Abstract
The pattern of sleep in the fur seal, a semiaquatic pinniped, has several striking behavioral and physiological adaptations that allow this species to inhabit both the land and water environment. These features include unihemispheric slow wave sleep (USWS, also being unihemispheric waking), the ability to maintain movement for stabilization of the sleep posture and to briefly open one eye while having a sleep electroencephalogram (EEG) in one hemisphere. In vivo microdialysis studies suggest that acetylcholine release is required for cortical activation during USWS, and that monoamines are not required for USWS. The need to breathe, to maintain efficient thermoregulation, and to avoid predation have shaped the sleep patterns in semiaquatic fur seals as in fully aquatic cetaceans.
Introduction (Sleep on land and in water)
Behaviorally, sleep in terrestrial mammals is usually described as 1) a state of immobility / behavioral quiescence in 2) a specific posture 3) with a reduced ability to respond to external stimuli. Terrestrial mammals can display two distinctive sleep stages - slow wave sleep (SWS) and rapid eye movement (REM) sleep, which are usually scored based on EEG (electroencephalogram), EMG (electromyogram) and EOG (electrooculogram) features [1-4].
Air-breathing animals sleeping in water encounter a number of challenges. First, animals need to prevent inhaling water into the lungs while breathing. This can be achieved by regularly emerging or continuously staying at the surface while keeping the nostrils / blowholes above the water. This requires the maintenance of motions or postures that are not a requirement for terrestrial mammals. Second, sleeping at the water surface, which is the easiest way to maintain regular breathing, is not always a safe place to sleep. The surface is where the animals are most often hunted by large ocean predators (e.g., killer whale, sharks, [5]). In addition, most cetaceans (fully aquatic mammals) are social animals with strong connections between individuals. They “sleep” in groups while remaining in motion and maintaining positions appropriate to their social status. Therefore, continuous monitoring of the environment for predators and conspecifics can be a life-sustaining requirement for aquatic mammals. The third important issue arises from the fact that thermal conductivity of water is 50-60 fold greater than air. This requires that aquatic mammals have some behavioral adaptations preventing excessive heat loss or overheating, by maintaining postures or motion and excluding prolonged periods of immobility when at the water surface.
Studies of sleep in cetaceans (Odontocete) reveal that these challenges are met via two striking features of their sleep, unihemispheric sleep (USWS) and the absence of rapid eye movement (REM) sleep. It has been proposed that USWS (being unihemispheric waking at the same time or UW) enables movement, which allows breathing without aspiration of water [6,7]. Movement also allows muscle thermogenesis and prevents overcooling while in cold water [8]. The other function of USWS in cetaceans is believed to be monitoring the surrounding environment, which enables analysis of the afferent information arising from the open eye, facilitating the avoidance of predators, maintaining pod coherence and protection of neonates [9]. The reasons why REM sleep in cetaceans is absent (or remains undetectable if present) are not clear [8]. REM sleep in land mammals features elevated arousal thresholds, impaired thermoregulation and muscle tone suppression [2,3]. Thus, long episodes of REM sleep at the surface of water would be obviously maladaptive or dangerous behavior for cetaceans.
Fur seals are member of the eared seals (Family Otariidae, Clade Pinnipedia, Order Carnivores; [10]). The northern fur seal (Callorhinuos ursinus L.) is one of the smallest and most pelagic fur seals. During the winter migratory season, northern fur seals migrate more than 2000 km to the wintering grounds and remain pelagic for up to 10 months before going back to land [11]. The aim of this review is to summarize the data on the adaptive features of the pattern of sleep in the fur seal allowing this animal to inhabit both terrestrial and aquatic environments.
