Abstract
Objectives
DNA fragmentation factors 40 and 45 (DFF40 and DFF45) are responsible for final DNA-laddering during apoptosis, whereas Bcl-2 (B-cell lymphoma 2) is an apoptosis inhibitor. Our aim was to investigate the expression of DFF40, DFF45, and Bcl-2 in uterine leiomyosarcomas (uLMS), leiomyomas (uLM), and the normal myometrium. Furthermore, the correlation between DFF40, DFF45, and Bcl-2 expression and clinicopathological parameters in leiomyosarcomas was assessed. Their prognostic value in disease-free survival (DFS) and overall survival (OS) was also calculated.
Materials and methods
This study included 53 cases of uLMS from patients matched for age and menopausal status with 53 cases of uLM and 53 controls of normal myometrium (uM). Case samples of uterine myometrium from leiomyosarcomas (uLMS-M) and leiomyomas (uLM-M) were also studied. Immunohistochemical scoring was undertaken for DFF40, DFF45, and Bcl-2.
Results
DFF40, DFF45, and Bcl-2 were significantly underexpressed in uLMS compared with uLMS-M and uM. In uLMS samples, no correlation between the analyzed proteins was observed. Negative DFF40 and Bcl-2, but not DFF45, staining was a predictor of poorer DFS and OS in women with uLMS. uLM showed DFF40 and Bcl-2 overexpression compared with uM and uLM-M, with a significant positive correlation between DFF40 and DFF45. No differences in DFF40, DFF45, and Bcl-2 expression were observed between the uLMS-M, uLM-M, and uM samples, with a significant positive correlation between DFF40 and DFF45 expression.
Conclusion
DFF40, DFF45, and Bcl-2 are significantly underexpressed in uLMS, but only a lack of DFF40 and Bcl-2 negatively influences DFS and OS. Disruption of DFF40 and DFF45 expression was observed in uLMS, but not in uLM or control and case myometrium; this may play a role in tumor pathogenesis.
Keywords: B-cell lymphoma 2, disease-free survival, DNA fragmentation factor 40, DNA fragmentation factor 45, uterine, immunohistochemistry, leiomyosarcoma, uterine leiomyoma, overall survival
Introduction
Uterine leiomyosarcomas (uLMS) are rare malignant tumors arising within the myometrium, and account for 1%–2% of all uterine corpus malignancies; however, cervical localization of uLMS has also been reported.1–3 These represent the most common subtype of uterine sarcoma, with a stable incidence over the past three decades.4,5 Prior pelvic irradiation, use of tamoxifen, and African-American ethnicity have been reported to increase the risk of uLMS.6–8
In contrast to uLMS, uterine leiomyoma (uLM) is the most common gynecological pathology, affecting up to 80% of the female population.9 Although possible progression from uLM to uLMS has been postulated, the risk of malignant transformation of uLM is low, accounting for less than 1% of all cases. Recent clonality studies have confirmed that most uLMS arise de novo.10–12 Proven clinical and pathological prognostic factors for uLMS are age, stage, grade (G), and mitotic index (MI), whereas prognostic biomarkers have not been consistently shown.5,13–15
Changes in the expression of Bcl-2, an anti-apoptotic protein, have been reported in several malignancies, including breast and cervical cancers, ovarian malignant epithelial tumors, endometrial carcinomas, as well as in uLMS; these tumors also show downregulated p53 and caspase-9 expression.16–22 Caspases (cysteine-aspartic proteases) can be activated via the mitochondrial or cytoplasmic pathways and as a result of cellular oxidative stress; their activation leads to activation of DNA fragmentation factors (DFF) in the nucleus, which are directly responsible for DNA fragmentation and cellular apoptosis.23 The DFF complex exists as a dimer composed of DFF40 (caspase-activated DNase; CAD) and DFF45 (inhibitor of caspase-activated DNase; ICAD).24 After DFF45 cleavage by caspase-3, the active form of DFF40 is released, which promotes DNA fragmentation (DNA laddering).24 However, DFF45 is not a simple inhibitor of DFF40, as its presence is essential for the proper folding of DFF40 into the active protein.24
The association between expression of these proteins and prognosis in various tumors has also been investigated. According to Hwang et al, Bcl-2 expression was a strong favorable prognostic factor in the HR(+)/HER2(−) subtype of breast cancer and a marginally significant favorable prognosticator in the HR(+)/HER2(+), but had no prognostic impact on other subtypes.29 In contrast, no significant correlation was seen between Bcl-2 expression and clinicopathological parameters of colorectal cancer.26 Lack of Bcl-2 expression was also shown to be an independent predictor of poor prognosis in endometrial cancers.30,31 In human uterine smooth muscle tumors, a significantly lower Bcl-2 expression was observed in leiomyosarcomas than in leiomyomas, and positive Bcl-2 expression has been proven to be an independent predictor of favorable prognosis in patients with leiomyosarcomas.32 Decreased DFF45 expression has been observed in renal, colorectal, and esophageal cancers, and it has been postulated that its downregulation (although unexpected at first glance) may play a role in tumor escape from apoptosis and may promote tumor progression and metastasis.33–35
The aim of this retrospective case–control study was to evaluate the expression of DFF40, DFF45, and Bcl-2 as potential prognostic biomarkers in uLMS.
Materials and methods
Case selection
In this retrospective study, 57 patients with primary uLMS were identified between 2000 and 2015. Inclusion criteria were patients older than 18 years with pathologically confirmed primary solitary uLMS; their histological diagnosis was reevaluated and confirmed. Patients who had additional malignancies, were smokers, had received hormonal treatment (including hormonal contraception) over the past 5 years, had been diagnosed with smooth muscle tumors of uncertain malignant potential or cellular and bizarre leiomyoma, or had missing clinical data were excluded. Finally, 53 uLMS cases were compared with 53 age-matched cases of solitary uLM and 53 matched cases of normal human uterine myometrium (uM). In cases of uLMS and uLM, additional myometrial samples were obtained at least 20 mm away from the primary tumor.
