Abstract
Induction of pro-inflammatory T cell immunity is augmented by innate dendritic cell (DC) maturation commonly initiated by Toll-like receptor (TLR) signaling. We demonstrate that ligation of TLR3, 4, and 9 induces murine DC production of complement components and local production of the anaphylatoxin C5a. In vitro, ex vivo, and in vivo analyses show that TLR-induced DC maturation as assessed by surface phenotype, expression profiling by gene array, and functional ability to stimulate T cell responses, requires autocrine C3a- and C5a-receptor (C3ar1/C5ar1) signaling. Studies employing bone marrow chimeric animals and Foxp3-GFP/ERT2-Cre/dTomato fate mapping mice show that TLR-initiated, DC autocrine C3ar1/C5ar1 signaling causes expansion of effector T cells and instability of regulatory T cells and contributes T cell-dependent transplant rejection. Together, our data position immune cell-derived complement production and autocrine/paracrine C3ar1/C5ar1 signaling as crucial intermediary processes that link TLR-stimulation to DC maturation and the subsequent development of effector T cell responses.
Keywords: complement TLR, complement, transplantation, alloimmunity, inflammation
Introduction
Toll-like receptors (TLRs) initiate innate immune activation which amplifies T and B cell immune responses. Surface-expressed TLRs (1, 2, 4–6, 11) and intracellular TLRs (3, 7, 8, 9) recognize conserved molecular patterns derived from pathogens (pathogen associated molecular patterns or PAMPs) (1–4) and potentially, some damaged/necrotic cells, e. g. HMGB1 which signals through TLR4 (5). TLR-initiated signaling is transmitted through linker proteins that include “Myeloid Differentiation primary response gene 88” (MyD88) and “TIR-domain-containing adapter-inducing interferon-β” (TRIF, TICAM1) to activate gene expression profiles driven by NF-κB, AP-1, and members of the interferon regulatory factor (IRF) family (4, 6–8) to induce DC maturation (4, 9). Important among processes associated with DC maturation are upregulation of MHC class II expression, induction of CD80, CD86 and CD40, and production of “innate” cytokines (e.g. type 1 interferon, IL-1, IL-6, IL-12, TNFα) (2, 6–8, 10, 11). The TLR activation educates DCs to initiate effector T cell (Teff) differentiation (e.g.Th1, Th2, Th17) and expansion, while limiting Foxp3+ T regulatory cell (Treg) generation, function and stability (12, 13) together providing protective, pathogen-reactive T cell immunity (4, 14).
Non-physiological TLR activation can overcome normal immunological homeostasis and result in pathological immune injury. For example HMGB1/TLR4 activation is a crucial mediator of ischemia reperfusion injury (15, 16) and experimental CpG/TLR9 ligation can promote Th1 and Th17 cell activation in rodent models of autoimmunity (17–20). In both solid organ and hematopoietic cell transplant systems, TLR9-initiated signals accelerate rejection or graft-vs.-host-disease (GVHD) (13, 21–25).
The complement system has been conventionally connected with innate immunity. In previous work (26–28), we found that early during interaction of murine DCs with cognate T cells both partners locally generate C3a and C5a which establish autocrine C3ar1/C5ar1 signaling loops with DC- and T cell-expressed receptors (C3ar1 and C5ar1). This process occurs in concert with downregulation of the cell surface C3/C5 convertase regulator decay accelerating factor (DAF or CD55). The resultant C3ar1/C5ar1 signaling in the DCs and cognate T cell partners plays an integral role in DC-induced, effector T cell (Teff) activation, including during GVHD (27–36).
The observations that C3ar1/C5ar1 signaling and TLR-signals each cause changes in DCs that promote their ability to induce Teff responses prompted the hypothesis that the two processes are linked, i.e. that TLR-enhancing effects on Teff immunity are dependent on C3ar1/C5ar1 signal transduction in DCs. We employ analyses of DCs interacting T cells along with murine models of alloimmunity to demonstrate that C3ar1/C5ar1 signaling in DCs is a requisite process connecting TLR-stimulation with both DC maturation and Teff activation at threshold PAMP concentrations that model physiological TLR signaling.
Materials and Methods
Mice
C57BL/6(B6), B6 CD45.1, B6 C3−/−, OTII, B6 Myd88−/−, B6 TRIF−/−, BALB/c were originally purchased from The Jackson Laboratory and maintained at Mount Sinai or Case Western. Factor D−/− (fD−/−) mice were a kind gift from Y. Xu (UAB, Birmingham AL). B10.D2 Hc0 (C5-deficient, C5def, Jackson) and BALB/c C3−/− (backcrossed >12 generations from B6 C3−/−) were crossed together to produce C3−/−C5def. B6 C3ar1−/−C5ar1−/−, C3ar1−/−C5ar1−/− Foxp3-GFP were produced as previously described (28, 37–39). Rosa(dTomato)xFoxp3CreERT2-GFP mice (40) were obtained from A. Rudensky (Sloan-Kettering Institute, New York, NY) and they were crossed with B6 C3ar1−/−C5ar1−/− mice to produce C3ar1−/−C5ar1−/−(dTomato)xFoxp3CreERT2-GFP mice. TEa mice(41) were a gift of J Bromberg (U Maryland). All mice were housed in the Icahn School of Medicine at Mount Sinai Center for Comparative Medicine or Case Western Reserve University in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments were performed using animals that were littermates, or were maintained in the same room and/or were co-housed within the same cages to limit potential effects of microbiome differences.
Antibodies and Reagents
Antibodies against CD4, CD8, CD11c and fixable viability dye (eBioscience), CD55 (BD Biosciences), CD88 (AbD serotec), CD80, CD86, MHC Class II (Miltenyi Biotec) were used for flow cytometry. CFSE was obtained from eBioscience. MR1 (anti mouse CD40L) was purchased from BioXCell (West Lebanon, NH). Peptides were synthesized by Genscript (Piscataway NJ).
Cell isolations
Spleen was passed through a 40μm strainer (BD Falcon) and lysed with red blood cell lysis buffer (Life Technologies, CA). T cell depletion from bone marrow suspensions and isolation of murine splenic naïve CD4+ T cells, total T cells/APCs were accomplished using magnetic beads and the AutoMACS Pro machine (Miltenyi Biotec, Auburn, CA). For splenic DC isolation, single cell suspensions of spleen cells were treated with Collagenase D (Roche) for 30 minutes at 37°C and incubated with CD11c microbeads kits (Miltenyi Biotec) following the manufacturer’s protocol. The purity of DCs after isolation was 85–95%. Isolated DCs were stimulated with CpG ODN 1826 or LPS (both from InvivoGen) for 18 hours in 96 wells if needed.
Mixed lymphocyte responses
Splenic DCs from untreated mice were stimulated in vitro with TLR ligands overnight, washed with PBS x3 and then co-cultured with naïve, allogeneic CD4+ T cells or unfractionated T cells labeled with CFSE for mixed lymphocyte reaction. Analogous studies were performed using splenic DCs isolated 4h after i.v. injections of CpG. On day 4, CFSE dilution was assessed for cellular proliferation and live cell numbers were counted in the well using flow cytometry. Cells were incubated in complete medium (RPMI + 10% FCS + L-Glutamine + sodium pyruvate + nonessential amino acids + Pen/Strep + β-mercaptoethanol) at 37°C. Splenic DCs were phenotyped for surface markers by flow cytometry. In some experiments cells were harvested at 18 h for analysis of cytokine gene expression by qPCR.
ELISPOT assays
Cytokine ELISPOT assays were performed using spleen cells co cultured with BALB/c APCs on IFNγ capture plates for 24hrs then analyzed as previously described (42).
Flow cytometry
Data were collected on a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (Tree Star, OR) or Cytobank (Cytobank Inc., CA). To measure recall immune responses posttransplant, spleen cells from heart transplant recipients were stimulated with donor cells overnight and then analyzed for intracellular IFNγ within the CD4 or CD8 gate by flow cytometry (32).
Heart transplant
Heterotopic heart transplants were performed as previously described by our lab (32, 43–45). For graft survival experiments, recipients were treated with anti-CD40L (anti-CD154) mAb MR1 (1mg on day 0 and 500μg on day7&14 i.p.) ±CpG ODN 1826 (100μg on day 1 and 50μg on day 3&5 i.p.). Heart graft function was monitored every other day by palpation and rejection was defined as the day on which a palpable heartbeat was no longer detectable and was confirmed by histology.
Real-time PCR
RNA isolation was performed using Trizol (Thermofisher) and cDNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) as per the manufacturer’s instructions. RT-PCR (TaqMan probes; Applied Biosystems) was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories). All the mouse PCR primers were purchased from Life Technologies. PCR products were normalized to the control gene (GAPDH) and expressed as fold increase compared with unstimulated cells using the ΔΔCt method.
C5a ELISA
Splenic APCs were cultured in serum-free HL-1 medium with either allogeneic or syngeneic splenic T cells with or without CpG (10μg) in 48 well plates for 48 hours. Culture supernatant fluids were concentrated with the use of Amicon Ultra-0.5, normal molecular weight limit of 10kDa (Millipore), and tested for C5a with Mouse Complement Component C5a Duo Set ELISA (R&D systems, Minneapolis, MN) as per manufacturer’s instructions.
BM Chimeras
6 to 8 week-old male B6 or BALB/c mice were fasted for 24 hours prior to irradiation. On day 0, recipients were irradiated with 650 rad twice with at least a 3 hour interval between treatments. Once irradiated, mice received adoptive transfer of T cell-depleted BM cells isolated from the various donors. 8–10 week later % chimerism was assessed by flow cytometry.