Sleep stages and amounts in fur seals while on land
When on land, SWS in seals can be bilateral, as in terrestrial mammals, meaning that EEG slow waves progress and disappear synchronously in two cortical hemispheres (Figure 1). SWS in the fur seal is also characterized by striking interhemispheric electroencephalogram (EEG) asymmetry [12-14]. These episodes are also called asymmetrical SWS or USWS. REM sleep in the fur seal has all the commonly described behavioral (muscle jerks, body twitches) and polygraphic features (reduced muscle tone, cortical activation, rapid eye movements, increased heart and breathing rate variability) of this stage described in the majority of terrestrial mammals [1-3]. The amount of sleep stages in fur seals depends on the animals' age [13]. The amounts of REM sleep and duration of REM sleep episodes drop substantially during the first months of the pup's lives and the number of episodes appear to decrease progressively with age (Figure 2). As opposed to REM sleep, the amount of SWS do not decline in pups over the first months of their lives but it appears to decline in adulthood. Shortly after birth the fur seal pups display predominantly BSWS. Within the next 2-3 months, by the time the pups are about to leave for their first migratory in the ocean, the proportion of ASWS in fur seal pups increases reaching on average 25% of SWS which is close to that in adults (Figure 2).
Figure 1.
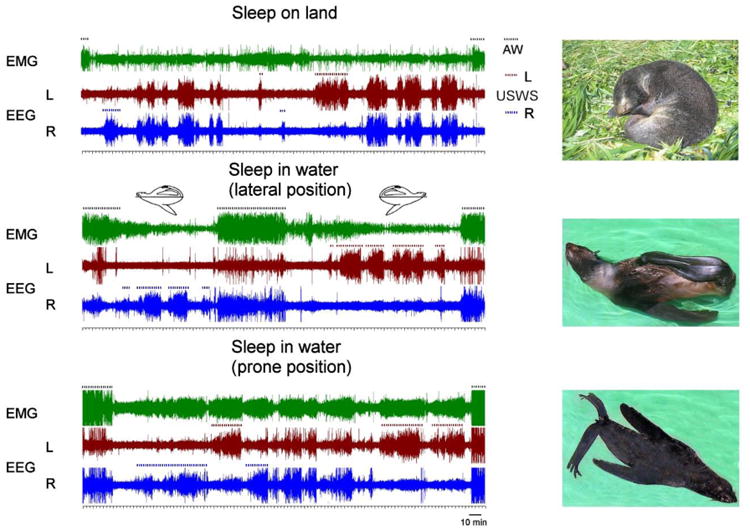
Representative polygrams of recording from fur seals sleeping on land, at the surface in the lateral (middle) and in a prone position (bottom). The duration of each polygram is approximately 6 hr. EMG – neck electromyogram, EEG – electroencephalogram in symmetrical left (L) and (R) fronto-occipital derivations. Episodes of unihemispheric sleep (USWS) in the left and right hemispheres and active wakefulness (AW) are marked by brown, blue and black dotted lines, respectively. Postures of fur seals are shown on the photographs.
Figure 2.
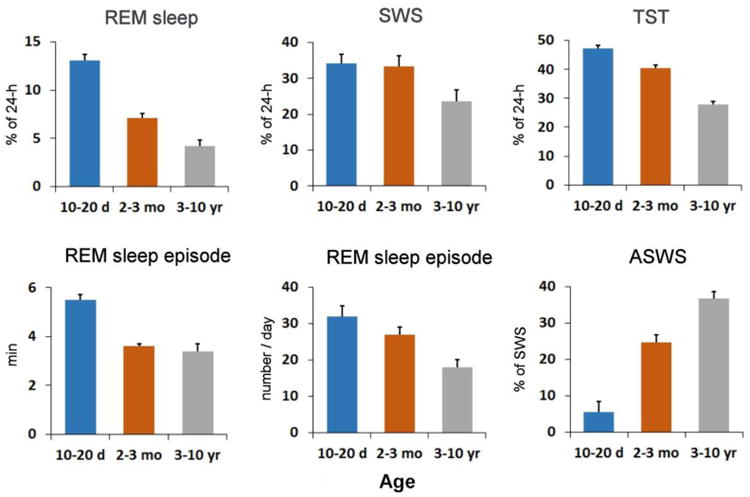
Sleep ontogeny in the fur seal. The diagrams show the amounts of sleep stages (REM sleep -rapid eye movement sleep, SWS - slow wave sleep, TST - total sleep time), duration and number of REM sleep episodes, and proportion/ ratio of asymmetrical SWS (ASWS) in fur seals of different ages (10-20 day old pups, 2-3 month old pups and in the group of juvenile males and females, 3-10 year old animals) while sleeping on land. The data are means + SEM calculated for 4, 4 and 7 individual fur seals from the 3 age groups.
