pathologies which alter tissue biomechanics of the esophagus, such as motility disorders, esophageal stricture (which has underlying fibrotic pathophysiology), or for determination of degree of inflammation in esophagitis and reflux disease. We propose that further study is required, with software updates to commercially available probes for translational application to practice. In particular to patient study in the outpatient setting aimed at determination of malignant extent of esophageal cancer as currently diagnosed with EUS and compared to the shear wave method and correlation to post surgical pathologic specimens for true tumor extent. This study would be fairly simple to conduct as the EUS probe is already indwelling for diagnostic workup preoperatively and is the current standard of care. Given operators are familiar with EUS use and application, and only software updates would be required in addition to taking probe geometry into account would be required from a theoretical perspective, implementation into patient study would not represent significant challenge.
Limitations
The esophagus is not homogenous in animals, in particular swine, as it traverses from the oral cavity to the stomach through the thorax [46]. These morphological changes, through relatively minor in a given section of tissue likely have underlying effects on mechanical wave transmission and subsequently, wave velocity which is used to determine many of the parameters we estimate in this work. Furthermore, we have attempted to generate reasonable lesions which simulate malignancy in order to determine if shear wave velocity and shear modulus are affected. It is possible that the degree of stiffness of malignant tissue compared to our phantom will be either greater or lesser than we have simulated, and consequently shear wave speed and moduli will vary accordingly. In other tissues RF ablated tissues have been shown to have increased stiffness compared to normal tissues [30], [32], [47], [48], while in our study this was this case it is unclear if this is generalizable in esophageal tissues as well. We have attempted to remove the possibility of anatomic sample-to-sample variation through using a single experienced operator to obtain specimens (JMA). Although tissues were frozen to −80 °C before testing and it is possible this may alter mechanical properties in other tissues this does not appear to occur to a marked degree, in particular soft tissue tubular structures [49]–[57]. Furthermore, we have attempted to homogenize the lesions which were generated by having two operators (JMA and IZN) agree on extent and comparability of simulated malignant lesions when ablation was performed. Furthermore, while all samples were from similar size swine, in vivo conditions are likely different in terms of baseline tissue strain and consequently stiffness, we attempted to ameliorate this by preparation on the specimen mounting to prestrain to in vivo length (~15%) to obtain homogenous in vivo based tissue strain across specimens. Despite attempts to obtain exact in vivo strain it is likely this does not reflect exact in vivo strain. During mechanical inversion which was non-destructive there may have been alterations in stiffness after inversion which were not evaluated. While there were low numbers of samples tested, we did use each esophageal specimen as their own control, which may reduce variability in the tested samples but limit generalizations derived from our data. Furthermore, due to small sample size the sample-to-sample variability may have been unequally distrubted amounst experimental groups and altered the results. Additionally, we have made several mathematical assumptions that may not replicate the physiologic reality, in particular tissue homogeneity and isotropic nature and furthermore on wave transmission which did not account for tubular esophageal morphology.
The measurements made were region-based measurements and to determine the spatial extent of the lesion, an imaging method would be ideal. Implementations of shear wave elastography are now available on many clinical scanners and could be used. However, this would have to be done in an ex vivo or intraoperative setting. This study was meant to demonstrate that there was contrast that could be detected in an esophagus with shear wave vibrometry. Efforts will be made in future work to make images from the Lamb wave reconstructions.
Conclusion
In this study we have demonstrated that simulated esophageal malignancy of varied stage may be detected and discriminated based on shear wave vibrometry through changes in group velocity and the elastic and viscous components of the shear modulus. This method may have future utility in diagnosis and prognostication of esophageal and gastrointestinal malignancies.
Acknowledgments
This work was supported in part by a grant from the National Heart, Lung, and Blood Institute (NHLBI) T32 HL105355 (Aho), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or NIH.
Footnotes
Conflicts
Mayo Foundation has a financial interest in technologies developed by some of the authors. Several authors report patent disclosures for multiple technologies.