Every uLMS was staged according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification, and the tumor grade and MI were calculated for all uLMS as the number of mitoses in 10 consecutive high-power fields (hpf) at 400× magnification that were also analyzed. Patients with stage I and II uLMS underwent total hysterectomy with bilateral salpingo-oophorectomy (BSO) and pelvic lymph node biopsy, whereas those with stages III and IV disease underwent complete cytoreductive surgery, which allowed us to obtain archive specimens of uLMS tissue and concomitant uterine myometrium. Women with uLM underwent supracervical hysterectomy with bilateral salpingectomy or salpingo-oophorectomy, if clinically justified, to obtain uLM and related uterine myometrium specimens. Additionally, uM was obtained from patients who suffered from recurrent or persistent cervical dysplasia with no otherwise diagnosed myometrial pathology, and total hysterectomy was undertaken as the final treatment. All specimens were collected premenstrually, in the first phase of the menstrual cycle, which was confirmed using histopathological examination of the concomitant endometrium. Clinical data were retrieved from medical files.
Immunohistochemistry
Detailed immunohistochemistry protocols have been presented in earlier reports.36,37 In brief, 3-µm sections of selected tissues were deparaffinized and rehydrated, then incubated in a target retrieval solution (Dako, Carpinteria, CA, USA) for 15 min at 98°C. They were then incubated with 3% hydrogen peroxide in methanol for 10 min, and washed with TRIS-buffered saline (pH 7.5) for a further 10 min. The sections were incubated with diluted normal serum as a blocking solution for 30 min. A standard immunohistochemical technique was performed using rabbit polyclonal antibodies to DFF40 (Abcam, Cambridge, UK) (1:100 dilution) and DFF45 (Abcam, Cambridge, UK) (1:50 dilution), and a monoclonal mouse anti-human antibody to Bcl-2 (1:200 dilution; Leica Microsystems GmbH, Leica Biosystems, Nussloch, Germany). Colon carcinoma (for Bcl-2), breast carcinoma (for DFF45), and ovary tissue (for DFF40) were used as positive controls. For the negative control, the same specimens and methods were used, but the primary antibodies were omitted.
Immunohistochemical scoring
DFF45, DFF40, and Bcl-2 staining was blindly and independently evaluated by two board-certified pathologists in 5 hpf of maximal staining intensity. The Remmele and Stanger immunoreactive score was used, and each sample was scored based on the intensity of staining (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining) and the number of stained cells (0, expression in up to 10% of the cells; 1+, expression in 10%–50% of the cells; 2+, expression in 51%–80% of the cells; and 3+, expression in more than 80% of the cells).38 The final immunoreactivity score was determined by multiplying the intensity scores by the extent of the positivity scores of the stained cells. A discrepancy between the observations by the two pathologists occurred in 18 (2.74%) cases, and the samples were verified again 2 weeks after the primary evaluation to prevent recall bias. In all cases, a consensus was achieved and no samples required exclusion from the analysis.
Statistical analyses
Clinical features presented non-normal distributions (confirmed using the Kolmogorov–Smirnov test) and are presented as the medians and interquartile range (IQR) or rough number and percentage (%). They were compared using the Mann–Whitney U test, or the Kruskal–Wallis analysis of variance test, depending on the number of groups. Post hoc tests were used where appropriate. Immunohistochemical scoring of tissue specimens was evaluated using the chi-square test.39 Data from the immunohistochemistry results are presented as numbers and percentage of immunoscoring. Spearman’s rank test was used to determine the correlation of immunoscoring and pathological features. A Kaplan–Meier survival curve was prepared according to the DFF40, DFF45, and Bcl-2 expression status. Cox’s proportional hazard model was used to identify survival predictors, and the Cox–Mantel test was used to compare overall survival (OS) (defined as the period between initial surgery and the time of death) and disease-free survival (DFS) (defined as the period between initial surgery and the time of recurrence). To evaluate the intra-rater agreement of the immunohis tochemistry results, the intraclass correlation coefficient (ICC) for a single histopathological evaluation with a 95% confidence interval (CI) was calculated. To randomize patients who underwent evaluation for intra-rater agreement, we used the Research Randomizer (www.randomizer.org), and 55 (20.91%) samples were chosen and reevaluated separately for DFF40, DFF45, and Bcl-2 after a 2-week interval from the primary evaluation to prevent recall bias. The Guidelines for Reporting Reliability and Agreement in Studies were used to verify these results.40 A p-value of less than 0.05 was considered significant. All calculations were conducted using STATISTICA version 12.0 (StatSoft, Inc., Tulsa, OK, USA) and MedCalc Statistical Software version 17.0.4 (MedCalc Software bvba, Ostend, Belgium).
Ethical approval and informed consent
This retrospective, case–control study was approved by the Jagiellonian University Review Board (decision no: 122.6120.360) with the required informed consents from participants. All procedures were carried out in accordance with the ethical standards of the institutional and national research committees and in the spirit of the principles of the 1964 Declaration of Helsinki and its later amendments.
Results
Clinicopathological variables
The median age of the 53 patients with uLMS was 51 years (IQR 8). The number of cases with each FIGO stage was as follows: 27 (50.95%) with Stage I, 5 (9.43%) with Stage II, 12 (22.64%) with Stage III, and 9 (16.98%) with Stage IV. The tumor grading (G) was as follows: G1 6 cases (11.32%); G2 10 cases (18.87%), and G3 37 cases (69.81%), with a median MI of 37.00 (IQR 30.00; range 12.00–123.00). The median OS was 58.00 months (IQR 54.00; range 6–155) and the median DFS was 31.00 months (IQR 34.00; range 2–155). As cases were matched according to age and menopausal status, there were no differences in the last two factors. The control group showed a significantly higher mean age of menarche compared with women with uLMS (p=0.002) and uLM (p=0.012; Table 1). No differences in parity and menstrual cycle characteristics were noticed, except for the type of menstrual bleeding between donors of uM, uLM, and uLMS specimens; women with uLMS and uLM reported statistically significantly more frequent abnormal vaginal spotting than controls (21/53 [39.62%] vs 6/53 [11.32%], p<0.001, and 26/53 [49.06%] vs 6/53 [11.32%], p<0.001, respectively; Table 1).
Table 1.