Tamoxifen treatment and Treg Fate mapping
Tamoxifen (Sigma-Aldrich) was dissolved in olive oil (Fluka) to a final concentration of 20mg/ml by shaking overnight at 37°C in a light blocking vessel. The dose of tamoxifen was determined by weight, approximately 75mg/kg body weight of a mouse.
Microarrays and analysis
We isolated splenic CD11c+DCs (using Miltenyi magnetic beads) from WT or C3ar1−/−C5ar1−/− mice 4hr after injection with CpG 100ug or vehicle control. The cells were immediately placed in in Trizol and sent to SUNY Albany Center for Functional Genomics. Total RNA was isolated by standard techniques (>150pg RNA obtained per sample). After quality control testing, the samples were processed using standard Affymetrix WT pico protocols, hybridized to Affymetrix Mouse Gene 2.0 ST arrays and the chips were scanned using GeneChip Scanner 7G (Affymetrix Inc.). The intensity data at the probeset level were extracted and normalized with the RMA algorithm (46) and data quality was assessed in Affymetrix Expression Console (Affymetrix Inc.). The Affymetrix control probesets or the probesets with low intensity across all samples were excluded from downstream analysis. The LIMMA test (47) was performed on normalized data between comparison groups and the differentially expressed genes (DEG) with p <0.05 were identified and visualized with heatmap. Gene Ontology enrichment analysis using Fisher-exact test was further performed on DEGs to investigate biological functions or pathways associated with DEGs. The data discussed in this publication have been deposited in NCBI’s Gene Exprssion Omnibus and are accessible through GEO Series accession number GSE98315 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE98315).
Statistics
Statistical significance was determined by Student’s T test (unpaired, two-tailed), two-way ANOVA (with Bonferroni post-tests to compare replicate means) or by log-rank (Mantel-Cox) test performed in GraphPad Prism 5 or Prism 6 with a significance threshold values of p<0.05. All experiments were repeated at least twice. Data are presented as mean values with SD. Error bars indicate mean± SEM and ns indicates p>0.05, not significant.
Results
TLR signaling requires endogenous immune cell complement production
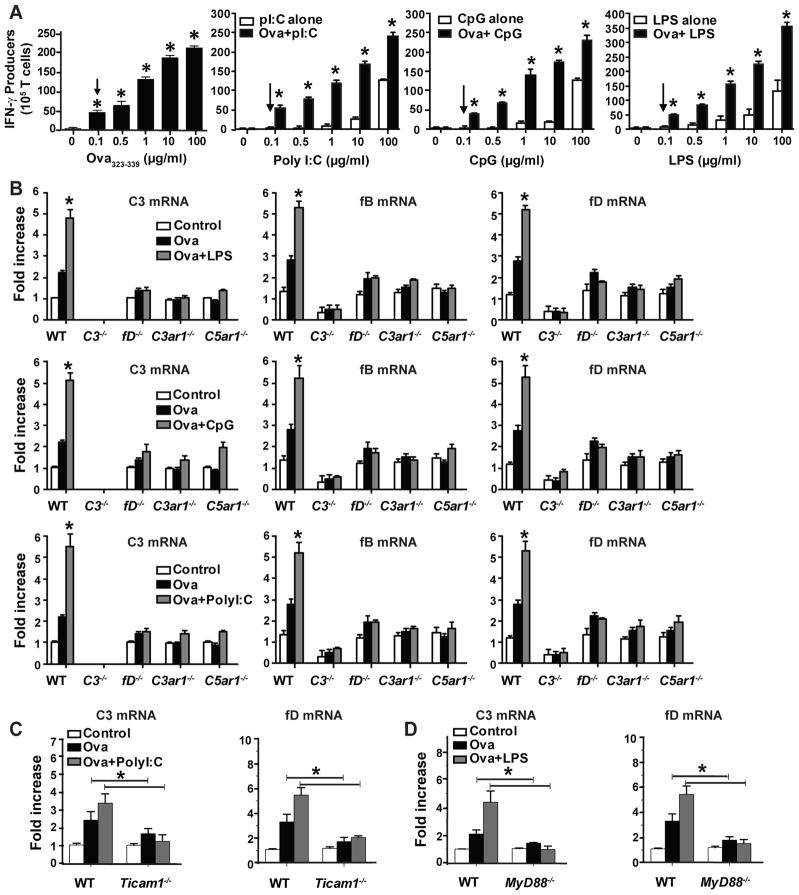
We first studied the effects of DC-produced complement on TLR3, 4 and 9 induction of IFNγ production by ova323–339+I-Ab-specific CD4+ OT-II T cells. To exclude effects of systemic complement on signaling through each of the TLRs, we performed in vitro studies using DCs as antigen presenting cells (APCs) in the absence of serum complement. We titrated ova323–339 and TLR ligand concentrations to establish threshold conditions for detection of IFNγ by the responding cells (Fig 1A). One tenth μg/ml of ova gave ~25% of the maximal IFNγ response, and 0.1 μg/ml of poly I:C, CpG, and LPS, did not generate IFNγ-producing cells in the absence of ova323-339 (Fig 1A). In the presence of antigen, the number of IFNγ-producing OT-II cells increased in a dose-dependent manner with addition of increasing the amounts of each PAMP (Fig 1A), such that the effect of TLR signaling on endogenous complement production and vice versa could be interrogated.
Figure 1.
TLR-stimulation induces complement gene upregulation via autocrine C3ar1/C5ar1 signaling in DCs. A. Purified OTII CD4+ cells (1x106) mixed with splenic CD11c+ DCs (2.5x105) were tested in IFNγ ELISPOT assays upon stimulation with varying concentrations of ova323-339 (left panel, arrow indicates lowest concentration of antigen to initiate a response) or various PAMPs with (black) without (white) antigen (right 3 panels, arrows indicate sub-threshold levels of each PAMP that did not induce IFNγ production). *p<0.05 compared to baseline, bars represent means and s.d. of triplicates. Each experiment was repeated at least once. B. Relative C3, fB and fD gene expression (RT-PCR) of cultures containing OT-II cells plus splenic APCs from WT or various complement component deficient animals as indicated ± 0.1 μg/ml ova323-339 ± 0.1 μg/ml pI:C or 0.1 μg/ml LPS or 0.1 μg/ml CpG for 1 h. Each bar shows mean and s.d. of triplicate values and is representative of at least 2 individual experiments. *p<0.05 compared to unstimulated baseline. C–D. Relative expression (qRT-PCR) of C3 (left) and fD (right) in RNA obtained from OTII cells mixed with DCs from WT, Ticam1−/− (C) or MyD88−/− (D) mice as indicated ± 0.1 μg/ml ova323-339 ± 0.1 μg/ml Poly I:C or 0.1 μg/ml LPS. *p<0.05. Each bar shows mean + s.d. of triplicate values and is representative of 3 individual experiments.
Using the above conditions ± threshold amounts of each PAMP, we determined effects of TLR stimulation on complement component gene expression by cells within the culture [noting DCs produce ~1000-fold more complement than T cells (28)]. The inclusion of each PAMP increased C3, factor B (fB) and factor D (fD) mRNA expression levels from OT-II cultures (Fig 1B) over levels induced by ova323-339 without a PAMP. Substitution of C3−/−, fD−/− C3ar1−/−, or C5ar1−/− DCs for WT DCs abolished the TLR-induced upregulations (Fig 1B), connecting autocrine C3ar1/C5ar1 signaling with TLR-signaling. Studies with DCs from MyD88−/− or Ticam1−/− (Trif−/−) mice showed that poly I:C-induced C3/fD (Fig 1C) mRNA upregulation is mediated via Ticam1, that LPS-induced DC C3/fD (Fig 1C) gene upregulation is mediated via MyD88, and CpG-induced C3 expression required MyD88 (not shown), together confirming that the PAMP/TLR-induced effects are transmitted through established canonical signaling pathways for each of the TLRs (48).
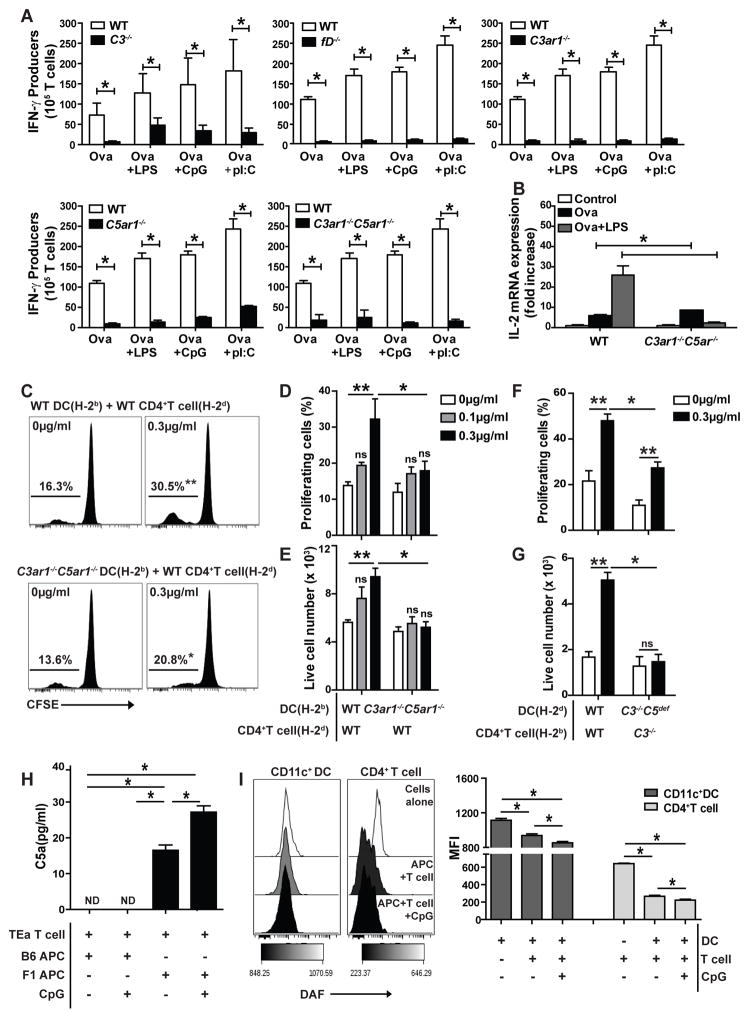
To test whether the TLR-induced increases in T cell IFNγ production and autocrine C3ar1/C5ar1 signaling in DCs are linked, we cultured OT-II cells with the defined threshold concentrations of antigen and PAMPs, using WT DCs or DCs from C3−/−, fD−/−, C3ar1−/−, C5ar1−/− or C3ar1−/−C5ar1−/− mice (Fig 2A). These assays showed that DC absence of each reduced OT-II IFNγ production by >80%. The same pattern was observed for LPS-stimulated, OT-II cell IL-2 RNA production (Fig 2B).
