Abstract
Introduced approximately 10 years ago, high-resolution manometry catheters have fostered interest in anorectal manometry. This review, which accompanies 2 articles in this issue of Neurogastroenterology and Motility, reviews the methods, clinical indications, utility, and pitfalls of anorectal manometry and revisits the American Gastroenterological Association (AGA) Medical Position Statement on Anorectal Testing Techniques, which was last published in 1999. High-resolution manometry provides a refined assessment of the anorectal pressure profile, obviates the need for station pull-through maneuvers, and minimizes movement artifacts. In selected cases, this refined assessment may be useful for identifying structural abnormalities or anal weakness. However, many manometry patterns that were previously regarded as abnormal are also observed in a majority of healthy patients, which substantially limits the utility of manometry for identifying defecatory disorders. It is our impression that most conclusions of the AGA medical position statement from 1999 remain valid today. High-resolution techniques have not substantially affected the number of publications on or management of anorectal disorders. The ongoing efforts of an international working group to standardize techniques for anorectal manometry are welcome. Although high-resolution manometry is more than an expensive hobby, improvements in catheter design and further research to rigorously define and evaluate these techniques are necessary to determine if they are worth every penny.
Keywords: anal, constipation, diagnosis, fecal incontinence, high-resolution, manometry
Graphical Abstract
High-resolution manometry (HRM) catheters provide better spatial resolution of anorectal pressures than standard (non-HRM catheters) and minimize movement artifacts during manometry.
However, with a few exceptions, HRM catheters have not increased the clinical utility of manometry to quantify anal weakness.
Further studies are necessary, in particular to improve the currently limited utility of HRM for diagnosing defecatory disorders.
Introduction
Anorectal manometry (ARM) evaluates anorectal sensorimotor mechanisms that are responsible for fecal continence and defecation (1). Until 2008, ARM was solely performed with non–high-resolution catheters that incorporated water-perfused, air-charged, or solid-state sensors. Anorectal high-resolution manometry (HRM) was introduced in 2008, followed by high-definition manometry (HDM) (2). Prompted by 2 recent articles on anorectal HRM in this journal (3,4), we review the methods and clinical indications for and utility and pitfalls of ARM and revisit the American Gastroenterological Association (AGA) Medical Position Statement on Anorectal Testing Techniques, which was last published in 1999 (5).
Equipment
The design of non-HRM, HRM, and HDM systems is detailed elsewhere (2,6,7). Whereas the non–high-resolution catheters have 3 or 6 unidirectional sensors, HRM or HDM catheters have multiple pressure sensors that straddle the entire anal canal and more proximal sensors inside a balloon placed in the rectum. Therefore, HRM and HDM catheters provide better spatial resolution of the sphincter pressure profile than non–high-resolution catheters. Station pull-through maneuvers are not required, which minimizes movement-related artifacts and shortens the procedure duration. In addition to line graphs, pressures can be displayed in color, which are easier to review. In contrast to HRM catheters, HDM catheters also display pressures recorded by individual sensors around the circumference of the catheter, which reflects axial symmetry.
Techniques
Similar to non-HRM, HRM and HDM assess anorectal pressures at rest and during maneuvers (eg, squeeze, cough, rectal distension, simulated evacuation) and the sensation of rectal distension. In the survey by Carrington et al (3), approximately 50% of 107 respondents reported that they were using HRM or HDM catheters. Amazingly, no two centers used identical protocols for patient preparation, setup, study, and data interpretation. No center observed the methods recommended in a consensus document published 15 years ago (6).
The study by Mion et al (4) adds to our database of normal values for HDM (2). Although pressures measured with different manometry techniques are significantly correlated (8–10), the correlations are far from perfect, probably because differences in techniques introduce another source of variability. This underscores the need for evaluating normal values and the diagnostic utility of each technique (2).
Resting Pressure
The resting anal pressure is generated by the resting tone of the internal and external anal sphincter and, to a lesser extent, by the hemorrhoidal plexus (1). Resting pressure should be recorded approximately 3 minutes after the catheter is inserted, allowing sphincter contraction related to catheter insertion to subside. Resting pressure is generally recorded for 20 to 30 seconds. With this short recording time, the measurement may be influenced by the ultraslow wave cycling activity.