Sleep in water
The fur seal has large flippers serving for locomotion in water and on land as well as for thermoregulation. The flippers are highly vascularized but not insulated with fur. When seals sleep in water they maintain two hind flippers and one fore flipper above the surface (Figure 1). The other front flipper paddles in water. This posture is called a “jug handle”. Keeping three flippers above the water in the air, fur seals reduce heat loss in cold water [11,15, 16]. When sleeping in water the fur seal's nostrils are in the air for most of the time allowing regular breathing except for periods when they submerge during short episodes of REM sleep [13]. When in water, seals can go without sleep for a day or two, so the variability in the amounts of sleep between days greatly increases. REM sleep episodes in water do not last longer than 1 min, and the total duration of REM sleep substantially decreases or even disappears for several continuous days. The composition of SWS also changes: the proportion of asymmetrical SWS/USWS in water increases while that of bilateral SWS decreases (Figure 1, [13]).
Comparative aspects of USWS
In addition to the northern fur seal, interhemispheric EEG asymmetry during SWS was also recorded in 3 other species of fur seals and sea lions [17,18], as well as in one young walrus (a pinniped belonging to the family Odobenidae; [19]) and in one Amazonian manatee (the order Sirenia; [20]). In contrast, no evidence for EEG asymmetry has ever been recorded in individuals of the 3rd family of Pinnipeds, namely the Phocidae also called “earless seals” or “true seals” [21-23]. Therefore, USWS is likely a feature of all toothed whales (Cetacea) and eared seals (Otariidae) but not of the true seals (Phocidae). In other words, USWS is not an obligatory feature of aquatic sleep.
In both the fur seal and bottlenose dolphin (the two most extensively studied species of Pinnipeds and Cetaceans) interhemispheric EEG asymmetry during SWS is recorded across the dorsal cortex, including sensory, motor and associative areas [6,7,14,24]. In the fur seal, the difference between the EEG power in the two cortical hemispheres is clearly detectable in the range of 1.2-16 Hz [14,24]. In the bottlenose dolphin the asymmetry in slow waves is paired with the asymmetry in sleep “spindles” [6,7]. In both the dolphin and fur seal EEG asymmetry during SWS is also recorded between subcortical areas [8, 25]. Thus, USWS in Cetaceans and Otariids appears to be both a cortical and subcortical phenomenon.
When fur seals that are sleeping on land are deprived of bilateral SWS they display repeated attempts to enter this state. At the same time the frequency and duration of the episodes of SWS with interhemispheric EEG asymmetry increase when compared to baseline conditions [26]. Even under these conditions, the expression of EEG asymmetry in fur seals is generally smaller than in Cetaceans. However, when fur seals sleep in water the difference between EEG power in symmetrical cortical areas becomes comparable with that in cetaceans reaching a 10 fold ratio [14,27], and the episodes of asymmetrical and USWS become longer lasting several hours (Figure 1) as in cetaceans [6-8].
Interhemispheric EEG asymmetry during SWS has also been recorded in a number of avian species [28-30]. This suggests that EEG asymmetry is likely a feature of sleep in many species of birds. At the same time, the expression of EEG asymmetry during SWS in the birds is highly variable between species. In general, the expression of asynchrony of EEG slow waves in the birds is smaller and the duration of episodes shorter than that in marine mammals.
USWS and motion/ postures
While immobility is one of the core features of sleep in terrestrial mammals, the ability to display EEG slow waves (or “sleep”) during swimming is one of the remarkable features of cetaceans. One of the first hypotheses on the function of USWS was to achieve some of the benefits of sleep, while allowing movement directed by the waking hemisphere to control movement to support breathing. However, subsequent visual observations on dolphins and porpoises revealed that the pattern of their swimming during USWS is indistinguishable from that of quiet waking [7].
In contrast, USWS in fur seals is accompanied by a characteristic posture. Fur seals sleep in water most of the time in a lateralized posture on the side while they paddle with only one front flipper to maintain the position (e.g., the left front flipper paddles while sleeping on the left side and the right front flipper when on the right side; [13]). While in this posture the moving front flipper is contralateral to the waking (in case of USWS) or more activated (displaying lower voltage EEG slow waves) hemisphere (Figure 1). These data indicate that USWS / UW in the fur seal permits motion, specifically allowing the waking hemisphere to adjust the intensity and direction of the flipper movements to maintain postural stability without frequent arousal and sleep interruptions.