Patient characteristics for uterine leiomyosarcoma (uLMS), uterine leiomyoma (uLM), and normal myometrium cases (uM)
| Uterine leiomyosarcomas (uLMS) n=53 | Uterine leiomyomas (uLM) n=53 | Uterine control myometrium (uM) n=53 | p-value | |
|---|---|---|---|---|
| Median age at therapy, years (IQR); min–max | 51.00 (8.00); 44.00–70.00 | n/a | ||
| Menopausal status, n (%) | n/a | |||
| Pre– | 30 (56.61%) | |||
| Post | 23 (43.39%) | |||
| FIGO stage, n (%) | n/a | n/a | n/a | |
| I | 27 (50.95%) | |||
| II | 5 (9.43%) | |||
| III | 12 (22.64%) | |||
| IV | 9 (16.98%) | |||
| Grade, n (%) | n/a | n/a | n/a | |
| 1 | 6 (11.32%) | |||
| 2 | 10 (18.87%) | |||
| 3 | 37 (68.81%) | |||
| Median primary tumor size, cm (IQR); min–max | 5.00 (7.00); 2.00–15.00 | 6.00 (2.00); 3.00–13.00 | n/a | 0.733 |
| Mitotic index, no of mitoses per 1 high power field, n (%) | n/a | n/a | n/a | |
| ≤50 | 38 (71.70%) | |||
| >50 | 15 (28.30%) | |||
| Adjuvant treatment, n (%) | n/a | n/a | n/a | |
| None | 21 (39.63%) | |||
| Radiotherapy (RTH) | 5 (9.43%) | |||
| Chemotherapy (CHT) | 22 (41.51%) | |||
| Combination of RTH and CHT | 5 (9.43%) | |||
| Median age of first menstrual period, years (IQR); min–max | 12.00 (2.00); 9.00–15.00 | 12.00 (2.00); 10.00–15.00 | 13.00 (1.00); 10.00–15.00 | p=0.009* |
| Median age of menopause, years (IQR); min–max (n=23) | 54.00 (2.00); 49.00–55.00 | 52.00 (4.00); 48.00–55.00 | 52.00 (1.00); 50.00–54.00 | p=0.061 |
| Median duration of menstrual cycle, days (IQR); min–max | 28.00 (2.00); 24.00–32.00 | 28.00 (1.00) 25.00–34.00 | 28.00 (0.00) 26.00–31.00 | p=0.408 |
| Median duration of menstruation, days (IQR), min–max | 4.00 (1.00); 3.00–7.00 | 4.00 (1.00); 3.00–7.00 | 4.00 (1.00); 3.00–6.00 | p=0.281 |
| Menstrual cycles, n (%) | p=0.164 | |||
| Regular | 36 (67.92%) | 32 (60.38%) | 42 (79.25%) | |
| Irregular | 17 (32.08%) | 21 (39.62%) | 11 (20.75%) | |
| Menstrual cycles, n (%) | p=0.510 | |||
| Painful | 13 (24.53%) | 15 (28.31%) | 9 (16.98%) | |
| Painless | 40 (75.47%) | 38 (71.69%) | 44 (83.08%) | |
| Type of menstrual bleeding, n (%) | p=0.038* | |||
| Scant | 4 (7.55%) | 2 (3.77%) | 4 (7.55%) | |
| Normal | 37 (69.81%) | 36 (67.92%) | 46 (86.79%) | |
| Heavy | 12 (22.64%) | 15 (28.31%) | 3 (5.66%) | |
| Parity, n (%) | p=0.403 | |||
| Nullipara | 5 (9.43%) | 3 (5.66%) | 2 (3.77%) | |
| Primipara | 4 (7.55%) | 9 (16.98%) | 5 (9.43%) | |
| Multipara | 44 (83.02%) | 41 (77.36%) | 46 (86.80%) |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics classification of uLMS; IQR, interquartile range.
DFF40, DFF45, and Bcl-2 expression in uterine leiomyosarcomas
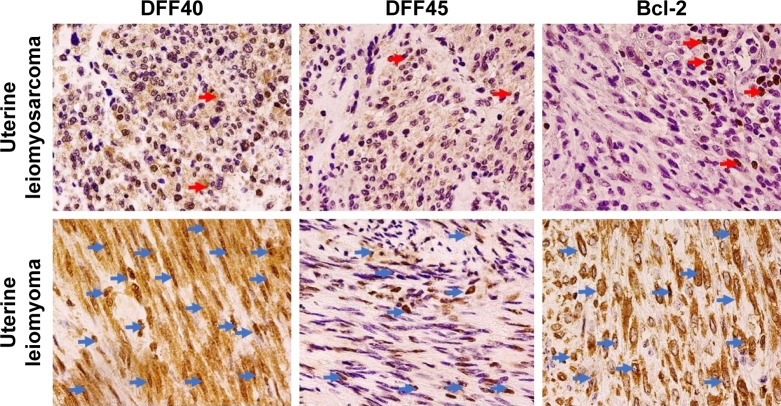
Bcl-2 showed cytoplasmic localization, while both DFF40 and DFF45 showed a nuclear pattern of expression (Figure 1). Only seven (13.21%) uLMS tissues had moderate expression of DFF40 and three (5.66%) showed moderate expression of Bcl-2. High expression of the studied proteins was absent from all uLMS samples (Table 2). DFF40 expression in uLMS was significantly lower than that in both uM (p<0.001) and uLMS-M (p<0.001). Similarly, DFF45 expression in uLMS was significantly lower than that in uM (p<0.001) and uLMS-M (p<0.001; Table 2). Bcl-2 expression in uLMS specimens was also significantly lower than that in uM (p<0.001) and uLMS-M (p=0.001; Table 2). In uLMS specimens, no correlations between DFF40 and DFF45, Bcl-2 and DFF40, or Bcl-2 and DFF45 expression were observed. Bcl-2 showed a significant inverse moderate correlation with tumor stage (R =−0.368; p=0.007) and MI (R =−0.301; p=0.029), but not with tumor diameter or grade. DFF40 but not DFF45 correlated inversely with the MI (R =−328; p=0.016), whereas both DFF40 and DFF45 correlated negatively with the stage of the disease (R =−0.300; p=0.029; R =−0.301; p=0.029, respectively); however, their expression did not correlate with tumor grade.
Figure 1.
Weak DFF40, DFF45, and Bcl-2 expression (400×) in uterine leiomyosarcomas (red arrows) compared to strong DFF40, Bcl-2, and moderate DFF45 expression in uterine leiomyomas (blue arrows).
Abbreviations: DFF40, DNA fragmentation factor 40; DFF45, DNA fragmentation factor 45; Bcl-2, B-cell lymphoma 2 protein.
Table 2.