Anal resting pressure is not uniform over the longitudinal extent of the anal canal. Although HRM and HDM catheters measure pressures over the entire length of the anal canal, only the highest pressure at any level of the anal canal at each point in time is used for analysis. Typically, maximum resting pressure is the highest pressure at any instant, whereas mean resting pressure is averaged across the duration of the maneuver (11). Hence, the length of the high-pressure zone is the only parameter of the longitudinal sphincter pressure profile provided by HRM and HDM. Among healthy people, resting anal pressure is lower in women than in men (12,13) and lower in older than in younger women (11,13,14).
Squeeze Pressure
The squeeze anal pressure measures voluntary contraction of the external anal sphincter (1). It is usually recorded for 30 seconds during maximal voluntary contraction. Endurance can be measured by assessing the duration of the squeeze response (11). The squeeze response depends on the extent of volitional participation.
The squeeze pressure is lower in women than in men (12,13) and lower in older than in younger women (11,13,14). Because HDM can assess contractile symmetry, it is useful for identifying contraction of the puborectalis muscle, which only generates forces on the posterior aspect of the anorectum (13,15). Pressures measured with HDM can distinguish between healthy women and women with fecal incontinence (16). Whether a sophisticated analysis of the HDM pressure profile, in addition to pressures alone, is helpful for identifying external sphincter or puborectalis injury is unknown.
Simulated Evacuation
During simulated evacuation, patients are asked to expel the manometry catheter, typically with an empty, and less frequently with an inflated, balloon. Traditional criteria to evaluate these maneuvers include an inadequate increase in rectal pressure (eg, <40 mm Hg), which reflects a poor propulsive force; impaired anal relaxation (≤20% of baseline pressure); or both. However, even asymptomatic healthy people—approximately 20% undergoing non-HRM and 80% undergoing HRM—have manometric abnormalities that have been used to diagnose defecatory disorders (DDs) (17).
The assessment of pressure changes during simulated evacuation is influenced by the type of recording catheter (18), the distension of the intrarectal balloon (19), the body position (19), the displacement of the catheter (18), and the nature of voluntary participation, perhaps because some people find it embarrassing to defecate in the laboratory (20).
Cough Reflex
Increased intra-abdominal pressure (eg, during coughing) is associated with contraction of the external anal sphincter. This reflex assesses the integrity of the sacral reflex arc. The reflex is preserved in patients with spinal lesions above the sacral level but is absent in patients with spinal sacral lesions (cauda equina) or pudendal neuropathy (7).
Rectoanal Inhibitory Reflex
Rapid rectal distension elicits an intrinsic reflex, mediated by the myenteric plexus, that relaxes the internal anal sphincter. Studies with non-HRM suggest that the reflex may be absent in Hirschsprung disease (21), in adults with acquired myenteric neuropathies (22,23), and for other technical reasons. The technical category includes markedly decreased anal resting pressure, external sphincter contraction that masks the relaxation of the internal anal sphincter, megarectum, or lower rectal resection (7).
Rectal Compliance and Sensation
The threshold volumes at which patients perceive the first sensation, the desire to defecate, the urge to defecate, and the perception of discomfort or pain can be measured during rectal distension. The rectal balloons supplied with some HRM catheters are relatively stiff. These balloons can be cleaned and reused, but the balloon stiffness varies over time (personal observation, A.E.B.). Hence, rectal compliance and pressure thresholds for rectal sensation cannot be reliably measured with anorectal manometry. These variables are more accurately measured with a barostat or manual distention of an infinitely compliant balloon (18,24).
Utility of Anorectal Manometry in Clinical Practice
In 1999, the AGA Medical Position Statement on Anorectal Manometry concluded that: 1) although ARM can reproducibly measure sphincter pressures, age- and sex-specific normal ranges must be established in each laboratory; 2) in fecal incontinence, ARM is indicated “to define functional weakness” and “to perform and predict response to biofeedback training”; 3) there are no controlled clinical trials validating the usefulness of ARM in the diagnosis and treatment of constipation, but ARM might be useful “to support findings of other tests and to perform, monitor outcome, and possibly predict response to biofeedback training”; and 4) ARM is indicated to diagnose Hirschsprung disease (25). These guidelines provide a foundation for reevaluating the clinical utility of anorectal manometry in the era of HRM.