This hypothesis is also supported by the data on the pattern of sleep in water in the Phocidae seals [21-23] and the walrus [19]. They do not display USWS. They sleep in water while holding their breath and floating motionlessly at the surface or at depth. They always wake up to initiate movement to surface for breathing. Therefore, bilateral SWS is incompatible with movement in the Phocidae seals and likely in the walrus, as in terrestrial mammals. This pattern of sleep allows them to sleep under the ice fields to minimize the time spent on the surface and thereby avoid predation (sharks, killer whales, and polar bears) and harsh weather (e.g., limited access to open water in the freezing seas).
When in water fur seals also can sleep in a prone (ventral) position (Figure 1). The amplitude and frequency of movement in this posture is smaller compared to the lateral posture. Sleep in the prone position can be either right or left USWS. Less often SWS can be bilateral but even in this case the interhemispheric EEG asymmetry remains highly pronounced. Thus, while asymmetrical SWS remains the major type of sleep in the fur seal while in the prone position, no obvious association is observed between interhemispheric EEG asymmetry and the pattern of movement in this posture. This suggests that maintaining of movement is not the only function of USWS in the fur seal.
USWS and eye state
EEG asymmetry during SWS in fur seals is frequently linked to asymmetry in eye state. When seals sleep on land these episodes occur at the onset of sleep. A delay in the development of slow wave activity in one of two hemispheres is associated with brief opening (1-2 sec) of the eye contralateral to the hemisphere with lower voltage activity. The eye contralateral to the hemisphere with higher voltage activity is closed at this time. Both eyes are usually closed during bilateral (both low and high voltage) SWS and during REM sleep (Figure 3). When fur seals sleep in water on the side, the eye directed to the water, where predation risk originates from sharks and killer whales, was observed to be briefly open while contralateral eye directed away from the water was more often closed. The eye facing water is also contralateral to the waking hemisphere when in the lateral position.
Figure 3.
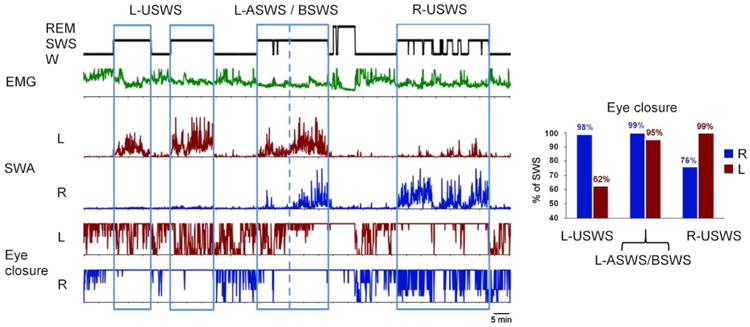
Association between sleep and eye states in a fur seal. The diagrams on the left side (from top to bottom) show behavioral states (W- wakefulness, SWS – slow wave sleep and REM – rapid eye movement sleep), integrated neck electromyogram (EMG), slow wave activity (SWA, EEG power in the range of 1.2–4 Hz) in the left (L) and right (R) cerebral hemispheres and the time of closure of the left (L) and right (R) eyes. All parameters were scored or calculated for consecutive 5-sec epochs. Time scale is 5 min. Blue boxes highlight episodes of unihemispheric and asymmetrical SWS (USWS and ASWS) in the left and right hemispheres (L-USWS and R-USWS), and an episode of L-ASWS transitioned into bilateral SWS (BSWS). The blue dotted line separates the margin between L-ASWS and BSWS. The diagram on the right side shows percentage of time each eye was closed during the two episodes of L-USWS combined, episode of L-ASWS / BSWS and the episode of R-USWS as marked on the left diagram.
In cetaceans USWS/ UW also correlates with unilateral eye closure / opening. For instance, in one beluga and one bottlenose dolphin studied, the eye contralateral to the deeply sleeping hemisphere was found to be primarily closed or in an intermediate state (40-60% and 31-46% of SWS respectively). At the same time the eye contralateral the waking hemisphere was open or in an intermediate state for 95-98% of the time [27,31].