Figure 2.
TLR-induced enhancement of in vitro monoclonal and polyclonal T cell responses requires C3/C5 and autocrine C3ar1/C5ar1 signaling in immune cells. A. Frequencies of IFNγ-producing OTII cells (ELISPOT) when cultured with DCs from WT, C3−/−, fD−/−, C5ar1−/−, C3ar1−/− or C3ar1−/−C5ar1−/− mice as indicated plus ova323-339 (0.1μg/ml) ± LPS (0.1μg/ml), CpG (0.5 μg/ml) or poly I:C (0.5 μg/ml). B. IL-2 gene expression (qRT-PCR) from cultures of OTII cells mixed with ova323-339 (0.1μg/ml) plus WT or C3ar1−/−C5ar1−/− DCs for 1 h. C–E. WT or C3ar1−/−C5ar1−/− splenic H-2b DCs were pre-stimulated ±CpG overnight as indicated and co-cultured with CFSE-labeled allogeneic naïve WT H-2d CD4+T cells for 4 days. Representative CFSE dilution plots (C), quantified CFSE-dilution (D) and live T cell numbers at the end of the culture period (E) are shown. F–G. WT or C3−/−C5def splenic H-2d DCs were prestimulated ±CpG overnight as indicated and co-cultured with CFSE-labeled allogeneic WT or C3−/− total CD4+ for 4 days. Quantified CFSE-dilution (F) and live T cell numbers on d4 (G) are shown. **p<0.05 compared to unstimulated controls, *p<0.05 compared to stimulated WT counterparts (2 way ANOVA or unpaired t test). ns indicates p>0.05, not significant. H. Concentrations of C5a detected in 48 h culture supernatants of TEa CD4+ T cells mixed with allogeneic (bxd) F1 APCs or syngeneic B6 APCs ±CpG (10μg/ml) as indicated. Assays were performed in serum free media. I. DAF expression on bxd F1 CD11c+ DC and TEa CD4+T cells in the absence or presence of CpG (10μg/ml) as indicated, 48 h after initiating cultures. Representative flow plots (left) and quantification (right). Each experiment was repeated at least two-three times with similar results. Error bars indicate mean± SEM. *p<0.05 (unpaired t test). ND indicates not detected.
To verify that the link between TLR-induced T cell responses and DC-derived complement pertains in a polyclonal system, we performed studies with B6 DCs and allogeneic BALB/c T cells, employing T cell proliferation (CFSE dilution) and expansion (live cell number) as readouts (Fig 2C–E). We determined that pre-treatment with 0.3 μg/ml CpG was the minimum threshold dose required for WT DCs to augment alloreactive T cell proliferation and expansion (not shown) in mixed lymphocyte responses (MLRs). Unstimulated allogeneic DCs induced proliferation of allogeneic T cells: 13–16% of the T cells in the culture underwent >4 cell divisions on day 4, consistent with previous observations (27, 29, 35, 37). Whereas CpG pre-treatment of WT DCs augmented alloreactive T cell proliferation and expansion (Fig 2C–E), CpG pre-treatment of C3ar1−/−C5ar1−/− DCs had no effect. Effects paralleling those with C3ar1−/−C5ar1−/− DCs occurred using C3/C5-deficient DCs (Fig 2F–G). In the case of WT DCs, CpG induced stronger allogeneic T cell proliferation/expansion of purified CD4+ T cells (note reversal of responder/stimulator strains). For BALB/c H-2d C3−/−C5def DCs pretreated with CpG and cultured with purified C3−/− T cells (removing all C3 and DC-derived C5), proliferation was severely blunted and T cell expansion was fully abrogated (Fig 2F–G). The same was true for purified, CpG-induced enhancements of CD8+ T cell responses and for LPS-induced enhancements of T cell proliferation/expansion (not shown). Thus, for both monoclonal T cells and polyclonal T cells responding to DCs in 2 different genetic backgrounds, TLR augmentation of T cell immunity in vitro was dependent on DC complement synthesis and C3ar1/C5ar1 signals.
To establish that the above interpretation applies in a disease relevant context, we used a monoclonal TCR transgenic system germane to alloimmune responses, i.e. TEa CD4+ cells (41) (reactive to I-Ab + I-Edα52-68), that have been employed in transplant models. We incubated TEa T cells with allogeneic (bxd)F1 APCs (that express I-Ab + I-Edα52-68) or syngeneic APCs as controls ± added CpG. (Fig 2H). C5a was detectable in 48 h supernatants of cultures containing allogeneic (bxd)F1 APCs (which undergo cognate interactions with the TEa cells). The amounts of C5a increased in cultures containing added CpG. In contrast, no C5a was detected in cultures containing control B6 APCs ± CpG. Flow cytometric analyses showed that CpG caused ~30–50% downregulation of DAF on both (bxd)F1 APCs and TEa CD4+ compared to cells incubated with media alone (Fig 2I). Together with previous findings using polyclonal T cells (26), these data show that TLR-induced, immune cell C5a production is connected to repression of DAF expression and that the process applies in general.
Immune cell C3ar1/C5ar1 signaling mediates TLR9-induced DC maturation in vivo
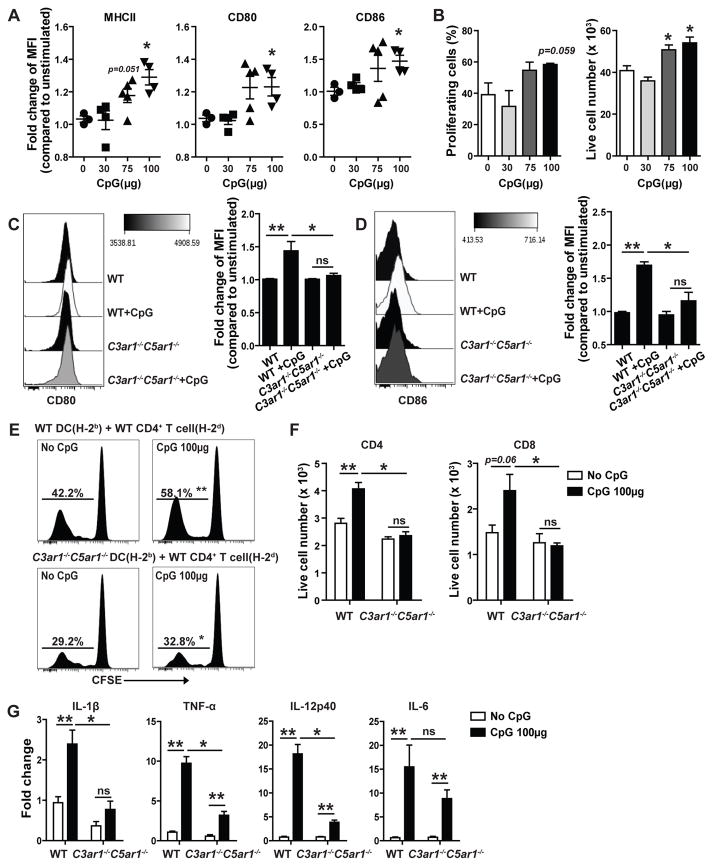
We next tested whether the interposition of C3ar1/C5ar1 signaling between TLR signals and DC maturation applies in vivo. We used TLR9 ligation because its ligation by CpG has been extensively studied in GVHD and solid organ transplant systems (13, 22–25). TLR9 stimulation also augments Teff induction following immunization (49) as well as heightens pathology in several autoimmune models (18, 19). To define threshold concentrations of CpG required to induce DC maturation markers and allogeneic T cell stimulatory activity in vivo, we intravenously injected varying amounts of CpG (0–100μg) into WT mice. We isolated splenic CD11c+ DCs 4h later and analyzied DC surface phenotypes and tested DC allostimulatory function by co-culturing the DCs with allogeneic, CFSE-labeled T cells (Fig 3A–B). These assays demonstrated that 100μg CpG was the minimum concentration that consistently upregulated CD80, CD86 and class II MHC on DC surfaces and enhanced proliferation and expansion of co-cultured allogeneic T cells. We next injected groups of B6 WT and C3ar1−/−C5ar1−/− mice with 100μg CpG, isolated splenic CD11c+ DCs 4 h later, and compared DC surface phenotypes and function ex vivo between the 2 groups (Fig 3C–G). These experiments showed that the absence of C3ar1/C5ar1 prevented CpG-induced upregulation of CD80/CD86 (Fig 3C–D) and class II MHC (WT:40%±2.8% increase vs. no CpG; C3ar1−/−C5ar1−/−:21%±5.0%, p<0.05 vs WT, data not shown), and abrogated CpG-induced, augmentation of allogeneic T cell proliferation and expansion (Fig 3E–F). DC CD40 expression levels were not altered by the CpG administration in either WT or in C3ar1−/−C5ar1−/− DC (not shown). For WT DCs, the CpG administration augmented mRNA expression of IL-1β, TNFα, IL-12p40, and IL-6. In contrast, DCs from C3ar1/C5ar1 mice showed diminished (TNFα, IL-12p40, and IL-6) and fully restrained upregulation of IL-1β (Fig 3G).