Fecal Incontinence
Anal resting and squeeze pressures measured with non-HRM and HDM are lower in incontinent patients than in healthy persons (4,16,24). Endoanal imaging, electromyography, and translumbar or transsacral magnetic stimulation are useful for determining the contribution of nerve and sphincter/pelvic floor injury to reduced squeeze pressure (1,24,25,26).
Normal anal pressures underscore the importance of other factors (eg, abnormal rectal sensation, diarrhea) to fecal incontinence. Rectal sensation may be reduced or increased in fecal incontinence (4,24,27,28). Pelvic floor retraining may improve these sensory disturbances and restore fecal continence (29).
Chronic Constipation
Because pelvic floor biofeedback therapy is superior to laxatives for DDs, the AGA Medical Position Statement on Chronic Constipation (30) recommends anorectal testing in constipated patients in whom a trial of laxatives and/or fiber supplementation has failed. A considerable proportion of patients with chronic constipation and irritable bowel syndrome with constipation have symptoms suggestive of DD. In addition to symptoms, the Rome IV criteria require features of impaired evacuation on 2 of 3 tests (ie, anorectal manometry, the rectal balloon expulsion test, and barium or magnetic resonance defecography) to diagnose a DD (31).
Traditional manometry criteria for DD include impaired anal relaxation, failure to increase rectal pressure, and a negative rectoanal gradient (ie, rectal pressure lower than anal pressure) during simulated evacuation. Confirming previous studies with HRM, Mion et al (4) observed that many asymptomatic healthy people have a negative rectoanal gradient during evacuation (11,17), perhaps because the test is generally conducted in the left lateral position. Unlike normal defecation, the urge to defecate induced by rectal distention is not preceded by a normal predefecatory motor pattern associated with anal relaxation (32). Patients may not understand the instructions provided during the test (33) or may not be keen to accomplish the task (20).
Despite these limitations, anal manometry is useful for diagnosing DD in some patients. Two subtypes of dyssynergia (ie, II and IV), which are characterized by inadequate rectal propulsion accompanied by a paradoxical simultaneous increase in anal pressure, or by a failure of the sphincter to relax, were significantly more frequent in DD than in healthy persons (17). However, the test characteristics (ie, a positive likelihood ratio of 1.8–2.3 and specificity of 72%–80%) suggest that manometry is of limited diagnostic utility. HRM patterns that suggest obstructed defecation or a large rectocele may be useful for selecting patients who require defecography (34,35). Indeed, the HRM pressure profile during simulated evacuation identified rectal prolapse with an accuracy of 96% and also identified 2 unique phenotypes in patients with rectal prolapse (35). Although an abnormal rectal balloon expulsion test predicts a successful response to biofeedback therapy, the utility of manometry phenotypes for predicting the response to biofeedback therapy in DD is unknown (36–38). A recent study using water-perfused manometry observed that patients with high rectal pressures and normal rectal balloon expulsion also benefited from biofeedback therapy (39).
Hirschsprung Disease
In infants and children, an absent rectoanal inhibitory reflex is 91% sensitive and 94% specific for diagnosing Hirschsprung disease (21). These figures are slightly but not significantly lower than rectal suction biopsy. When the reflex is present, it excludes the diagnosis of Hirschsprung disease.
In adults, undiagnosed Hirschsprung disease is rare (7). Studies with non-HRM suggest that the rectoanal inhibitory reflex may be absent in patients with megacolon and rectum of normal caliber who have chronic intestinal pseudo-obstruction (22,23). In patients with megarectum, the rectoanal inhibitory reflex may be absent because the rectal balloon does not adequately distend the rectum.