References
- 1.Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998 Nov.83(10):2049–2053. [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50(3):368–372. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg. Oncol. Clin. N. Am. 2002 Apr.11(2):235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand. J. Gastroenterol. 2002 Dec.37(12):1359–1365. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 5.Wang G-Q, Jiao G-G, Chang F-B, Fang W-H, Song J-X, Lu N, Lin D-M, Xie Y-Q, Yang L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann. Thorac. Surg. 2004 May;77(5):1740–1744. doi: 10.1016/j.athoracsur.2003.10.098. [DOI] [PubMed] [Google Scholar]
- 6.Tachibana M, Hirahara N, Kinugasa S, Yoshimura H. Clinicopathologic features of superficial esophageal cancer: results of consecutive 100 patients. Ann. Surg. Oncol. 2008 Jan.15(1):104–116. doi: 10.1245/s10434-007-9604-4. [DOI] [PubMed] [Google Scholar]
- 7.Hulscher JBF, van Sandick JW, de Boer AGEM, Wijnhoven BPL, Tijssen JGP, Fockens P, Stalmeier PFM, ten Kate FJW, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJB. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl. J. Med. 2002 Nov.347(21):1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 8.Dubecz A, Gall I, Solymosi N, Schweigert M, Peters JH, Feith M, Stein HJ. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac. Oncol. 2012 Feb.7(2):443–447. doi: 10.1097/JTO.0b013e3182397751. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Hofstetter WL, Rashid A, Swisher SG, Correa AM, Ajani JA, Hamilton SR, Wu T-T. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am. J. Surg. Pathol. 2005 Aug.29(8):1079–1085. [PubMed] [Google Scholar]
- 10.Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann. Surg. Oncol. 2008 Nov.15(11):3278–3288. doi: 10.1245/s10434-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 11.van Vliet EPM, Heijenbrok-Kal MH, Hunink MGM, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br. J. Cancer. 2008 Feb.98(3):547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TW. Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg. Clin. N. Am. 2000 Aug.10(3):471–485. [PubMed] [Google Scholar]
- 13.Silverstein FE, Martin RW, Kimmey MB, Jiranek GC, Franklin DW, Proctor A. Experimental evaluation of an endoscopic ultrasound probe: in vitro and in vivo canine studies. Gastroenterology. 1989 Apr.96(4):1058–1062. doi: 10.1016/0016-5085(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology. 1982 Feb.82(2):228–231. [PubMed] [Google Scholar]
- 15.Zhang M, Nigwekar P, Castaneda B, Hoyt K, Joseph JV, di Sant’Agnese A, Messing EM, Strang JG, Rubens DJ, Parker KJ. Quantitative characterization of viscoelastic properties of human prostate correlated with histology. Ultrasound Med. Biol. 2008 Jul.34(7):1033–1042. doi: 10.1016/j.ultrasmedbio.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Jalkanen V, Andersson BM, Bergh A, Ljungberg B, Lindahl OA. Prostate tissue stiffness as measured with a resonance sensor system: a study on silicone and human prostate tissue in vitro. Med. Biol. Eng. Comput. 2006 Jul.44(7):593–603. doi: 10.1007/s11517-006-0069-6. [DOI] [PubMed] [Google Scholar]
- 17.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason. Imaging. 1998 Oct.20(4):260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 18.Cross SE, Jin Y-S, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007 Dec.2(12):780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 19.Byun S, Son S, Amodei D, Cermak N, Shaw J, Kang JH, Hecht VC, Winslow MM, Jacks T, Mallick P, Manalis SR. Characterizing deformability and surface friction of cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2013 May;110(19):7580–7585. doi: 10.1073/pnas.1218806110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaminathan V, Mythreye K, O’Brien ET, Berchuck A, Blobe GC, Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011 Aug.71(15):5075–5080. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrmann A, Staunton JR, Nandakumar V, Banyai N, Davies PCW, Ros R. AFM stiffness nanotomography of normal, metaplastic and dysplastic human esophageal cells. Phys. Biol. 2011 Feb.8(1):015007. doi: 10.1088/1478-3975/8/1/015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenner J, Stacer AC, Winterroth F, Johnson TD, Luker KE, Luker GD. Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep. 2014 Jan.4:5512. doi: 10.1038/srep05512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat. Rev. Cancer. 