Figure 3.
TLR9-induced DC maturation in vivo requires autocrine immune cell C3ar1/C5ar1 expression. A–B. In vivo CpG titration. A. Quantification of normalized changes in DC expression (flow cytometry, MFI) of MHCII, CD80 and CD86 on splenic DCs isolated 4 h after injection with various doses of CpG or vehicle control from H-2b WT mice. B. Quantification of 96 h CFSE-dilution (left) and live cell numbers (right) of CFSE-labeled WT H-2d T cells mixed with WT H-2b DCs isolated 4 h after injection with various doses of CpG or control, gated on CD4+T cells. Pooled data from two independently done experiments with similar results (Each time 2–3 animals/group). Error bars indicate mean± SEM. *p<0.05 compared to unstimulated controls (unpaired t test), NS indicates p>0.05, not significant. C–D. Representative flow plots and quantification of normalized changes in DC expression (flow cytometry, MFI) of CD80 (C) and CD86 (D) on splenic DCs isolated 4 h after injection with 100μg CpG or vehicle control from H-2b WT vs C3ar1−/−C5ar1−/− mice. E–F. Representative 96 h CFSE-dilution plots (E) and quantified live cell numbers at the end of the cultures (F) of CFSE-labeled WT H-2d T cells mixed with WT or C3ar1−/−C5ar1−/− H-2b DCs isolated 4 h after injection with CpG or control, gated on CD4+ or CD8+ T cells. G. Relative gene expression (qPCR) of Tnf-a, Il-1b, Il-12p40 and Il-6 in WT or C3ar1−/−C5ar1−/− splenic DCs isolated 4 h after injection with CpG or vehicle control. Each experiment was repeated at least three times with similar results (Each time 2–3 animals/group). Error bars indicate mean± SEM. **p<0.05 compared to unstimulated controls (unpaired t test), *p<0.05 (two-way ANOVA) compared to stimulated WT counterparts. ns indicates p>0.05, not significant.
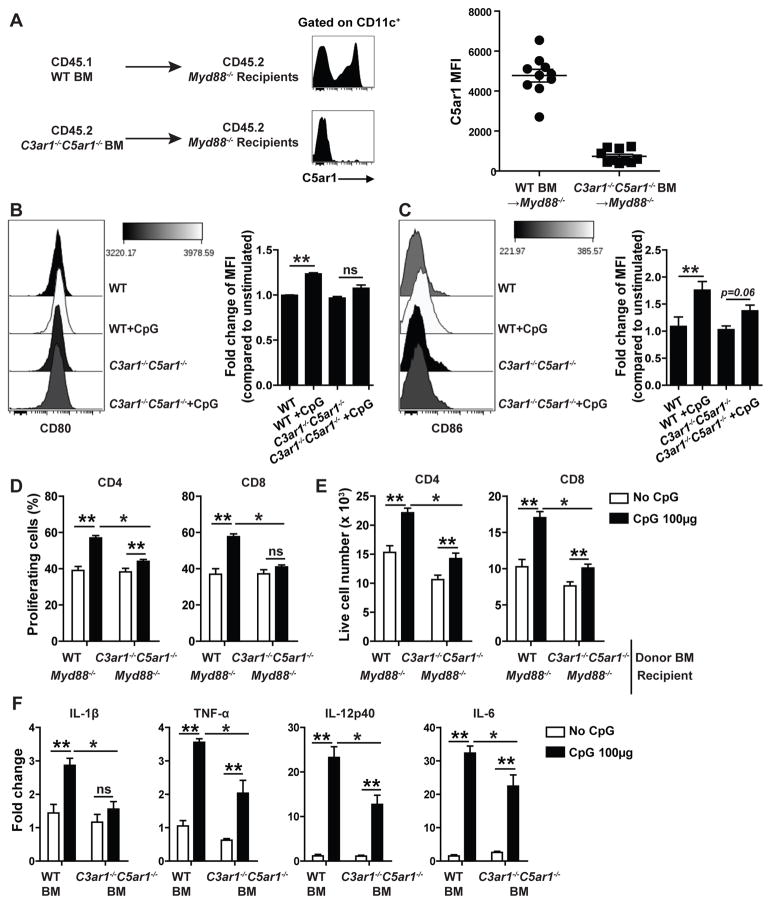
We next performed two sets of studies to exclude any contribution C3ar1/C5ar1 or MyD88 signaling in non-BM-cells in the effects. We transplanted BM from B6 WT or C3ar1−/−C5ar1−/− mice into congenic, lethally irradiated, B6 MyD88−/− recipients. As CpG/TLR9 signaling is MyD88-dependent (3, 19), in these chimeras only the transplanted BM-derived cells are capable of responding to CpG through MyD88. Nine weeks later (after confirming chimerism, Fig 4A), we injected groups of the chimeras with 100μg of CpG and analyzed DC phenotypes and function (Fig 4B–F). These assays demonstrated that the added CpG induced maturation of WT DCs as assessed by CD80/CD86 surface expression, ability to stimulate alloreactive T cells, and ability to upregulate proinflammatory cytokine gene expression, whereas it had much lesser effects on the C3ar1−/−C5ar1−/− DCs.
Figure 4.
BM cell-derived autocrine C3ar1/C5ar1 signaling mediates TLR9-induced DC maturation in vivo. A. Representative flow plots (left) and quantification (right) of expression (flow cytometry, MFI) of C5ar1 on CD11c+ cells from recipient peripheral blood isolated 10 weeks after WT→Myd88−/− or C3ar1−/−C5ar1−/−→Myd88−/− BM transplants. B–C. Representative flow plots and quantification of normalized changes in DC expression (flow cytometry, MFI) of CD80 (B) and CD86 (C) on splenic DCs isolated 4 h after injection with 100 μg CpG or vehicle control from WT→Myd88−/− or C3ar1−/−C5ar1−/−→Myd88−/− BM chimeras. D–E. Quantified % proliferation (D) and live cell numbers (E) on day 4 of CFSE-labeled WT H-2d T cells mixed with splenic DCs isolated from H-2b WT→Myd88−/− or C3ar1−/−C5ar1−/−→Myd88−/− BM chimeras 4h after injection with CpG or control, gated on CD4+ or CD8+ T cells. F. Relative gene expression (qPCR) of Il-1b,Tnf-a, Il-12p40 and Il-6 in the splenic DCs isolated 4 h after injection with CpG or vehicle control from the BM chimeras mentioned in A–E. Representative data of two independently performed experiments (Each time 2–3 animals/group). Error bars indicate mean± SEM. **p<0.05 compared to unstimulated controls and *p<0.05 compared to stimulated WT counterparts (unpaired t test or two-way ANOVA). ns indicates p>0.05, not significant.
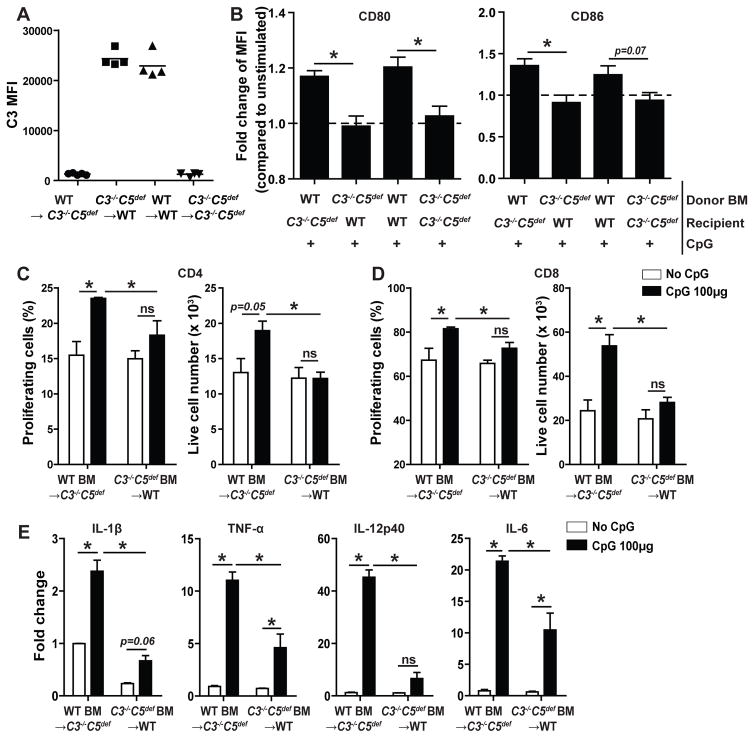
In the second approach, we tested whether the TLR effects conform to our prior findings (26–29, 36) that Teff responses (without TLR stimulation) depend upon immune cell-derived complement rather than serum/liver-derived complement. We constructed WT BM→ C3−/−C5def, C3−/−C5def BM→ WT, and control WT→WT and C3−/−C5def→ C3−/−C5def BM chimeras (H-2d), and verified >90% donor chimerism 10 weeks later (Fig 5A). We then injected groups of chimeras with CpG or vehicle, isolated splenic DCs 4 h later, and analyzed them as above (Fig 5B–E). These assays showed that while DCs from CpG-treated WT BM chimeras upregulated surface expression of CD80/86, these costimulatory molecules remained unchanged on DCs from CpG-treated chimeras possessing C3−/−C5def BM, regardless of host C3/C5 expression (Fig 5B). Culturing of the isolated DCs with allogeneic T cells (Fig 5C–D), showed that the in vivo CpG administration augmented the ex vivo T cell proliferation/expansion when DCs from mice with WT BM were used but not when DCs from mice with C3−/−C5def BM were used, regardless of serum complement. The absence of C3/C5 in BM cells likewise blunted CpG-induced upregulation of IL-1β, TNF-α, IL-12p40 and IL-6 RNA (Fig 5E).