Limitations of HRM
Although HRM catheters provide better spatial resolution than non-HRM catheters, they have several limitations. The catheter diameter and spacing of sensors along the longitudinal axis varies among catheters. In some catheters (eg, ManoScan [Given Imaging Ltd] and UniTip [Unisensor]), there are no sensors across an approximately 4-cm distance between the lower panel of 10 sensors—which are generally situated in the anal canal and occasionally extend into the lower rectum—and the rectal balloon sensors. Hence, pressures are not measured in the lower rectum. Unlike non-HRM anorectal probes that are generally 4- to 6-mm in diameter and are flexible, the HDM probe is rigid, does not conform to the anorectal angle, and has a diameter of 10.75 mm, which may be uncomfortable and induce artifact (13). HRM pressure sensors are less durable than water-perfused sensors. Although malfunctioning sensors can be recognized on the tracing, inexperienced observers may overlook the same. “The pressures recorded with ManoScan HRM catheters vary over time (40,41). Although pressure drift has been attributed to the difference between the temperature at which the catheter is calibrated (ie, room temperature) and the body temperature, in vitro experiments in a water bath at constant temperature and an in vivo study with esophageal HRM observed that the drift increased linearly over time (40,41). Moreover, [pressure drift] is not remedied by the software algorithm (“thermal compensation”) designed to correct this when the catheter is removed from the body at the end of the study” (42). This suggests the drift is not solely explained by temperature differences. In approximately 15% of studies, the partial correction of pressure drift could potentially have altered the clinical interpretation of rectoanal pressures at rest and during squeeze and evacuation (42). This pressure drift is not related to wear and tear; to the contrary, it is greatest for new catheters and decreases over time. It is higher for sensors that are exposed to greater pressures (eg, in the anus rather than the rectum). Although there are more normal data for HRM and HDM than for non-HRM catheters, additional data in healthy people will provide a more precise estimate of normal values.
Effect of HRM on Publications
Through a literature search, we compared the effect of HRM on publications dealing with anorectal and esophageal manometry. Using the Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE databases, we searched annually, from 1975 to 2016, for articles pertaining to esophageal and anorectal manometry, separately, with the terms “manometr*” and descriptors for esophageal or, separately, anorectal diseases. Case reports and reviews were excluded.
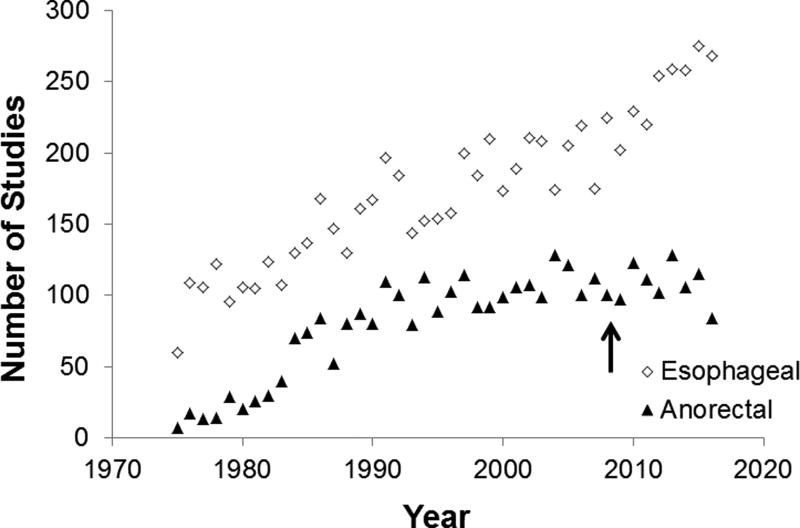
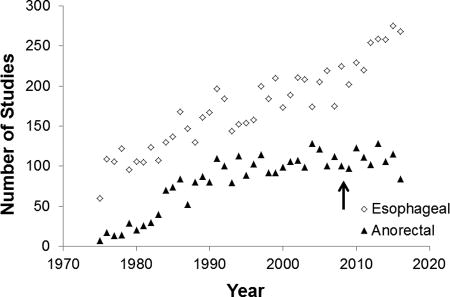
Between 1975 and 2016, the number of publications related to anorectal and esophageal manometry increased, respectively, from 7 to 115 and from 60 to 275 per year (Figure). Articles on esophageal motility have increased sharply since 2009, which was shortly after publication of the first paper on a widely available HRM technique (44). Findings from high-resolution techniques provided the impetus for the Chicago classification of esophageal motility disorders, which is now in its third iteration (44). In contrast, the number of publications on anorectal manometry has been stable between 1997 and 2016. Introduced in 2008, high-resolution techniques have not substantially influenced the number of publications on or management of anorectal disorders.