Immunoexpression of DFF40, DFF45, and Bcl-2 in uterine leiomyosarcomas (uLMS)
| Immunoexpression* | Uterine leiomyosarcomas (uLMS) | Uterine myometrium from hysterectomy specimen for leiomyosarcomas (uLMS-M) | Uterine leiomyomas (uLM) | Uterine myometrium from hysterectomy specimen for leiomyomas (uLM-M) | Uterine control myometrium (uM) |
|---|---|---|---|---|---|
| DFF40 | |||||
| Negative | 23 (43.40%) | 4 (7.55%) | 0 (0.00%) | 3 (5.66%) | 4 (7.55%) |
| Low | 23 (43.40%) | 25 (47.17%) | 16 (30.19%) | 28 (52.83%) | 30 (56.60%) |
| Moderate | 7 (13.20%) | 18 (33.96%) | 18 (33.96%) | 18 (33.86%) | 16 (30.19%) |
| High | 0 (0.00%) | 6 (11.32%) | 19 (35.85%) | 4 (7.55%) | 3 (5.66%) |
| DFF45 | |||||
| Negative | 31 (58.49%) | 5 (9.43%) | 1 (1.89%) | 3 (5.66%) | 3 (5.66%) |
| Low | 22 (41.51%) | 42 (79.25%) | 41 (77.36%) | 35 (66.04%) | 40 (75.47%) |
| Moderate | 0 (0.00%) | 6 (11.32%) | 9 (16.98%) | 14 (26.41%) | 10 (18.87%) |
| High | 0 (0.00%) | 0 (0.00%) | 2 (3.77%) | 1 (1.89%) | 0 (0.00%) |
| Bcl-2 | |||||
| Negative | 25 (47.17%) | 11 (20.75%) | 0 (0.00%) | 13 (24.53%) | 9 (16.98%) |
| Low | 25 (47.17%) | 22 (41.51%) | 15 (28.30%) | 15 (28.30%) | 20 (37.74%) |
| Moderate | 3 (5.66%) | 18 (33.97%) | 17 (32.08%) | 19 (35.85%) | 21 (39.62%) |
| High | 0 (0.00%) | 2 (3.77%) | 21 (39.62%) | 6 (11.32%) | 3 (5.66%) |
Notes: Data presented as n (%). Uterine myometrium from hysterectomy specimens for leiomyosarcomas (uLMS-M); leiomyomas (uLM-M); uterine myometrium from hysterectomy specimen for leiomyomas (uLM-M) and uterine control. Samples of uLMS-M and uLM-M were obtained 1 cm from the primary tumor margin.
Immunoexpression was described as following: negative: 0; low: 1–2; moderate: 3–4; and high: 6–9.
Abbreviations: DFF40, DNA fragmentation factor 40; DFF45, DNA fragmentation factor 45; Bcl-2, B-cell lymphoma 2 protein.
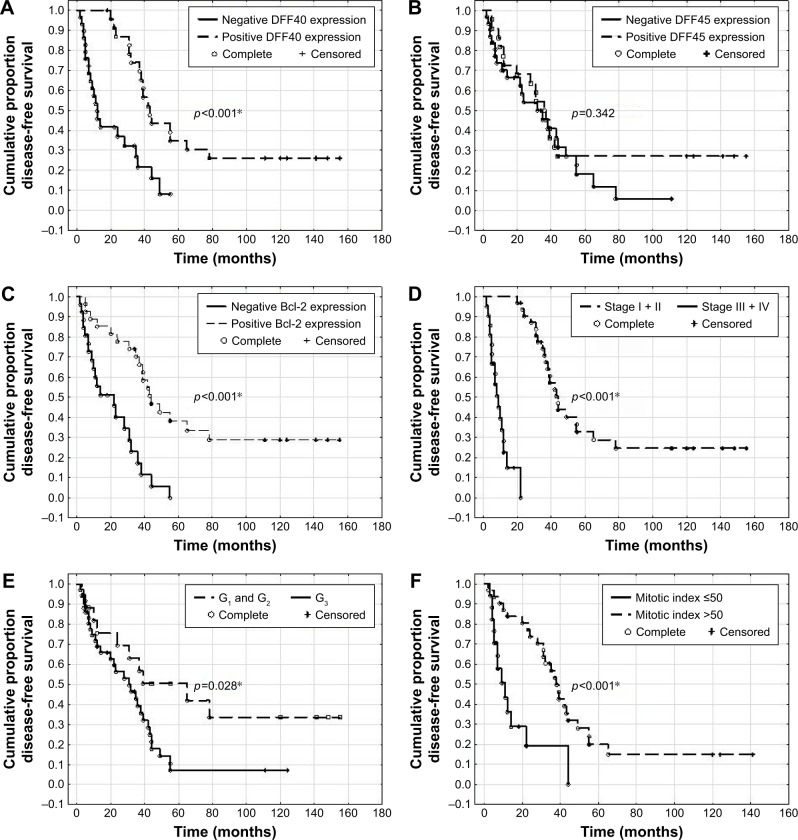
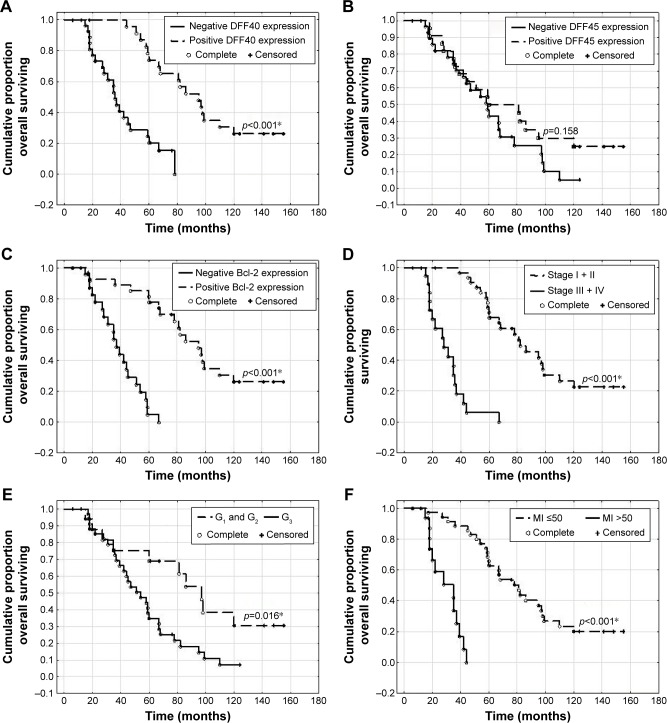
Women with uLMS that was negative for DFF40 and Bcl-2 staining showed significantly shorter OS than the OS of those whose specimens showed positive staining (p<0.001 and p<0.001, respectively), while there was no relationship between DFF45 expression and OS (Figure 2). OS in patients with stages III and IV disease (p<0.001), a G3 tumor (p=0.016), and an MI over 50 was poorer (Figure 2). Similarly, patients whose specimens tested negative for DFF40 and Bcl-2 (but not DFF45; p=0.342) expression showed significantly shorter DFS (p<0.001 and p<0.001, respectively; Figure 3). Patients with advanced stages of disease and higher grades and MI also had poorer DFS (p<0.001, p=0.028, and p<0.001, respectively; Figure 2).