2009 Mar.9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012 Feb.196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011 Mar.4(2):165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakkad SM, Solaiyappan M, Argani P, Sukumar S, Jacobs LK, Leibfritz D, Bhujwalla ZM, Glunde K. Collagen I fiber density increases in lymph node positive breast cancers: pilot study. J Biomed. Opt. 2012 Nov.17(11):116017. doi: 10.1117/1.JBO.17.11.116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson L, Czene K, Rosenberg L, Humphreys K, Hall P. Possible influence of mammographic density on local and locoregional recurrence of breast cancer. Breast Cancer Res. 2013 Jan.15(4):R56. doi: 10.1186/bcr3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009 Nov.139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 1998 Nov.24(9):1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 30.Varghese T, Zagzebski JA, Lee FT. Elastographic imaging of thermal lesions in the liver in vivo following radiofrequency ablation: preliminary results. Ultrasound Med. Biol. Jan.28(11–12):1467–1473. doi: 10.1016/s0301-5629(02)00656-7. [DOI] [PubMed] [Google Scholar]
- 31.Kwiecinski W, Provost J, Dubois R, Sacher F, Haïssaguerre M, Legros M, Nguyen-Dinh A, Dufait R, Tanter M, Pernot M. Quantitative evaluation of atrial radio frequency ablation using intracardiac shear-wave elastography. Med. Phys. 2014 Nov.41(11):112901. doi: 10.1118/1.4896820. [DOI] [PubMed] [Google Scholar]
- 32.Mariani A, Kwiecinski W, Pernot M, Balvay D, Tanter M, Clement O, Cuenod CA, Zinzindohoue F. Real time shear waves elastography monitoring of thermal ablation: in vivo evaluation in pig livers. J Surg. Res. 2014 May;188(1):37–43. doi: 10.1016/j.jss.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Luo L, Chen J, Wang J, Zhou H, Li M, Jin Z, Chen N, Miao H, Lin M, Dai W, Ahuja AT, Wang Y-XJ. Acoustic Radiation Force Impulse Elastography for Efficacy Evaluation after Hepatocellular Carcinoma Radiofrequency Ablation: A Comparative Study with Contrast-Enhanced Ultrasound. Biomed Res. Int. 2014;2014:7. doi: 10.1155/2014/901642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasai C, Namekawa K, Koyano A, Omoto R. Real-Time Two-Dimensional Blood Flow Imaging Using an Autocorrelation Technique. IEEE Trans. Sonics Ultrason. 1985 May;32(3):458–464. [Google Scholar]
- 35.Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med. Biol. 2008 Apr.34(4):546–558. doi: 10.1016/j.ultrasmedbio.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nenadic IZ, Urban MW, Mitchell SA, Greenleaf JF. Lamb wave dispersion ultrasound vibrometry (LDUV) method for quantifying mechanical properties of viscoelastic solids. Phys. Med. Biol. 2011 Apr.56(7):2245–2264. doi: 10.1088/0031-9155/56/7/021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brum J, Bernal M, Gennisson JL, Tanter M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys. Med. Biol. 2014 Feb.59(3):505–523. doi: 10.1088/0031-9155/59/3/505. [DOI] [PubMed] [Google Scholar]
- 38.Nenadic IZ, Qiang B, Urban MW, de Araujo Vasconcelo LH, Nabavizadeh A, Alizad A, Greenleaf JF, Fatemi M. Ultrasound bladder vibrometry method for measuring viscoelasticity of the bladder wall. Phys. Med. Biol. 2013 Apr.58(8):2675–2695. doi: 10.1088/0031-9155/58/8/2675. [DOI] [PubMed] [Google Scholar]
- 39.Couade M, Pernot M, Prada C, Messas E, Emmerich J, Bruneval P, Criton A, Fink M, Tanter M. Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med. Biol. 2010 Oct.36(10):1662–1676. doi: 10.1016/j.ultrasmedbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Bernal M, Nenadic I, Urban MW, Greenleaf JF. Material property estimation for tubes and arteries using ultrasound radiation force and analysis of propagating modes. J Acoust. Soc. Am. 2011 Mar.129(3):1344–1354. doi: 10.1121/1.3533735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Song P, Urban MW, Greenleaf JF, Chen S. Shear wave speed measurement using an unfocused ultrasound beam. Ultrasound Med. Biol. 2012 Sep.38(9):1646–1655. doi: 10.1016/j.ultrasmedbio.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urban MW, Qiang B, Song P, Nenadic IZ, Chen S, Greenleaf JF. Investigation of the effects of myocardial anisotropy for shear wave elastography using impulsive force and harmonic vibration. Phys. Med. Biol. 2016 Jan.61(1):365–382. doi: 10.1088/0031-9155/61/1/365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aho JM, Qiang B, Wigle DA, Tschumperlin DJ, Urban MW. Nondestructive measurement of esophageal biaxial mechanical properties utilizing sonometry. Phys. Med. Biol. 2016 Jul.61(13):4781–4795. doi: 10.1088/0031-9155/61/13/4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Y, Gregersen H, Kassab GS. A two-layered mechanical model of the rat esophagus. Experiment and theory. Biomed. Eng. Online. 2004 Nov.3(1):40. doi: 10.1186/1475-925X-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu X, Gregersen H. Regional distribution of axial strain and circumferential residual strain in the layered rabbit oesophagus. J Biomech. 2001 Feb.34(2):225–233. doi: 10.1016/s0021-9290(00)00176-7. [DOI] [PubMed] [Google Scholar]