Figure.
Number of Publications Incorporating Esophageal and Anorectal Manometry Between 1975 and 2016. The arrow indicates the year of the first publication regarding high-resolution manometry.
Conclusions
HRM and HDM provide a refined assessment of the anorectal pressure profile, obviate the need for station pull-through maneuvers, and minimize movement artifacts. In selected cases, this refined assessment may be useful for identifying structural abnormalities or anal weakness. However, many manometry patterns that were once regarded as abnormal are also observed in many healthy persons, which substantially limits the utility of manometry for identifying DD. It is our impression that most conclusions of the AGA Medical Position Statement from 1999 remain valid today. HRM and HDM have had a lesser impact on the diagnosis and management of anorectal disorders than esophageal disorders.
The ongoing efforts of the international working group to standardize techniques are welcome. Although HRM is more than an expensive hobby, improvements in catheter design and further research to rigorously define and evaluate these techniques are necessary to determine if they are worth every penny.
Key Points.
High-resolution manometry (HRM) catheters provide better spatial resolution of anorectal pressures than standard (non-HRM catheters) and minimize movement artifacts during manometry.
However, with a few exceptions, HRM catheters have not increased the clinical utility of manometry to quantify anal weakness.
Further studies are necessary, in particular to improve the currently limited utility of HRM for diagnosing defecatory disorders.
Acknowledgments
Funding: This study was supported by a grant from DK078924, and by Grant Number 1 UL1 RR024150-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Abbreviations
- AGA
American Gastroenterological Association
- ARM
anorectal manometry
- DD
defecatory disorder
- HDM
high-definition manometry
- HRM
high-resolution manometry
Footnotes
Author Contributions:
G.B. and A.E.B. wrote the paper and approved the final version of this manuscript.
Disclosures: No competing interests declared.
References
- 1.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006 Jul;18(7):507–19. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee TH, Bharucha AE. How to perform and interpret a high-resolution anorectal manometry test. J Neurogastroenterol Motil. 2016 Jan 31;22(1):46–59. doi: 10.5056/jnm15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington EV, Heinrich H, Knowles CH, Rao SS, Fox M, Scott SM International Anorectal Physiology Working Party Group. Methods of anorectal manometry vary widely in clinical practice: results from an international survey. Neurogastroenterol Motil. 2017 Jan 18;18:18. doi: 10.1111/nmo.13016. [DOI] [PubMed] [Google Scholar]
- 4.Mion F, Garros A, Brochard C, Vitton V, Ropert A, Bouvier M, et al. 3D high-definition anorectal manometry: values obtained in asymptomatic volunteers, fecal incontinence and chronic constipation: results of a prospective multicenter study (NOMAD) Neurogastroenterol Motil. 2017 Mar 02;02:02. doi: 10.1111/nmo.13049. [DOI] [PubMed] [Google Scholar]
- 5.Diamant NE, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999 Mar;116(3):735–60. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002 Oct;14(5):553–9. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 7.Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: review of collective experience. Am J Gastroenterol. 2002 Feb;97(2):232–40. doi: 10.1111/j.1572-0241.2002.05450.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007 Apr;102(4):850–5. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 9.Kang HR, Lee JE, Lee JS, Lee TH, Hong SJ, Kim JO, et al. Comparison of high-resolution anorectal manometry with water-perfused anorectal manometry. J Neurogastroenterol Motil. 2015 Jan 01;21(1):126–32. doi: 10.5056/jnm14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty S, Feuerhak KJ, Zinsmeister AR, Bharucha AE. Reproducibility of high-definition (3D) manometry and its agreement with high-resolution (2D) manometry in women with fecal incontinence. Neurogastroenterol Motil. 2017 Mar;29(3) doi: 10.1111/nmo.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noelting J, Ratuapli SK, Bharucha AE, Harvey DM, Ravi K, Zinsmeister AR. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012 Oct;107(10):1530–6. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HJ, Jung KW, Han S, Kim JW, Park SK, Yoon IJ, et al. Normal values for high-resolution anorectal manometry/topography in a healthy Korean population and the effects of gender and body mass index. Neurogastroenterol Motil. 2014 Apr;26(4):529–37. doi: 10.1111/nmo.12297. [DOI] [PubMed] [Google Scholar]