EEG asymmetry in parallel with asymmetrical eye state was also recorded in one walrus [19] as well as in several bird species [28, 30]. During such sleep episodes birds opened the eye which was contralateral to the hemisphere with low voltage EEG slow waves as in cetaceans and in the fur seal. In birds the open eye was also directed toward potential threats [28] or the direction of their fly [30]. All of the above supports the idea that USWS in marine mammals and birds universally serves to monitor the surroundings via processing of the visual information in the waking hemisphere. At the same time, the waking hemisphere during USWS in marine mammals appears to be capable of processing multimodal information, including auditory [26] and somatosensory (from the vibrissae and moving flipper when sleeping in the lateral position).
Neurochemistry of USWS
To date we have examined the release of four neurotransmitters in sleep and waking states in the fur seal. In terrestrial animals cortical and subcortical release of monoamines (histamine, serotonin and noradrenaline) decreases at the transition from active wakefulness (the state with the greatest levels of both muscle tone and activity), to quiet wakefulness, bilateral SWS and then decreases further in REM sleep (the state of immobility with the most substantial reduction in muscle tone; [32-34]). This was also the case in the fur seal [35, 36]. Also similar to terrestrial mammals [37,38], cortical release of acetylcholine (Ach) in the seal during waking and REM sleep was greater than in bilateral SWS [39]. During USWS the release of Ach in the waking hemisphere in the fur seal was always greater than in the sleeping hemisphere (Figure 4). There was no such asymmetry in the release of norepinephrine, serotonin or histamine.
Figure 4.
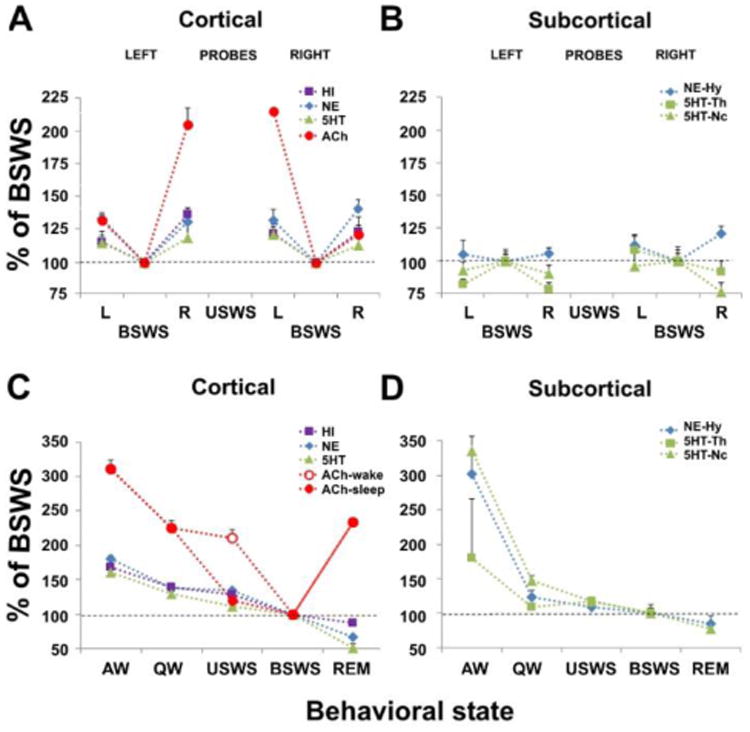
Release of neurotransmitters during sleep and waking states in the fur seal. (A,B) Cortical and subcortical release of histamine (HI), norepinephrine (NE), serotonin (5HT) and acetylcholine (ACh) during bilateral slow wave sleep (BSWS) and unihemispheric slow wave sleep (USWS) in the left (L) and right (R) hemispheres in fur seals. Each point represents the mean of the percent change in neurotransmitter level relative to BSWS which is calculated separately for the probes located in the left and right cortical hemispheres and subcortical structures (Hy, hypothalamus; Th, thalamus; Nc, caudate nucleus). (C,D) Cortical and subcortical release of HI, NE, 5HT, and ACh during waking and sleep states in fur seals. Each point represents the mean of the percent change in neurotransmitter level relative to BSWS. The release of HI, NE, and 5HT were not lateralized during USWS. It is shown as average for all left and right probes. The release of ACh was lateralized during USWS. For USWS it is shown separately for the waking (open circle) and sleeping (closed circle) hemispheres. During USWS, ACh release is higher on the waking side than on the sleeping side. The release of cortical HI, NE, and 5HT as well as subcortical NE and 5HT during USWS are not lateralized and are comparable to the release of ACh on the sleeping side. The data for cortical ACh and 5HT release were collected during the prior studies (reproduced from Lyamin et al., 2016).