- 46.Yokosawa S, Koike T, Kitagawa Y, Hatta W, Uno K, Abe Y, Iijima K, Imatani A, Ohara S, Shimosegawa T. Identification of the layered morphology of the esophageal wall by optical coherence tomography. World J. Gastroenterol. 2009 Sep.15(35):4402–4409. doi: 10.3748/wjg.15.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varghese T, Shi H. Elastographic imaging of thermal lesions in liver in-vivo using diaphragmatic stimuli. Ultrason. Imaging. 2004 Jan.26(1):18–28. doi: 10.1177/016173460402600102. [DOI] [PubMed] [Google Scholar]
- 48.Bharat S, Techavipoo U, Kiss MZ, Liu W, Varghese T. Monitoring stiffness changes in lesions after radiofrequency ablation at different temperatures and durations of ablation. Ultrasound Med. Biol. 2005 Mar.31(3):415–422. doi: 10.1016/j.ultrasmedbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg. Am. 2003 Nov.85-A(11):2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Kiefer GN, Sundby K, McAllister D, Shrive NG, Frank CB, Lam T, Schachar NS. The effect of cryopreservation on the biomechanical behavior of bovine articular cartilage. J Orthop. Res. 1989;7(4):494–501. doi: 10.1002/jor.1100070406. [DOI] [PubMed] [Google Scholar]
- 51.Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, Bugbee WD. The effects of storage on fresh human osteochondral allografts. Clin. Orthop. Relat. Res. 2004 Jan.(418):246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 52.Szarko M, Muldrew K, Bertram JE, Ball S, Amiel D, Williams S, Tontz W, Chen A, Sah R, Bugbee W, Tomford W, Springfield D, Mankin H, Jomha N, Law G, McGann L, Kiefer G, Sundby K, McAllister D, Shrive N, Frank C, Lam T, Schachar N, Schachar N, McGann L, Muldrew K, Hurtig M, Novak K, Schachar N, McGann L, Muldrew K, Acker J, Elliot J, McGann L, Muldrew K, Novak K, Yang H, Zernicke R, Schachar N, McGann L, Hayes W, Bodine A, Light L, McLellan G, Light L, McLellan G, Klenerman L, Simon S, Paul I, Mansour J, Munro M, Abernethy P, Radin E, Fulcher G, Hukins D, Shepherd D, Hori R, Mockros L, Spirt A, Mak A, Wassell R, Williams S, Amiel D, Ball S, Allen T, Wong V, Chen A, Sah R, Bugbee W, Kubo T, Arai T, Namie K, Takahashi K, Hojo T, Inoue S, Ueshima K, Shiga T, Yutani Y, Hirasawa Y, Gosline J, French C, Sokoloff L, Maly J, Bender K, Pendleton S, Wainwright S, Biggs W, Currey J, Gosline J, Pastoureau P, Chamel A, Pietila K, Kantomaa T, Pirttiniemi P, Poikela A, Myers E, Mow V, Li L, Buschmann M, Shirazi-Adl A, Park S, Nicoll S, Mauck R, Ateshian G. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Musculoskelet. Disord. 2010 Dec.11(1):231. doi: 10.1186/1471-2474-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stemper BD, Yoganandan N, Stineman MR, Gennarelli TA, Baisden JL, Pintar FA. Mechanics of Fresh, Refrigerated, and Frozen Arterial Tissue. J Surg. Res. 2007;139(2):236–242. doi: 10.1016/j.jss.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Venkatasubramanian RT, Grassl ED, Barocas VH, Lafontaine D, Bischof JC. Effects of freezing and cryopreservation on the mechanical properties of arteries. Ann. Biomed. Eng. 2006 May;34(5):823–832. doi: 10.1007/s10439-005-9044-x. [DOI] [PubMed] [Google Scholar]
- 55.Virues Delgadillo JO, Delorme S, El-Ayoubi R, DiRaddo R, Hatzikiriakos SG. Effect of freezing on the passive mechanical properties of arterial samples. J Biomed. Sci. Eng. 2010;03(07):645–652. [Google Scholar]
- 56.Pukacki F, Jankowski T, Gabriel M, Oszkinis G, Krasinski Z, Zapalski S. The Mechanical Properties of Fresh and Cryopreserved Arterial Homografts. Eur. J. Vasc. Endovasc. Surg. 2000 Jul.20(1):21–24. doi: 10.1053/ejvs.2000.1120. [DOI] [PubMed] [Google Scholar]
- 57.L’Italien GJ, Maloney RD, Abbott WM. The preservation of the mechanical properties of venous allografts by freezing. J Surg. Res. 1979 Oct.27(4):239–243. doi: 10.1016/0022-4804(79)90136-7. [DOI] [PubMed] [Google Scholar]