- 13.Coss-Adame E, Rao SS, Valestin J, Ali-Azamar A, Remes-Troche JM. Accuracy and reproducibility of high-definition anorectal manometry and pressure topography analyses in healthy subjects. Clin Gastroenterol Hepatol. 2015 Jun;13(6):1143–50. doi: 10.1016/j.cgh.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Yang X, Xu C, Zhang Y, Zhang X. Normal values and pressure morphology for three-dimensional high-resolution anorectal manometry of asymptomatic adults: a study in 110 subjects. Int J Colorectal Dis. 2013 Aug;28(8):1161–8. doi: 10.1007/s00384-013-1706-9. [DOI] [PubMed] [Google Scholar]
- 15.Raizada V, Bhargava V, Karsten A, Mittal RK. Functional morphology of anal sphincter complex unveiled by high definition anal manometery and three dimensional ultrasound imaging. Neurogastroenterol Motil. 2011 Nov;23(11):1013–9. doi: 10.1111/j.1365-2982.2011.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zifan A, Ledgerwood-Lee M, Mittal RK. A predictive model to identify patients with fecal incontinence based on high-definition anorectal manometry. Clin Gastroenterol Hepatol. 2016 Dec;14(12):1788–96. doi: 10.1016/j.cgh.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossi U, Carrington EV, Bharucha AE, Horrocks EJ, Scott SM, Knowles CH. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar;65(3):447–55. doi: 10.1136/gutjnl-2014-308835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauter M, Heinrich H, Fox M, Misselwitz B, Halama M, Schwizer W, et al. Toward more accurate measurements of anorectal motor and sensory function in routine clinical practice: validation of high-resolution anorectal manometry and Rapid Barostat Bag measurements of rectal function. Neurogastroenterol Motil. 2014 May;26(5):685–95. doi: 10.1111/nmo.12317. [DOI] [PubMed] [Google Scholar]
- 19.Rao SS, Kavlock R, Rao S. Influence of body position and stool characteristics on defecation in humans. Am J Gastroenterol. 2006 Dec;101(12):2790–6. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 20.Duthie GS, Bartolo DC. Anismus: the cause of constipation? Results of investigation and treatment. World J Surg. 1992 Sep-Oct;16(5):831–5. doi: 10.1007/BF02066978. [DOI] [PubMed] [Google Scholar]
- 21.de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006 May;42(5):496–505. doi: 10.1097/01.mpg.0000214164.90939.92. [DOI] [PubMed] [Google Scholar]
- 22.Faussone-Pellegrini MS, Fociani P, Buffa R, Basilisco G. Loss of interstitial cells and a fibromuscular layer on the luminal side of the colonic circular muscle presenting as megacolon in an adult patient. Gut. 1999 Nov;45(5):775–9. doi: 10.1136/gut.45.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basilisco G, Gebbia C, Peracchi M, Velio P, Conte D, Bresolin N, et al. Cerebellar degeneration and hearing loss in a patient with idiopathic myenteric ganglionitis. Eur J Gastroenterol Hepatol. 2005 Apr;17(4):449–52. doi: 10.1097/00042737-200504000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Bharucha AE, Fletcher JG, Harper CM, Hough D, Daube JR, Stevens C, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54(4):546–55. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharucha AE, Daube J, Litchy W, Traue J, Edge J, Enck P, et al. Anal sphincteric neurogenic injury in asymptomatic nulliparous women and fecal incontinence. Am J Physiol Gastrointest Liver Physiol. 2012 Jul 15;303(2):G256–62. doi: 10.1152/ajpgi.00099.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SS, Coss-Adame E, Tantiphlachiva K, Attaluri A, Remes-Troche J. Translumbar and transsacral magnetic neurostimulation for the assessment of neuropathy in fecal incontinence. Dis Colon Rectum. 2014 May;57(5):645–52. doi: 10.1097/DCR.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruana BJ, Wald A, Hinds JP, Eidelman BH. Anorectal sensory and motor function in neurogenic fecal incontinence: comparison between multiple sclerosis and diabetes mellitus. Gastroenterology. 1991 Feb;100(2):465–70. doi: 10.1016/0016-5085(91)90217-9. [DOI] [PubMed] [Google Scholar]