The primary phenomena of USWS is interhemispheric slow wave EEG asymmetry. Activity of the Ach system is known to be more related to EEG arousal in animals than activity of the monoamine system [37, 38]. This may explain why Ach, as opposed to monoamines, is found to be lateralized during USWS in the fur seal. We can suggest that the activity of the Ach system can be controlled asymmetrically from both sides of the cerebral cortex. At the same time, the activity of other systems may either not be controlled laterally or it does not have an effect on the cortical asymmetry of EEG. This does not imply that other neurotransmitters do not contribute to the control of other aspects of USWS. For instance, we cannot fully exclude that over short intervals of time asymmetric release of the monoamines might occur in the fur seal [39]. However, we see no changes in the concentration of monoamines in relation to asymmetric sleep or waking states in the fur seal.
Conclusions
The fur seal displays several remarkable behavioral and physiological adaptations allowing sleep both on land and in water under the temperature conditions of the subpolar areal of this species. As a semiaquatic animal, the fur seal provides an opportunity to examine the physiological and behavioral substrates of switching between the typical terrestrial type of sleep (such as bilateral SWS, immobility, regular breathing and REM sleep) and fully aquatic mode of sleep as shown by cetaceans (USWS, sleep in motion, interrupted pattern of breathing and absence or substantial reduction of REM sleep).
Highlights.
The fur seal displays both REM and nonREM sleep
Unihemispheric sleep in the fur seal facilitates maintenance of an asymmetrical sleep posture and vigilance
Acetylcholine release is required for cortical activation during unihemispheric sleep
Acknowledgments
The work on this review was supported by grants from National Science Foundation (0919929), National Institute of Health MH064109, DA034748, Russian Fund for Basic Research (13-04- 1704, 14-04-32075), Medical Research Service of the Dept. of Veterans Affairs and Utrish Dolphinarium Ltd.
Footnotes
Conflict of interest statement: We have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Oleg I. Lyamin, Department of Psychiatry and Biobehavioral Sciences, and Brain Research Institute, University of California Los Angeles, Los Angeles, CA
Lev M. Mukhametov, Severtsov Institute of Ecology and Evolution, RAS, Moscow, RUSSIA
Jerome M. Siegel, Utrish Dolphinarium Ltd., Moscow, Russia
References
Papers of particular interest, published within the period of review, have been highlighted as: • of special interest
- 1.Siegel JM. Do all animals sleep? Trends in Neurosciences. 2008;31:208–213. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel JM. REM sleep: a biological and psychological paradox. Sleep Med Rev. 2011;15:139–42. doi: 10.1016/j.smrv.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel JM. Rapid eye movement sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Mechanisms. Vol. 2016. WB Saunders; Philadelphia, PA: pp. 78–95. [Google Scholar]
- 4.Joiner WJ. Unraveling the Evolutionary Determinants of Sleep. Curr Biol. 2016;26:R1073–R1087. doi: 10.1016/j.cub.2016.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heithaus MR, Frid A. Optimal diving under the risk of predation. J Theor Biol. 2003;223:79–92. doi: 10.1016/s0022-5193(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 6.Mukhametov LM, Supin AYa, Polyakova IG. Interhemispheric asymmetry of the ectroencephalographic sleep pattern in dolphins. Brain Res. 1977;134:581–584. doi: 10.1016/0006-8993(77)90835-6. [DOI] [PubMed] [Google Scholar]
- 7.Mukhametov LM. Sleep in marine mammals. Exp Brain Res. 1984;8:227–238. [Google Scholar]
- 8.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008;32:1451–84. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lilly JC. Animals in aquatic environments: adaptations of mammals to the ocean. In: Dill DB, editor. Handbook of Physiology-Environment. Vol. 1964. American Physiology Society; Washington, DC: pp. 741–747. [Google Scholar]