Figure 5.
TLR9-induced DC maturation in vivo requires immune cell-derived complement proteins C3/C5. A. C3 zymosan assay. Recipient sera from each BM chimera group were tested for the presence of serum C3 10 week after BM transplants. Quantification of C3 deposition on zymosan particles (flow cytometry, MFI) is shown. B. Normalized changes in DC expression (flow cytometry, MFI) of CD80 (left) and CD86 (right) on splenic DCs isolated 4 h after injection with 100μg CpG or vehicle control from H-2d WT→ C3−/−C5def, C3−/−C5def→ WT, WT→WT and C3−/−C5def → C3−/−C5def BM chimeras. C–D. Quantified %proliferation (left panels) and live cell number (right panels) on day 4 of CFSE-labeled WT H-2b CD4+ (C) and CD8+ (D) T cells mixed with DCs isolated from H-2d WT→ C3−/−C5def, C3−/−C5def→ WT BM chimeras 4 h after injection with CpG or vehicle control. E. Relative gene expression (qPCR) of Il-1b, Tnf-a, Il-12p40 and Il-6 in splenic DCs isolated from H-2d WT→ C3−/−C5def, C3−/−C5def→ WT BM chimeras 4 h after injection with CpG or vehicle control (n=2–4/group). Error bars indicate mean± SEM. *p<0.05 (2 way ANOVA or unpaired t test), ns indicates p>0.05, not significant.
CpG-induced changes in DC transcriptomes are dependent upon C3ar1/C5ar1 signaling
To broadly assess the role of C3ar1/C5ar1 signaling on TLR-induced DC maturation in vivo, we compared the effects of in vivo CpG injection on genes expressed by splenic DCs from WT and C3ar1−/−C5ar1−/− mice using microarrays (Fig 6). Previous array studies by others performed using in vitro TLR9-stimulated DCs, showed that 0.1–10 μg/ml of CpG upregulates DC expression of proinflammatory cytokines, type 1 interferons and costimulatory signals and that many effects are NFκB (RelB)-dependent (50–52). Other work on murine spleen cell gene expression following in vivo administration of high dose (400 μg) CpG showed the maximal responses were detected 3–4 h post-injection and resulted in IFNγ- and TNFα-initiated inflammatory processes (53, 54).
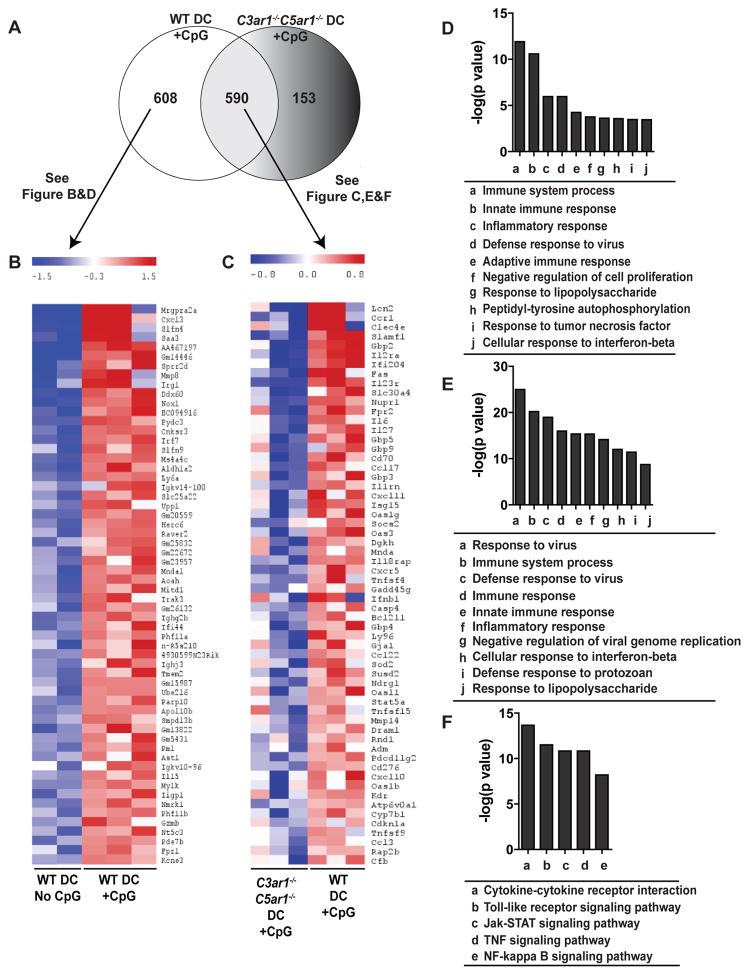
Figure 6.
Autocrine C3ar1/C5ar1 signaling in DC crucially regulates TLR9-induced changes in gene expression pathways related to inflammation and immune cell signaling. RNA isolated from splenic DCs of WT or C3ar1−/−C5ar1−/− mice 4 h after injection with CpG (100μg i.v.) or vehicle control were profiled by Affymetrix Mouse microarrays. A. Venn diagram of CpG-induced upregulated genes found in WT DCs and/or C3ar1−/−C5ar1−/− DCs. B Heat map depicting relative quantities of top 60 of the 608 genes uniquely upregulated by CpG-stimulated WT DCs (n=3) compared to levels WT DCs from untreated mice (n=2). C. Heat map depicting relative quantities of top 60 of the 590 genes upregulated by CpG in WT and C3ar1−/−C5ar1−/−DC compared to respective untreated controls (n=3/group). D. List of top 10 most significant GO terms by p values of the unique CpG-upregulated genes shown in B (DAVID database analysis). E–F List of top 10 most significant GO terms (E) and KEGG pathways (F, DAVID database analysis) for genes shown in C.
Building upon these findings and using our above identified threshold CpG doses for inducing DC maturation (Fig 3A) our new analyses show that at 4 h, 100 μg CpG induces upregulation of ~1200 WT DC genes (Fig 6A). These genes mapped to the GO terms “immune system process, response to virus, innate immune response, defense response to virus, inflammatory responses, immune response, negative regulation of viral genome replication, cellular responses to interferon-beta, defense response to protozoan and responses to lipopolysaccharide” (not shown). Included genes are related to the type 1 interferon pathway (IFNb and multiple IRFs), various cytokines and chemokines (e.g. CCL17, CCL22, CCL3, CCL4, CCL5, CXCL1, CXCL10, CXCL11, CXCL13, CXCL3, CXCL9, IL10, IL15, IL27, IL6, IL12A, IL12B, TNF), signaling pathway genes known to be downstream of TLRs (NFKBIA, CD40, LY96, IKBKE, MYD88, CD80, CD86, MAP3K8, PIK3R5, PIK3R1), and complement components (C3, CFB). Of the 1198 upregulated genes, 608 of them (51%) were induced by TLR9 in DCs from WT mice but not in DCs from CpG-treated C3ar1−/−C5ar1−/− mice (Fig 6A). These TLR9-induced, C3ar1/C5ar1-dependent genes mapped to the terms: innate immunity, immune systems processes, inflammatory response and defense response to virus (Fig 6B, D). While the remaining 590/1198 genes (49%) were upregulated in both WT and C3ar1−/−C5ar1−/− mice (including cytokine pathway-, TLR pathway-, TNF pathway-, Jak-STAT pathway-related genes, Fig 6C,E–F), greater increases for the majority of them occurred in the WT DCs, indicating that C3ar1/C5ar1 signaling amplifies the induction these other TLR9-induced DC genes. One hundred fifty-three genes were uniquely upregulated in DCs from CpG-treated C3ar1−/−C5ar1−/− mice. In contrast to the upregulated genes unique to CpG-treated WT DCs, the majority of the upregulated genes in CpG-treated C3ar1−/−C5ar1−/− DCs mapped to non-inflammatory pathways including “regulation of myeloid differentiation, regulation of protein kinase cascade, negative regulation of cell differentiation, and regulation of MAPPKKK cascade.” Together with the phenotyping and functional data delineated above, the findings support the conclusion that autocrine C3ar1/C5ar1 signaling is a crucial intermediary required for TLR-induced DC activation.