Figure 2.
Disease-free survival (DFS) in women with leiomyosarcomas depending on DFF40 (A), DFF45 (B), and Bcl-2 (C) expression and disease stage (D) according to FIGO classification, tumor grade (E), and mitotic index (F); *p<0.05 is statistically significant.
Abbreviations: DFF40, DNA fragmentation factor 40; DFF45, DNA fragmentation factor 45; Bcl-2, B-cell lymphoma 2 protein; FIGO, International Federation of Gynecology and Obstetrics.
Figure 3.
Overall survival (OS) in women with leiomyosarcomas depending on DFF40 (A), DFF45 (B), and Bcl-2 (C) expression, and disease stage (D) according to FIGO classification, tumor grade (E), and mitotic index (F); *p<0.05 is statistically significant.
Abbreviations: DFF40, DNA fragmentation factor 40; DFF45, DNA fragmentation factor 45; Bcl-2, B-cell lymphoma 2 protein; FIGO, International Federation of Gynecology and Obstetrics.
Negative DFF40 and Bcl-2 expression, but not DFF45, were adverse prognostic factors for DFS and OS in women with uLMS. Further analysis showed that an advanced disease stage, high tumor grade, and elevated MI were associated with a reduction in OS and DFS (Table 3).
Table 3.
Cox’s proportional hazard regression analysis of the prognostic value of clinical and immunohistochemical variables in 53 women with uterine leiomyosarcomas
| Prognostic factor | Disease free survival (DFS)
|
Overall survival (OS)
|
||
|---|---|---|---|---|
| HR (CI) | p-value | HR (CI) | p-value | |
| Age | 0.973 (0.921–1.029) | 0.348 | 0.964 (0.910–1.020) | 0.206 |
| Stage 3+ | 9.024 (1.196–81.968) | <0.001# | 14.153 (6.061–33.049) | <0.001# |
| Grade 3 | 2.226 (1.064–4.656) | 0.031# | 2.396 (1.148–4.999) | 0.019# |
| MI >50 | 4.674 (2.184–10.014) | <0.001# | 18.601 (5.703–60.670) | <0.001# |
| Negative DFF40 staining | 3.254 (3.254–6.459) | 0.007# | 6.315 (2.712–14.704) | <0.001# |
| Negative DFF45 staining | 1.359 (0.715–2.583) | 0.349 | 1.587 (0.829–3.044) | 0.164 |
| Negative Bcl-2 staining | 3.893 (1.940–7.812) | <0.001# | 11.476 (4.454–29.564) | <0.001# |
Note:
p, significant p value.
Abbreviations: DFF40, DNA fragmentation factor 40; DFF45, DNA fragmentation factor 45; Bcl-2, B-cell lymphoma 2 protein; CI, confidence intervals; HR, hazard ratio; MI, mitotic index.
DFF40, DFF45, and Bcl-2 expression in uLM
uLM showed marked nuclear expression of DFF40/DFF45 and cytoplasmic expression of Bcl-2 (Figure 2). DFF40 expression in uLM was significantly higher than that in uM and uLM-M (p=0.001 and p=0.005, respectively; Table 2). No significant differences in DFF45 scoring between uLM and uM, nor between uLM and uLM-M were observed (Table 2). In addition, uLM showed the highest Bcl-2 expression, as compared with uM (p<0.001) and uLM-M (p<0.001). In uLM, we found a significant positive correlation between DFF40 and DFF45 expression (R =0.388; p=0.004) and between DFF40 and Bcl-2 expression (R =0.630; p<0.001); however, there was no correlation between DFF45 and Bcl-2 expression. Bcl-2 expression correlated positively and significantly with the diameter of the leiomyoma (R =0.435; p<0.001).
DFF40, DFF45, and Bcl-2 expression in uterine myometrium
Myometrial samples showed a nuclear pattern of DFF40/DFF45 and cytoplasmic of Bcl-2 staining. Only four (7.55%) samples of the control uM were DFF40-negative, whereas 30 (56.60%) presented with low, 16 (30.19%) with moderate, and three (5.66%) with high DFF40 expression. Similarly, uLMS-M and uLM-M showed predominately low and moderate DFF40 expression and scantly low and high expression (Table 2). No significant differences were observed in DFF40 nor DFF45 scoring between uM, uLMS-M, and uLM-M (Table 2). Bcl-2 showed mainly moderate expression in uM and uLM-M, and low in uLMS-M (Table 2). No significant differences were observed in Bcl-2 expression between the uM, uLM-M, and uLMS-M groups.
In uM, a moderate positive and significant correlation between DFF40 and DFF45 expression was observed (R =0.762; p<0.001), with no correlation between Bcl-2 and DFF40 or Bcl-2 and DFF45. In uLM-M, a strong positive and significant correlation between DFF40 and DFF45 was also proven (R =0.893; p<0.001), with no association observed between Bcl-2 and DFF40 or Bcl-2 and DFF45. Additionally, in uLMS-M specimens, the only correlation observed was that between DFF40 and DFF45 (R =0.703; p<0.001).
Validation of the intra-rater reliability for immunohistochemistry scoring
An excellent intra-rater agreement was confirmed with regard to the immunoscoring of DFF40, DFF45, and Bcl-2 expression, and the following values were noted:
The first pathologist (ie, observer 1 vs 1) for the assessment of DFF40 showed, ICC 0.976 [confidence interval (CI) 0.959–0.986]; for DFF45, ICC 0.934 (CI 0.900–0.961); and for Bcl-2, ICC 0.952 (CI 0.919–0.972).
The second pathologist (ie, observer 2 vs 2) for the assessment of DFF40, ICC 0.934 (CI 0.889–0.961); for DFF45, ICC 0.898 (CI 0.831–0.929); and for Bcl-2, ICC 0.984 (CI 0.972–0.990).