- 10.Berta A, Churchill M. Pinniped Taxonomy: evidence for species and subspecies. Mammal Review. 2002;42:207–234. [Google Scholar]
- 11.Gentry RL. Behavior and Ecology of the Northern Fur Seal. Vol. 1998. Princeton University Press; Princeton, New Jersey: p. 398. [Google Scholar]
- 12.Mukhametov LM, Lyamin OI, Polyakova IG. Interhemispheric asynchrony of the sleep EEG in northern fur seals. Experientia. 1985;41:1034–5. doi: 10.1007/BF01952128. [DOI] [PubMed] [Google Scholar]
- 13.Lyamin OI, Mukhametov LM. Organization of sleep in the northern fur seal. In: Sokolov VE, Aristov AA, Lisitzina TU, editors. In: The Northern Fur Seal Systematic, Morphology, Ecology, Behavior. Vol. 1998. Nauka; Moscow: pp. 280–302. [Google Scholar]
- 14.Lyamin OI, Lapierre JL, Kosenko PO, Mukhametov LM, Siegel JM. Electroencephalogram asymmetry and spectral power during sleep in the northern fur seal. J Sleep Res. 2008;17:154–65. doi: 10.1111/j.1365-2869.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartholomew GE, Wilke F. Body temperature in the northern fur seal, Callorhinus ursinus. J Mammal. 1956;37:227–237. [Google Scholar]
- 16.Castellini M. Thermoregulation. In: Perrin WF, Wursig B, Thewissen JGM, editors. Encyclopedia of Marine Mammals. Vol. 2009. Acs; NY: pp. 1166–1171. [Google Scholar]
- 17.Lyamin OI, Chetyrbok IS. Unilateral EEG activation during sleep in the cape fur seal, Arctocephalus pusillus. Neurosci Lett. 1992;143:263–266. doi: 10.1016/0304-3940(92)90279-g. [DOI] [PubMed] [Google Scholar]
- 18.Lyamin OI, Mukhametov LM, Chetyrbok IS, Vassiliev AV. Sleep and wakefulness in the southern sea lion. Behav Brain Res. 2002;128:129–138. doi: 10.1016/s0166-4328(01)00317-5. [DOI] [PubMed] [Google Scholar]
- 19.Lyamin OI, Kosenko PO, Vyssotski AL, Lapierre JL, Siegel JM, Mukhametov LM. Study of sleep in a walrus. Dokl Biol Sci. 2012;444:188–91. doi: 10.1134/S0012496612030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhametov LM, Lyamin OI, Chetyrbok IS, Vassilyev AA, Diaz RP. Sleep in an Amazonian manatee, Trichechus inunguis. Experientia. 1992;48:417–419. doi: 10.1007/BF01923447. [DOI] [PubMed] [Google Scholar]
- 21.Mukhametov LM, Supin AYa, Polyakova IG. The sleep in Caspian seals (Phoca caspica) Zh Vysshei Nervnoi Deyatelnosti Imeni I P Pavlova. 1984;34:259–264. [PubMed] [Google Scholar]
- 22.Lyamin OI. Sleep in the harp seal (Pagophilus groenlandica). Comparisons of sleep on land and in water. J Sleep Res. 1993;2:170–174. doi: 10.1111/j.1365-2869.1993.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 23.Castellini MA, Milsom WK, Berger RJ, Costa DP, Jones DR, Castellini JM, Rea LD, Bharma S, Harris M. Patterns of respiration and heart rate during wakefulness and sleep in elephant seal pups. Am J Physiol. 1994;266:R863–R869. doi: 10.1152/ajpregu.1994.266.3.R863. [DOI] [PubMed] [Google Scholar]
- 24.Lyamin OI, Pavlova IF, Kosenko PO, Mukhametov LM, Siegel JM. Regional differences in cortical electroencephalogram (EEG) slow wave activity and interhemispheric EEG asymmetry in the fur seal. J Sleep Res. 2012;21:603–11. doi: 10.1111/j.1365-2869.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosenko P, Lapierre J, Mukhametov L, Siegel J, Lyamin O. Subcortical EEG asymmetry during slow wave sleep in the fur seal. Sleep. 2012;35:A26. doi: 10.1111/j.1365-2869.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM, Siegel JM. Fur seals display a strong drive for bilateral slow-wave sleep while on land. J Neurosci. 2008;28:12614–21. doi: 10.1523/JNEUROSCI.2306-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyamin OI, Mukhametov LM, Siegel JM, Nazarenko EA, Polyakova IG, Shpak OV. Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav Brain Res. 2002;129:125–129. doi: 10.1016/s0166-4328(01)00346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rattenborg NC, Lima SL, Amlaner CJ. Facultative control of avian unihemispheric sleep under the risk of predation. Behav Brain Res. 1999;105:163–72. doi: 10.1016/s0166-4328(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Rattenborg NC, Amlaner CJ, Lima SL. Unilateral eye closure and interhemispheric EEG asymmetry during sleep in the pigeon (Columba livia) Brain Behav Evol. 2001;58:323–32. doi: 10.1159/000057573. [DOI] [PubMed] [Google Scholar]