BM cell C3ar1/C5ar1 signaling is crucial for TLR9-induced cardiac allograft rejection
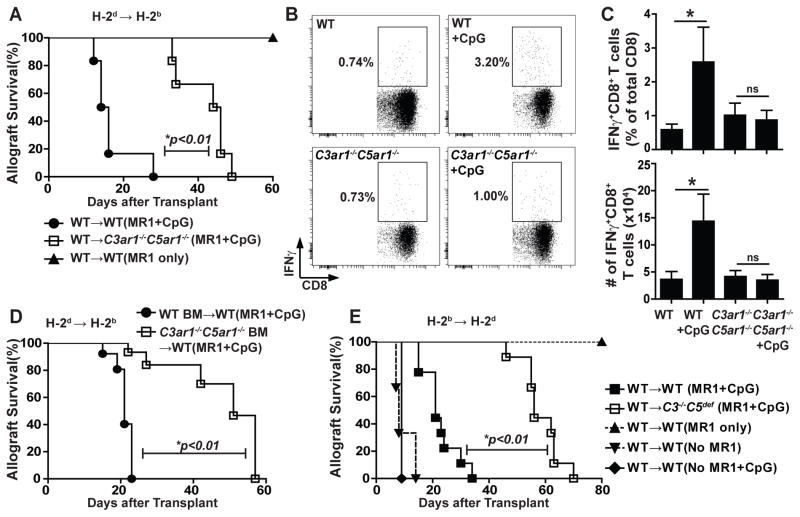
Prior work showed that costimulatory blockade with anti-CD40L mAb (MR1) delays T cell-mediated cardiac allograft rejection and can promote Treg-dependent allograft tolerance (44, 55, 56). CpG administration reverses the effects of MR1, inhibits regulatory T cell (Treg) function, augments alloreactive Teff expansion and causes rapid transplant rejection (13, 23). To test the importance of the above described connection between TLR activation and DC-derived complement activation in a clinically relevant system, we employed this transplantation model. We transplanted groups of B6 WT and C3ar1−/−C5ar1−/− recipients with allogeneic BALB/c hearts and treated them with MR1 ± CpG (Fig 7). Consistent with previous reports (13, 23), allografts transplanted into MR1-treated WT recipients survived >90 days, while post-transplant CpG administration caused cessation of graft heartbeat (with histological cellular rejection, not shown) with a median survival time (MST) of 15 d (p<0.01 vs MR1-treated WT controls). In contrast, allografts transplanted into MR1+CpG-treated C3ar1−/−C5ar1−/− recipients survived longer: MST 45 days (p<0.01 vs MR1+CpG WT controls, Fig 7A). In the absence of MR1, all WT and C3ar1−/−C5ar1−/− recipients rejected their grafts by day 9 (not shown).
Figure 7.
CpG-induced cardiac allograft rejection and enhanced alloimmunity in vivo are immune cell C3ar1/C5ar1 signaling dependent. A. Survival of H-2d WT allografts transplanted into H-2b WT or C3ar1−/−C5ar1−/− recipients + MR1 (d0) ± CpG (d 1,3 and 5) as indicated (n=6/group), *p<0.01 by log-rank (Mantel-Cox) test. B–C. Representative flow plots depicting % donor-reactive, IFNγ-producing CD8+ T cells in the recipient spleen (B) with quantification (C, top) and total number (C, bottom) on d14 post-transplant for WT or C3ar1−/−C5ar1−/− recipients of WT allografts treated with MR1±CpG as indicated. Pooled data from 4 independently done experiments with similar results (each time 2–3 animals/group) are shown. *p<0.05 (unpaired t test), ns indicates not significant. D. Survival of WT H-2d hearts transplanted into H-2b C3ar1−/−C5ar1−/−→ WT or WT→WT BM chimeras +MR1 (d0) and CpG treatment (d1, 3 and 5) (n=5/group). E. Survival of H-2b WT allografts transplanted into H-2d WT or C3−/−C5def recipients ± MR1 (d0, 7) ± CpG (d 1, 3, 5) as indicated (n=9/group for WT or C3−/−C5def→ WT+MR1+CpG, n=3–5/group for the rest), *p<0.01 by log-rank (Mantel-Cox) test.
We repeated the transplant experiments and quantified donor-reactive CD8+ T cells on day 14 (all allografts beating). Whereas CpG administration to MR1-treated WT recipients augmented frequencies and total numbers of splenic, donor-reactive IFNγ-producing CD8+ T cells (Fig 7B–C), CpG administration had no effect on the low frequencies or total numbers of donor-reactive T cells detected in the MR1-treated C3ar1−/−C5ar1−/− recipients. No donor-reactive antibodies were detected in the sera of any MR1-treated recipients (not shown).
To test the hypothesis that the prolonged allograft survival of CpG-treated C3ar1−/−C5ar1−/− mice is causally linked with the interconnection of TLR signaling and C3ar1/C5ar1 signaling in immune cells, we performed transplant studies in (CD45.2 H-2b) C3ar1−/−C5ar1−/− BM→(CD45.1) WT chimeras and H-2b WT→WT controls. Ten weeks after documenting ≥90% donor chimerism (not shown), we transplanted the chimeras with allogeneic hearts and treated them with MR1±CpG (Fig 7D). Heart grafts transplanted into the chimeras with C3ar1−/−C5ar1−/− BM survived longer (MST 51d, MST 21d, p<0.01) than those transplanted into chimeras with WT BM. Similar results (Fig 7E) were obtained in MR1-treated, H-2d C3−/−C5def recipients (MST 56d, p<0.01 vs WT control) compared to WT recipients+MR1/CpG (MST 21d,) and in corresponding BM chimeras (WT BM→WT CpG+MR1 MST: 34d vs C3−/−C5def BM→WT CpG+MR1: 50d, p<0.05, n=3–4/group, not shown), assigning the CpG reversal of MR1 costimulatory blockade to heightened immune cell C3ar1/C5ar1 signaling.
TLR9-induced Foxp3 downregulation in Treg is dependent on C3ar1/C5ar1 signaling
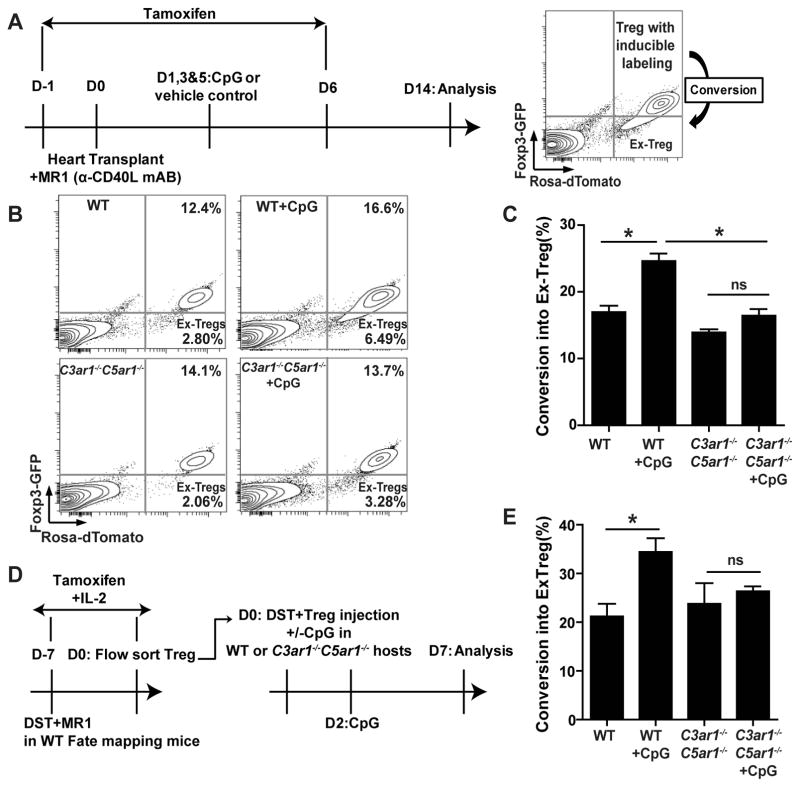
Prior work by others showed that costimulatory blockade with MR1 induces donor-reactive Treg, which prolong cardiac allograft survival/tolerance (55), and that TLR9 disrupts Foxp3 stability in Treg (12, 13). In view of these findings, we tested the hypothesis that TLR9 signaling impairs Foxp3 stability through a C3ar1/C5ar1-dependent mechanism. We used B6 Rosa(dTomato)xFoxp3CreERT2-GFP(40) “fate mapping” mice in which Foxp3+ cells constitutively express GFP, and tamoxifen treatment induces expression of dTomato under the Foxp3 promoter (GFP+dTomato+). dTomato expression without Foxp3 expression, i.e. GFPnegdTomato+ phenotype, thereby identifies T cells that were previously Foxp3+ but that have lost Foxp3 expression (termed ex- Treg)(40) (Fig 8A).
Figure 8.
CpG-induced Treg instability is C3ar1/C5ar1-dependent. A. Schematic of experimental design for B–C (left) and flow cytometry depiction of induced Treg (Foxp3GFP+dTomato+) and ex-Treg (Foxp3GFPnegdTomato+) (right). B–C. Representative flow plots (B) and quantified conversion rates (C, % ex-Treg/ total labeled Treg population) gated on live CD4+ T cells in WT or C3ar1−/−C5ar1−/− Treg “fate-mapping” mice on d 14 after allocardiac transplantation and subsequent MR1±CpG treatment. Pooled data from five independently done experiments with similar results (each time 2–4 animals/group) are shown. Error bars indicate mean± SEM. *p<0.05 (unpaired t tests). ns indicates p>0.05, not significant. D. Schematic of experimental design for E. E. Quantification of conversion rates (% ex-Treg/ total labeled Treg population) gated on splenic live CD4+ T cells in WT or C3ar1−/−C5ar1−/− hosts on d7 after Treg transfer and ±CpG treatment. Pooled data from two independently done experiments with similar results (each time 2–3 animals/group) are shown. Error bars indicate mean± SEM. *p<0.05 (unpaired t tests). ns indicates p>0.05, not significant.