Discussion
To our knowledge, a comprehensive investigation of DFF40 and DFF45 immunoexpression along with Bcl-2 staining in leiomyosarcomas and leiomyomas of the human uterus has not been reported previously. Many studies have confirmed Bcl-2 underexpression in uLMS and have identified it as a predictor of poor OS; in contrast, uLM shows increased expression of this protein.17,32,41–43 Our outcomes confirmed that the highest Bcl-2 expression was present in uLM, and the lowest in uLMS. Similarly, Bcl-2-negative uLMS cases presented with a significantly shorter DFS and OS. Additionally, uLM specimens showed a lack of correlation between Bcl-2 and DFF40 or DFF45 expression.
As we found no studies concerning DFF40/DFF45 expression in uLMS, our findings can only be discussed in the context of other malignancies. Both DFF40 and DFF45 were underexpressed in uLMS, and DFF40-negative cases presented with significantly shorter DFS and OS; in contrast, DFF45 expression was not a predictive factor of OS or DFS. Sánchez-Osuna et al reported that DFF40-deficient high-grade glioblastomas showed incomplete apoptosis, despite the correct activation of executioner caspases.44 Bagheri et al reported that DFF40 overexpression sensitized breast cancer cells to doxorubicin, concluding that modulation of DFF40 levels may be a beneficial strategy for treatment of chemoresistant cancers.45 Additionally, Boon and Sim reported that the overexpression of only DFF40 in nasopharyngeal carcinomas did not result in increased apoptosis of head and neck squamous carcinoma in response to oxidative stress, as DFF45 was required for proper DFF40 folding.46
DFF45 deficiency was also reported as a cause of abnormal cell apoptosis; this seems counterintuitive, as DFF45 is an inhibitor of DFF40 and, thus, the lack of an inhibitor should result in increased activity of DNAse. However, it is important to note that DFF45 also acts as a chaperone for DFF40 and its presence is mandatory for proper DFF40 folding, which is, in turn, essential to the functional activation of DFF40. Errami et al proved that DFF45-negative colon cancer cells were more resistant to apoptosis than was normal colon tissue.35 Furthermore, Konishi et al showed that esophageal squamous cancer cells exhibit abundant DFF45 expression, which correlated negatively with tumor grade and enhanced lymphatic spread.34 The only study to investigate DFF45 expression in gynecological malignancies, conducted by Brustmann, showed that DFF45 upregulation in serous ovarian cancer was correlated with aggressive tumor behavior.47 Another study also showed increasing DFF45 immunoreactivity from normal endometrium through normal endometrial and atypical hyperplasia to endometrial adenocarcinomas.48 The results of DFF45 expression in different malignancies are inconclusive. In our study, we observed altered DFF45 staining in uLMS that correlated only with the stage of the disease, but did not influence DFS or OS.
Apoptosis is mainly triggered by either membrane death receptors or via the mitochondrial pathway, involving activation of many proapoptotic proteins and deactivation of anti-apoptotic factors.49,50 Furthermore, under persistent exposure to damaging agents, the endoplasmatic reticulum can initiate apoptosis through calcium release and activation of caspase 12.51 These mechanisms activate a cascade of caspases that exist mainly in the cytosol as pro-enzymes; a cross-talk between apoptotic factors then finally results in caspase-3 activation that cleaves DFF45, releasing DFF40, which is directly responsible for the last step of apoptosis: DNA laddering.24 Increased Bcl-2 expression in leiomyomas may explain an uncontrolled tumor proliferation, and confirms the monoclonal hypothesis of uterine fibroid formation.9 In contrast to high Bcl-2 expression, we would rather have expected decreased DFF40 levels in uLM, as this protein results in the DNA damage. In uLMS, low levels of DFF40 were intuitively predicted, although we expected higher levels of Bcl-2 as an anti-apoptotic protein. These apparently inconsistent and counterintuitive results are in agreement with other reports showing increased expression of some oncogenes in benign proliferative disorders and their depletion in malignancies.27
Based on these findings, we hypothesize that benign tumors and malignancies may have developed different mechanisms to avoid apoptosis. In uLM, Bcl-2 overexpression seems important for preventing cells from undergoing apoptosis. High DFF40 levels make leiomyoma cells more susceptible to apoptosis; however, the proapoptotic signal seems to be abrogated at earlier levels of the apoptotic cascade, before activation of DFF40/DFF45, and Bcl-2 overexpression can enhance these mechanisms. Second, an exclusive increase in DFF40 expression, without a DFF45 increase, may be not sufficient for proper DFF40 activation, as the presence of DFF45 is essential for DFF40 to acquire its biological function. Unbalanced DFF40/DF45 expression may be another mechanism allowing leiomyoma cells to escape apoptosis. In uLMS, significant decreases in DFF40 and DFF45 expression seem sufficient to prevent DNA laddering. Under these circumstances, functioning of factors involved in transmitting of pro- and anti-apoptotic signals (including low Bcl-2 expression) seems secondary.
In this study, we confirmed that DFF40, DFF45, and Bcl-2 underexpression in uLMS can enhance apoptosis dysregulation. Moreover, in malignant tissues, no correlation was found between DFF40 and DFF45 expression. Dysregulation of DFF40 and DFF45 expression may be another mechanism underlying cell escape from apoptosis, as the presence of DFF45 is mandatory for proper DFF40 function.
The main strength of the study is that it provides a comprehensive analysis of DFF40 and DFF45, simultaneously with Bcl-2 evaluation, in uLMS. We correlated DFF40 and DFF45 expression with clinical malignancies and pathological features, showing that DFF40 and Bcl-2, but not DFF45, may serve as independent prognostic factors of DFS and OS. Moreover, protein expression in uLMS was not only compared with their expression in normal human myometrium, but also with cases of leiomyomas and myometrium from corresponding cases. In this analysis, no differences between case and control myometrium were seen in DFF40, DFF45, and Bcl-2 expression; these observations agree with the clonal theory of uLMS and uLM, which confirms the very low risk of malignant transformation of uLM. Finally, we observed a positive correlation between DFF40 and DFF45 expression but not Bcl-2, in both control and case myometrial samples.
The use of immunohistochemistry, which is a semi-quantitative method, may be considered a potential limitation of the study, especially for comparing DFF40 and DFF45 expression in the same tissue. We acknowledge that an immunostaining analysis is subjective and, for that reason, each sample was evaluated by two highly experienced pathologists. This methodology is widely accepted and has been employed by many other studies for investigating the expression of DFF45 and Bcl-2.25–29,32 As complete inter-rater agreement was achieved, the intra-rater disparity was the only potential bias in our sample assessment. However, as this disparity showed an almost-perfect correlation, intra-rater bias can safely be excluded.