- 28.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ‘idiopathic’ faecal incontinence. Gut. 1992 Jun;33(6):807–13. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wald A, Tunuguntla AK. Anorectal sensorimotor dysfunction in fecal incontinence and diabetes mellitus: modification with biofeedback therapy. N Engl J Med. 1984 May 17;310(20):1282–7. doi: 10.1056/NEJM198405173102003. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha AE, Dorn SD, Lembo A, Pressman A American Gastroenterological Association. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211–7. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 31.Rao SS, Bharucha AE, Chiarioni G, Felt-Bersma R, Knowles C, Malcolm A, et al. Functional anorectal disorders. Gastroenterology. doi: 10.1053/j.gastro.2016.02.009. Epub 2016 Mar 25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinning PG, Bampton PA, Andre J, Kennedy ML, Lubowski DZ, King DW, et al. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. Gastroenterology. 2004 Jul;127(1):49–56. doi: 10.1053/j.gastro.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 33.Heinrich H, Fruehauf H, Sauter M, Steingotter A, Fried M, Schwizer W, et al. The effect of standard compared to enhanced instruction and verbal feedback on anorectal manometry measurements. Neurogastroenterol Motil. 2013 Mar;25(3):230–7. doi: 10.1111/nmo.12038. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich H, Sauter M, Fox M, Weishaupt D, Halama M, Misselwitz B, et al. Assessment of obstructive defecation by high-resolution anorectal manometry compared with magnetic resonance defecography. Clin Gastroenterol Hepatol. 2015 Jul;13(7):1310–7. doi: 10.1016/j.cgh.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Prichard DO, Lee T, Parthasarathy G, Fletcher JG, Zinsmeister AR, Bharucha AE. High-resolution anorectal manometry for identifying defecatory disorders and rectal structural abnormalities in women. Clin Gastroenterol Hepatol. 2017 Mar;15(3):412–20. doi: 10.1016/j.cgh.2016.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (anismus) Neurogastroenterol Motil. 2004 Oct;16(5):589–96. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 37.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129(1):86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Ratuapli SK, Bharucha AE, Noelting J, Harvey DM, Zinsmeister AR. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology. 2013 Feb;144(2):314–22. doi: 10.1053/j.gastro.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazor Y, Hansen R, Prott G, Kellow J, Malcolm A. The importance of a high rectal pressure on strain in constipated patients: implications for biofeedback therapy. Neurogastroenterol Motil. 2017 Mar;29(3):e12940. doi: 10.1111/nmo.12940. [DOI] [PubMed] [Google Scholar]
- 40.Robertson EV, Lee YY, Derakhshan MH, Wirz AA, Whiting JR, Seenan JP, et al. High-resolution esophageal manometry: addressing thermal drift of the manoscan system. Neurogastroenterol Motil. 2012 Jan;24(1):61–4. e11. doi: 10.1111/j.1365-2982.2011.01817.x. [DOI] [PubMed] [Google Scholar]
- 41.Babaei A, Lin EC, Szabo A, Massey BT. Determinants of pressure drift in manoscan esophageal high-resolution manometry system. Neurogastroenterol Motil. 2015 Feb;27(2):277–84. doi: 10.1111/nmo.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parthasarathy G, McMaster J, Feuerhak K, Zinsmeister AR, Bharucha AE. Determinants and clinical impact of pressure drift in manoscan anorectal high resolution manometry system. Neurogastroenterol Motil. 2016 Sep;28(9):1433–7. doi: 10.1111/nmo.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandolfino JE, Ghosh SK, Lodhia N, Kahrilas PJ. Utilizing intraluminal pressure gradients to predict esophageal clearance: a validation study. Am J Gastroenterol. 2008 Aug;103(8):1898–905. doi: 10.1111/j.1572-0241.2008.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, et al. International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015 Feb;27(2):160–74. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]