We transplanted allogeneic BALB/c hearts into groups of B6 WT and C3ar1−/−C5ar1−/− Treg “fate mapping” recipient mice. We treated both groups with post-transplant MR1 (to induce Treg and prolong allograft survival) and with tamoxifen to induce Foxp3-GFP+dTomato+ Treg. We then administered CpG or vehicle to parallel groups of the heart graft recipients and analyzed their spleen cells 14 d post-transplantation (Fig 8A). The CpG administration increased ex-Treg formation from ~16 to 25% in WT recipients (p<0.05, Fig 8B–C). In contrast, ex-Treg formation in the C3ar1−/−C5ar1−/− fate mapping mice did not increase following CpG treatment (p=ns).
To test whether Treg extrinsic, systemic C3ar1/C5ar1 signaling mediates in vivo Treg instability following CpG stimulation, we induced dTomato+Foxp3-GFP+ Treg in WT B6 “fate mapping” mice by injecting them with allogeneic BALB/c spleen cells plus MR1 in the presence of tamoxifen and IL-2 (Fig 8D). Seven days later, we sorted the Foxp3-GFP+dTomato+ WT Treg and adoptively transferred them into WT or C3ar1−/−C5ar1−/− hosts. We then administered BALB/c spleen cells ± CpG DNA to the adoptive hosts and analyzed the splenic T cells on day 14. These assays showed that CpG significantly increased “ex-Treg” formation in WT adoptive hosts but had no effect on “ex-Treg” formation in the C3ar1−/−C5ar1−/− adoptive recipients (Fig 8E).
Discussion
Our findings demonstrate that autocrine C3ar1/C5ar1 signaling in DCs is a requisite process in TLR-mediated DC maturation required for induction and amplification of Teff cell responses. TLR3, 4 and 9 ligations cause MyD88- and/or TICAM1-dependent complement gene (i.e. C3 and fB) upregulation in splenic DCs in concert with downregulating cell surface DAF which together augment T cell proliferation and survival. The latter is likely mediated in part by C3ar1/C5ar1-initiated signals in T cells that we previously linked to PI-3Kγ-dependent alterations in Bcl-2, caspase-3 and Fas expression (27, 28, 31, 57, 58).
Using in vitro, ex vivo and in vivo analyses, our results uniquely demonstrate that this TLR-initiated complement production and C3ar1/C5ar1 signaling by immune cells is required for TLR-induced a) increases in DC costimulatory molecules, b) pro-inflammatory cytokine secretion by DCs, c) upregulation of DC gene pathways broadly related to inflammation and immune responses, d) DC-dependent augmentation of Teff proliferation/expansion, and e) Treg instability. Observations that CpG administration induced DC maturation in WT→MyD88−/− BM chimeras but did not in C3ar1−/−C5ar1−/− →MyD88−/− BM chimeras indicate that in vivo effects of TLR9 operates via direct effects on immune cells.
We newly document profound and expansive effects of autocrine C3ar1/C5ar1 signaling on TLR9-induced DC maturation in vivo by comparative gene expression analyses. We attempted to model physiological concentrations of CpG in vivo by titrating to threshold effects and by performing studies in MyD88 chimeras. Under the tested conditions we found that 50% of the upregulated genes were fully dependent on C3ar1/C5ar1 signaling and increases in the remainder of the genes were impaired in the absence of C3ar1/C5ar1 signaling. While the latter result suggests that some CpG-induced changes may be, at least partially, complement-independent, it is possible that exogenous (intravenous) CpG administration at any dose is “super-physiological” and induces gene expression changes that are partially dependent on complement, while DC maturation in response to physiological TLR ligation by a pathogen is fully complement-dependent. The findings by others that CpG administration at similar doses to those employed herein induces curative anti-tumor immunity (59, 60) suggest that the observed CpG-induced anti-tumor processes may also require autocrine C3ar1/C5ar1 signaling in DCs.
Our data confirm TLR9-induced upregulation of pathways linked to DC maturation, anti-viral immunity and type 1 IFNs, among others in WT mice. They also show that the absence of C3ar1/C5ar1 entirely prevented or severely blunted these changes shown to be essential for induction of effective immune responses to pathogens, model antigens and autoantigens (50–52). TLR-initiated signals alter gene expression by inducing upregulation and intranuclear transport of various transcription factors that include several IRFs, AP-1 and NFκB (6, 11, 13, 61), the latter of which has been independently linked to transcriptional regulation of fB (62). Our microarray data suggest that while many effects of TLR9 are fully dependent upon autocrine C3ar1/C5ar1 signaling, other signals initiated by C3ar1, C5ar1 and TLR9 may interact additively or synergistically to optimally induce upregulation and/or intranuclear transport of transcription factors required DC maturation. One candidate target for this signaling nexus is NFκB, as NFκB is required for DC maturation (51, 63) and is upregulated following ligation of C3ar1 (64, 65), C5ar1 (66, 67) or TLR ligation alone (68) in various cell types.
The 153 TLR9-induced genes uniquely upregulated in DCs from C3ar1−/−C5ar1−/− mice mapped to regulatory/inhibitory pathways including “regulation of myeloid leukocyte differentiation, regulation of protein kinase cascade, and negative regulation of cell differentiation” (DAVID database analysis, not shown). The fewer genes downregulated by TLR9 in WT (~250) or C3ar1−/−C5ar1−/− DCs (~140, not shown) mapped to basic pathways related to DNA repair, cell cycle, and cell division (DAVID database analysis) possibly implicating a role for C3ar1/C5ar1 signaling in regulating these fundamental cellular processes.
Although past studies have linked complement activation to TLR effects on APCs or T cells (69–72) they have not mechanistically connected the linkage with complement production by APCs themselves. These previously described effects have been attributed to “crosstalk” among systemic complement activation fragments and immune cells. Examples of reported linkages between complement and TLR signaling in APCs are a) synergistic effects of C5a and TLR4 signaling on IL-8/IL-6 production by human monocytes (73), b) C5a augmentation of LPS induced IL-10 production by human monocytes (74), c) synergism of C5a and TLR4 signaling in cytokine production by monocytes (73) and d) ability of C5a and TLR ligands to substitute for each other in enabling immune complexes to evoke IL-10 production by human monocytes (74). Examples of reported linkages between complement and APC TLR effects on elicited T cell responses are a) attenuation in C3−/− mice of T cell priming/migration in response to influenza virus (75), b) impairment in C3−/− mice of CD8 T cell expansion during viral infection (76) and more IL-6, TNF-α, and IL-1β(72) in response to TLR4 and TLR9 ligations in Daf1−/− mice (26) and increased cytokine responses derive from increased locally produced C5a/C3a and potentiated DC C5ar1/C3ar1 signal transduction (27, 28, 31). Our findings suggest re-interpretation of these observations is required, and implicate TLR-induced, DC-complement dependent mechanisms.
TLR stimulation or bacterial infection (13, 22, 23) at the time of transplantation prevents costimulatory blockade-(MR1)-induced transplant tolerance. Our results provide mechanistic insight by showing that CpG-induced acceleration of allograft rejection is mediated by TLR-induced, immune cell-autocrine C3ar1/C5ar1 signaling that augments Teff and downregulates Foxp3 expression in Treg. Our finding that TLR9-induced Treg inhibition is C3ar1/C5ar1-dependent also explains previous reports that TLR9-induced enhancements of T cell responses in vivo reflects MyD88-dependent blockade of Treg suppression (12). Our results also explain other reported connections of defective TLR signaling in mice deficient in complement components (77, 78). Since emerging evidence suggests that early post-transplant sterile inflammation is triggered by DAMP/TLR ligations (15, 79) our findings raise the possibility that peri-transplant blockade of C3ar1/C5ar1 signaling could attenuate the deleterious effects of early inflammation on enhancing alloimmunity so as to improve graft survival and function.
In summary, our data position immune cell-derived C3a/C3ar1 and C5a/C5ar1 interactions as crucial downstream intermediary steps between TLR-stimulation and DC maturation required for the development and amplification of T cell immune responses. In addition to this fundamental insight, the data support the need for testing complement inhibitors (80) for human TLR-driven disease.
Acknowledgments
The authors thank Peter Boros, Jinhua Liu, and Yansui Li (Mount Sinai) for their microsurgery support, Robert Fairchild (Cleveland Clinic) for his scientific advice, and Kevin Kelley and the Mouse Genetics and Gene Targeting Core at the Icahn School of Medicine at Mount Sinai for mouse re-derivation and production.
Footnotes
Disclosure of Conflicts of Interest
None
Contributions
Initial findings were obtained by J. Lui and M.E. Medof and published in abstract form. P. Heeger and M.E. Medof jointly and interactively led and funded the project, designed the overall direction of the studies, analyzed, reviewed and interpreted data and wrote and edited the manuscript. J. Sheen performed the majority of the experiments included in the manuscript, analyzed and interpreted data, constructed the majority of the figures, and contributed to writing and editing the manuscript. M. Strainic performed experiments and edited the manuscript. W. Zhang and Z. Yi performed the statistical analysis and interpretation of the microarray data, created the associated microarray figures, and contributed to editing of the manuscript.