Conclusion
This current study provides important evidence regarding the underexpression of DFF40/DFF45 and Bcl-2 in uLMS, and shows that DFF40 and Bcl-2 may serve as prognostic factors for DFS and OS. This comprehensive evaluation provides more insight into other findings concerning DFF40, DFF45, and Bcl-2 expression in malignancies.
Acknowledgments
The authors thank Dr H Molak-Olczak, who independently and blindly analyzed the immunoexpression of the investigated proteins as a second specialist in histopathology.
Footnotes
Author contributions
TB was the chief investigator who designed the study, selected the cases, conducted data analysis, and drafted the manuscript. KP and KO participated in the creation of the study design, specimen evaluation, and the selection of eligible cases. KO performed the protein immunoexpression assessment. AC performed statistical analysis and critically reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Zhiqiang L, Bin S, Min F, Yufang L. Leiomyosarcoma of cervical stump following subtotal hysterectomy: a case report and review of literature. Eur J Gynaecol Oncol. 2016;37(1):148–151. [PubMed] [Google Scholar]
- 3.Khosla D, Gupta R, Srinivasan R, Patel FD, Rajwanshi A. Sarcomas of uterine cervix: clinicopathological features, treatment, and outcome. Int J Gynecol Cancer. 2012;22(6):1026–1030. doi: 10.1097/IGC.0b013e31825a97f6. [DOI] [PubMed] [Google Scholar]
- 4.Boll D, Verhoeven RH, van der Aa MA, et al. Incidence and survival trends of uncommon corpus uteri malignancies in the Netherlands, 1989–2008. Int J Gynecol Cancer. 2012;22(4):599–606. doi: 10.1097/IGC.0b013e318244cedc. [DOI] [PubMed] [Google Scholar]
- 5.Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10(12):1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb S. Tamoxifen may increase risk for uterine sarcoma. Br Med J. 2002;325:7. doi: 10.1136/bmj.325.7354.7/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naaman Y, Shveiky D, Ben-Shachar I, Shushan A, Mejia-Gomez J, Benshushan A. Uterine sarcoma: prognostic factor and treatment evaluation. Isr Med Assoc J. 2011;13(2):76–79. [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasms. Version 2. 2016. [Accessed December 7, 2016]. Accessed from: www.nccn.org.
- 9.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 10.Mittal K, Joutovsky A. Areas with benign morphologic and immunohistochemical features are associated with some uterine leiomyosarcomas. Gynecol Oncol. 2007;104(2):362–365. doi: 10.1016/j.ygyno.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Mittal K. Precursor lesions for uterine leiomyosarcoma. Hum Pathol. 2007;38(8):1289. doi: 10.1016/j.humpath.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Zhang C, Hao J, et al. Use of X-chromosome inactivation pattern to determine the clonal origins of uterine leiomyoma and leiomyosarcoma. Hum Pathol. 2006;37(10):1350–1356. doi: 10.1016/j.humpath.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Gadducci A, Sartori E, Landoni F, et al. The prognostic relevance of histological type in uterine sarcomas: a Cooperation Task Force (CTF) multivariate analysis of 249 cases. Eur J Gynaecol Oncol. 2002;23(4):295–289. [PubMed] [Google Scholar]
- 14.Raspollini MR, Amunni G, Villanucci A, et al. Estrogen and progesterone receptors expression in uterine malignant smooth muscle tumors: correlation with clinical outcome. J Chemother. 2003;15(6):596–602. doi: 10.1179/joc.2003.15.6.596. [DOI] [PubMed] [Google Scholar]
- 15.Smirnova IS, Yakovleva TK, Rosanov YM, Aksenov ND, Pospelova TV. Analysis of p53-dependent mechanisms in the maintenance of genetic stability in diploid tumourigenic line SK-UT-1B of human uterine leiomyosarcoma. Cell Biol Int. 2001;25(11):1101–1115. doi: 10.1006/cbir.2001.0709. [DOI] [PubMed] [Google Scholar]
- 16.Kułak K, Bobiński M, Polak G, Jedrych BJ, Kotarski J, Bednarek W. Ocena ekspresji kaspazy 9 i bialka p53 w miesakach macicy. [Expression of caspase 9 and p53 in uterine leiomyosarcomas] Ginekol Pol. 2014;85(8):600–604. doi: 10.17772/gp/1778. Polish. [DOI] [PubMed] [Google Scholar]
- 17.Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K, Mayerhofer K. Bcl-2 expression and other clinicopathologic parameters in uterine leiomyosarcoma. Wien Klin Wochenschr. 2004;116(4):135–139. doi: 10.1007/BF03040751. [DOI] [PubMed] [Google Scholar]
- 18.Kowalewska M, Bakula-Zalewska E, Chechlinska M, et al. microRNAs in uterine sarcomas and mixed epithelial-mesenchymal uterine tumors: a preliminary report. Tumour Biol. 2013;34(4):2153–2160. doi: 10.1007/s13277-013-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlin JN, Zhou QC, Leitao MM, et al. Molecular subtypes of uterine leiomyosarcoma and correlation with clinical outcome. Neoplasia. 2015;17(2):183–189. doi: 10.1016/j.neo.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cekanova M, Fernando RI, Siriwardhana N, et al. BCL-2 family protein, BAD is down-regulated in breast cancer and inhibits cell invasion. Exp Cell Res. 2015;331(1):1–10. doi: 10.1016/j.yexcr.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhry P, Srinivasan R, Patel FD. Differential expression of Fas family members and Bcl-2 family members in benign versus malignant epithelial ovarian cancer (EOC) in North Indian population. Mol Cell Biochem. 2012;368(1–2):119–126. doi: 10.1007/s11010-012-1350-7. [DOI] [PubMed] [Google Scholar]
- 22.Dorjgochoo T, Xiang YB, Long J, et al. Association of genetic markers in the BCL-2 family of apoptosis-related genes with endometrial cancer risk in a Chinese population. PLoS One. 2013;8(4):e60915. doi: 10.1371/journal.pone.0060915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao PL, Jiang Y, Wee BY, Porter AG. Activation of caspase-1 in the nucleus requires nuclear translocation of pro-caspase-1 mediated by its prodomain. J Biol Chem. 1998;273(37):23621–23624. doi: 10.1074/jbc.273.37.23621. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima K, Kikuchi J, Koshiba S, Kigawa T, Kuroda Y, Yokoyama S. Solution structure of the DFF-C domain of DFF45/ICAD. A structural basis for the regulation of apoptotic DNA fragmentation. J Mol Biol. 2002;321(2):317–327. doi: 10.1016/s0022-2836(02)00588-0. [DOI] [PubMed] [Google Scholar]