- 30•.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell'Omo G, Lipp HP, Wikelski M, Vyssotski AL. Evidence that birds sleep in mid-flight. Nat Commun. 2016;7:12468. doi: 10.1038/ncomms12468. Here, using electroencephalogram recordings of great frigatebirds (Fregata minor) flying over the ocean for up to 10 days, the authors show that frigatebirds can sleep with either one hemisphere at a time or both hemispheres simultaneously. During asymmetrical / unihemispheric slow wave sleep the more awake EEG activity in the hemisphere opposite the direction of the turn indicates that the frigatebirds had the eye toward the direction of the turn open, presumably to watch where they were going. Also unexpectedly, frigatebirds sleep for only 0.69 h per day (7.4% of the time spent sleeping on land), indicating that ecological demands for attention usually exceed the attention afforded by sleeping unihemispherically. In addition to establishing that birds can sleep in flight, the results of this study challenge the view that they sustain prolonged flights by obtaining normal amounts of sleep on the wing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyamin OI, Mukhametov LM, Siegel JM. Association between EEG asymmetry and eye state in Cetaceans and Pinnipeds. Arch Ital Biol. 2004;142:557–568. [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 33.Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, Suaud-Chagny MF, Gottesmann C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–899. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 34.Chu M, Huang ZL, Qu WM, Eguchi N, Yao MH, Urade Y. Extracellular histamine level in the frontal cortex is positively correlated with the amount of wakefulness in rats. Neurosci Res. 2004;49:417–20. doi: 10.1016/j.neures.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Lapierre JL, Kosenko PO, Kodama T, Peever JH, Mukhametov LM, Lyamin OI, Siegel JM. Symmetrical serotonin release during asymmetrical slow-wave sleep: implications for the neurochemistry of sleep-waking states. J Neurosci. 2013;33:2555–61. doi: 10.1523/JNEUROSCI.2603-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Lyamin OI, Lapierre JL, Kosenko PO, Kodama T, Bhagwandin A, Korneva SM, Peever JH, Mukhametov LM, Siegel JM. Monoamine release during unihemispheric sleep and unihemispheric waking in the fur seal. Sleep. 2016;39:625–36. doi: 10.5665/sleep.5540. A number of neurotransmitters have been shown to be released at greater levels in waking than in sleep. It has been assumed that these neurotransmitters work together to produce the forebrain and brainstem activation that underlies waking. However, another possibility is that these “waking neurotransmitters” have roles, unrelated to EEG arousal. Examining the fur seal, an animal that can sleep with one side of the brain at a time, the authors found that the release of norepinephrine, histamine and serotonin are minimal and bilaterally symmetric when one side of the brain is asleep and the other awake. Of the transmitters most strongly implicated in waking control, only acetylcholine was elevated on the waking side. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172:601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- 38.Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 39.Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM, Siegel JM. Cortical acetylcholine release is lateralized during asymmetrical slow-wave sleep in northern fur seals. J Neurosci. 2007;27:11999–2006. doi: 10.1523/JNEUROSCI.2968-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