References
- 1.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 3.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends in immunology. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 4.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 5.Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino K, Sugiyama T, Matsumoto M, Tanaka T, Saito M, Hemmi H, Ohara O, Akira S, Kaisho T. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 8.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 9.Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3:373–385. doi: 10.2174/1566524033479726. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 12.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, Li P, Zhang J, Ansari JM, Hancock WW, Sayegh MH, Koulmanda M, Strom TB, Turka LA. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181:1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa HT, Williams LP, Segal BM. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J Immunol. 2002;169:2781–2787. doi: 10.4049/jimmunol.169.5.2781. [DOI] [PubMed] [Google Scholar]
- 19.Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Bruck W, Becher B. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tigno-Aranjuez JT, Jaini R, Tuohy VK, Lehmann PV, Tary-Lehmann M. Encephalitogenicity of complete Freund’s adjuvant relative to CpG is linked to induction of Th17 cells. J Immunol. 2009;183:5654–5661. doi: 10.4049/jimmunol.0900645. [DOI] [PubMed] [Google Scholar]
- 21.Braza F, Brouard S, Chadban S, Goldstein DR. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol. 2016;12:281–290. doi: 10.1038/nrneph.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Ahmed E, Wang T, Wang Y, Ochando J, Chong AS, Alegre ML. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraverty R, Flutter B, Fallah-Arani F, Eom HS, Means T, Andreola G, Schwarte S, Buchli J, Cotter P, Zhao G, Sykes M. The host environment regulates the function of CD8+ graft-versus-host-reactive effector cells. J Immunol. 2008;181:6820–6828. doi: 10.4049/jimmunol.181.10.6820. [DOI] [PubMed] [Google Scholar]
- 25.Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH, Murphy WJ, Serody JS, Hemmi H, Akira S, Levy RB, Blazar BR. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008;112:3508–3516. doi: 10.1182/blood-2007-09-113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–5802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou W, Medof ME, Heeger PS, Sacks S. Graft-derived complement as a mediator of transplant injury. Curr Opin Immunol. 2007;19:569–576. doi: 10.1016/j.coi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, Heeger PS, Tuohy VK, Medof ME. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov V, Raedler H, Yuan S, Leisman S, Kwan WH, Lalli PN, Medof ME, Heeger PS. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008;181:4580–4589. doi: 10.4049/jimmunol.181.7.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9:1784–1795. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin M, Yin N, Murphy B, Medof ME, Segerer S, Heeger PS, Schroppel B. Immune cell-derived c3 is required for autoimmune diabetes induced by multiple low doses of streptozotocin. Diabetes. 2010;59:2247–2252. doi: 10.2337/db10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant. 2013;13:2530–2539. doi: 10.1111/ajt.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, Medof ME, Merad M, Heeger PS. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting Edge: Receptors for C3a and C5a Modulate Stability of Alloantigen-Reactive Induced Regulatory T Cells. J Immunol. 2013 doi: 10.4049/jimmunol.1300847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 42.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 43.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 44.Conde P, Rodriguez M, van der Touw W, Jimenez A, Burns M, Miller J, Brahmachary M, Chen HM, Boros P, Rausell-Palamos F, Yun TJ, Riquelme P, Rastrojo A, Aguado B, Stein-Streilein J, Tanaka M, Zhou L, Zhang J, Lowary TL, Ginhoux F, Park CG, Cheong C, Brody J, Turley SJ, Lira SA, Bronte V, Gordon S, Heeger PS, Merad M, Hutchinson J, Chen SH, Ochando J. DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance. Immunity. 2015;42:1143–1158. doi: 10.1016/j.immuni.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalache S, Lakhani P, Heeger PS. Effects of preexisting autoimmunity on heart graft prolongation after donor-specific transfusion and anti-CD154. Transplantation. 2014;97:12–19. doi: 10.1097/TP.0b013e3182a77eba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic acids research. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 49.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iparraguirre A, Tobias JW, Hensley SE, Masek KS, Cavanagh LL, Rendl M, Hunter CA, Ertl HC, von Andrian UH, Weninger W. Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide. J Leukoc Biol. 2008;83:610–620. doi: 10.1189/jlb.0807511. [DOI] [PubMed] [Google Scholar]
- 51.Shih VF, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD, Yu T, Fagerlund R, Asagiri M, Zuniga EI, Hoffmann A. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu D, Sanin DE, Everts B, Chen Q, Qiu J, Buck MD, Patterson A, Smith AM, Chang CH, Liu Z, Artyomov MN, Pearce EL, Cella M, Pearce EJ. Type 1 Interferons Induce Changes in Core Metabolism that Are Critical for Immune Function. Immunity. 2016;44:1325–1336. doi: 10.1016/j.immuni.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klaschik S, Tross D, Klinman DM. Inductive and suppressive networks regulate TLR9-dependent gene expression in vivo. J Leukoc Biol. 2009;85:788–795. doi: 10.1189/jlb.1008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klinman DM, Klaschik S, Tomaru K, Shirota H, Tross D, Ikeuchi H. Immunostimulatory CpG oligonucleotides: Effect on gene expression and utility as vaccine adjuvants. Vaccine. 2010;28:1919–1923. doi: 10.1016/j.vaccine.2009.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 56.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 57.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+T cells. Immunity. 2008;28:423–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandy AG, Nurkkala M, Josefsson A, Eriksson K. Therapeutic dendritic cell vaccination with Ag coupled to cholera toxin in combination with intratumoural CpG injection leads to complete tumour eradication in mice bearing HPV 16 expressing tumours. Vaccine. 2007;25:6037–6046. doi: 10.1016/j.vaccine.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 60.Cho EI, Tan C, Koski GK, Cohen PA, Shu S, Lee WT. Toll-like receptor agonists as third signals for dendritic cell-tumor fusion vaccines. Head Neck. 2010;32:700–707. doi: 10.1002/hed.21241. [DOI] [PubMed] [Google Scholar]
- 61.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray PJ, Muller M, Decker T. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol. 2004;173:7416–7425. doi: 10.4049/jimmunol.173.12.7416. [DOI] [PubMed] [Google Scholar]
- 62.Huang Y, Krein PM, Muruve DA, Winston BW. Complement factor B gene regulation: synergistic effects of TNF-alpha and IFN-gamma in macrophages. J Immunol. 2002;169:2627–2635. doi: 10.4049/jimmunol.169.5.2627. [DOI] [PubMed] [Google Scholar]
- 63.Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, Verthuy C, Davanture S, Azukizawa H, Flores-Langarica A, Dalod M, Lawrence T. Homeostatic NF-kappaB Signaling in Steady-State Migratory Dendritic Cells Regulates Immune Homeostasis and Tolerance. Immunity. 2015;42:627–639. doi: 10.1016/j.immuni.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Ahamed J, Venkatesha RT, Thangam EB, Ali H. C3a enhances nerve growth factor-induced NFAT activation and chemokine production in a human mast cell line, HMC-1. J Immunol. 2004;172:6961–6968. doi: 10.4049/jimmunol.172.11.6961. [DOI] [PubMed] [Google Scholar]
- 65.Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, Nemenoff RA, Quigg RJ, Holers VM. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819–1828. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- 66.Allendorf DJ, Yan J, Ross GD, Hansen RD, Baran JT, Subbarao K, Wang L, Haribabu B. C5a-mediated leukotriene B4-amplified neutrophil chemotaxis is essential in tumor immunotherapy facilitated by anti-tumor monoclonal antibody and beta-glucan. J Immunol. 2005;174:7050–7056. doi: 10.4049/jimmunol.174.11.7050. [DOI] [PubMed] [Google Scholar]
- 67.Kastl SP, Speidl WS, Kaun C, Rega G, Assadian A, Weiss TW, Valent P, Hagmueller GW, Maurer G, Huber K, Wojta J. The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF-kappaB activation. J Thromb Haemost. 2006;4:1790–1797. doi: 10.1111/j.1538-7836.2006.02046.x. [DOI] [PubMed] [Google Scholar]
- 68.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 69.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–1015. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawlisch H, Kohl J. Complement and Toll-like receptors: key regulators of adaptive immune responses. Mol Immunol. 2006;43:13–21. doi: 10.1016/j.molimm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 71.Raby AC, Holst B, Davies J, Colmont C, Laumonnier Y, Coles B, Shah S, Hall J, Topley N, Kohl J, Morgan BP, Labeta MO. TLR activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur J Immunol. 2011;41:2741–2752. doi: 10.1002/eji.201041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 2007;30:178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- 74.DiMartino SJ, Yuan W, Redecha P, Ivashkiv LB, Salmon JE. Insoluble immune complexes are most effective at triggering IL-10 production in human monocytes and synergize with TLR ligands and C5a. Clin Immunol. 2008;127:56–65. doi: 10.1016/j.clim.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 76.Suresh M, Molina H, Salvato MS, Mastellos D, Lambris JD, Sandor M. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J Immunol. 2003;170:788–794. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 77.Spirig R, van Kooten C, Obregon C, Nicod L, Daha M, Rieben R. The complement inhibitor low molecular weight dextran sulfate prevents TLR4-induced phenotypic and functional maturation of human dendritic cells. J Immunol. 2008;181:878–890. doi: 10.4049/jimmunol.181.2.878. [DOI] [PubMed] [Google Scholar]
- 78.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176:3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 79.Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and Damage-Associated Molecular Patterns (DAMPs) Am J Transplant. 2016 doi: 10.1111/ajt.13963. [DOI] [PubMed] [Google Scholar]
- 80.Ricklin D, Lambris JD. New milestones ahead in complement-targeted therapy. Semin Immunol. 2016;28:208–222. doi: 10.1016/j.smim.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]