- 25.Schorr K, Li M, Krajewski S, Reed JC, Furth PA. Bcl-2 gene family and related proteins in mammary gland involution and breast cancer. J Mammary Gland Biol Neoplasia. 1999;4(2):153–164. doi: 10.1023/a:1018773123899. [DOI] [PubMed] [Google Scholar]
- 26.Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 2015;19(2):69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T, Nomura S, Sakai T, Nariya S. Expression of bcl-2 oncoprotein in gastrointestinal and uterine carcsinomas and their premalignant lesions. Hum Pathol. 1997;28(3):309–315. doi: 10.1016/s0046-8177(97)90129-5. [DOI] [PubMed] [Google Scholar]
- 28.Geisler JP, Geisler HE, Wiemann MC, Zhou Z, Miller GA, Crabtree W. Lack of bcl-2 persistence: an independent prognostic indicator of poor prognosis in endometrial carcinoma. Gynecol Oncol. 1998;71(2):305–307. doi: 10.1006/gyno.1998.5192. [DOI] [PubMed] [Google Scholar]
- 29.Hwang KT, Han W, Kim J, et al. Prognostic influence of BCL2 on molecular subtypes of breast cancer. J Breast Cancer. 2017;20(1):54–64. doi: 10.4048/jbc.2017.20.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porichi O, Nikolaidou ME, Apostolaki A, et al. BCL-2, BAX and P53 expression profiles in endometrial carcinoma as studied by real-time PCR and immunohistochemistry. Anticancer Res. 2009;29(10):3977–3982. [PubMed] [Google Scholar]
- 31.Geisler JP, Geisler HE, Wiemann MC, Zhou Z, Miller GA, Crabtree W. Lack of bcl-2 persistence: an independent prognostic indicator of poor prognosis in endometrial carcinoma. Gynecol Oncol. 1998;71(2):305–307. doi: 10.1006/gyno.1998.5192. [DOI] [PubMed] [Google Scholar]
- 32.Zhai YL, Nikaido T, Toki T, Shiozawa A, Orii A, Fujii S. Prognostic significance of bcl-2 expression in leiomyosarcoma of the uterus. Br J Cancer. 1999;80(10):1658–1664. doi: 10.1038/sj.bjc.6690578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajandram R, Razack AH, Ng KL, Gobe GC. Decreased expression of inhibitor of caspase-activated DNase (ICAD) in renal cell carcinoma – tissue microarray of human samples. J Kidney Cancer VHL. 2016;3(1):1–11. doi: 10.15586/jkcvhl.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konishi S, Ishiguro H, Shibata Y, et al. Decreased expression of DFF45/ICAD is correlated with a poor prognosis in patients with esophageal carcinoma. Cancer. 2002;95(12):2473–2478. doi: 10.1002/cncr.10987. [DOI] [PubMed] [Google Scholar]
- 35.Errami Y, Brim H, Oumouna-Benachour K, et al. ICAD deficiency in human colon cancer and predisposition to colon tumorigenesis: linkage to apoptosis resistance and genomic instability. PLoS One. 2013;8(2):e57871. doi: 10.1371/journal.pone.0057871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banas T, Basta P, Knafel A, et al. DFF45 expression in human endometrium is associated with menstrual cycle phases and decreases after menopause. Gynecol Obstet Invest. 2012;73:177–182. doi: 10.1159/000331647. [DOI] [PubMed] [Google Scholar]
- 37.Banas T, Skotniczny K, Basta A. DFF45 expression in ovarian endometriomas. Eur J Obstet Gynecol Reprod Biol. 2009;146(1):87–91. doi: 10.1016/j.ejogrb.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8(3):138–140. Article in German. [PubMed] [Google Scholar]
- 39.Preacher KJ. Calculation for the chi-square test: an interactive calculation tool for chi-square tests of goodness of fit and independence [Computer software] [Accessed January 2, 2017]. Available from: http://quantpsy.org.
- 40.Kottner J, Audige L, Brorson S, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. Int J Nurs Stud. 2011;48(6):661–671. doi: 10.1016/j.ijnurstu.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Uekuri C, Akasaka J, et al. The biology of uterine sarcomas: a review and update. Mol Clin Oncol. 2013;1(4):599–609. doi: 10.3892/mco.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiser AL, Anderson SE, Nonaka D, et al. Apoptotic and cell cycle regulatory markers in uterine leiomyosarcoma. Gynecol Oncol. 2006;101(1):86–91. doi: 10.1016/j.ygyno.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 43.D’Angelo E, Espinosa I, Ali R, et al. Uterine leiomyosarcomas: tumor size, mitotic index, and biomarkers Ki67, and Bcl-2 identify two groups with different prognosis. Gynecol Oncol. 2011;121(2):328–333. doi: 10.1016/j.ygyno.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Osuna M, Martínez-Escardó L, Granados-Colomina C, et al. An intrinsic DFF40/CAD endonuclease deficiency impairs oligonucleosomal DNA hydrolysis during caspase-dependent cell death: a common trait in human glioblastoma cells. Neuro Oncol. 2016;18(7):950–961. doi: 10.1093/neuonc/nov315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagheri F, Safarian S, Eslaminejad MB, Sheibani N. Sensitization of breast cancer cells to doxorubicin via stable cell line generation and overexpression of DFF40. Biochem Cell Biol. 2015;93(6):604–610. doi: 10.1139/bcb-2015-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boon SS, Sim SP. Inhibitor of caspase-activated DNase expression enhances caspase-activated DNase expression and inhibits oxidative stress-induced chromosome breaks at the mixed lineage leukaemia gene in nasopharyngeal carcinoma cells. Cancer Cell Int. 2015;15:54. doi: 10.1186/s12935-015-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brustmann H. DNA fragmentation factor (DFF45): expression and prognostic value in serous ovarian cancer. Pathol Res Pract. 2006;202(10):713–720. doi: 10.1016/j.prp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Brustmann H. Poly(ADP-ribose) polymerase (PARP) and DNA-fragmentation factor (DFF45): expression and correlation in normal, hyperplastic and neoplastic endometrial tissues. Pathol Res Pract. 2007;203(2):65–72. doi: 10.1016/j.prp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Nagata S. Apoptosis by death factor. Cell. 1997;88(3):355–356. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 50.Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2(8):589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 51.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]